子宫的快速恢复是缩短怀孕间隔的关键,是提高繁殖效率的重要因素,决定着牧场的经济效益。因此,为保障牧场的经济效益,缩短子宫复旧所需的时间,是生产者及相关领域研究人员亟待解决的问题。而明确子宫复旧调控机制是解决上述问题的前提。子宫复旧过程涉及子宫组织的修复、更新和重塑,其中潜在介质包括细胞因子、基质降解酶和生长因子[1]。分娩后,随着胎衣的排出,子宫处于退化状态,子宫内膜和子宫肌层的组织被修复和重塑[2]。目前,尚不清楚子宫组织修复的具体分子调控机制,但是可以肯定的是,干细胞在产后子宫组织修复中扮演着重要的角色。其次,子宫复旧不仅涉及组织修复,还涉及到子宫肌层重塑以及子宫形态的恢复,产后子宫迅速恢复到妊娠前的水平需要子宫肌层的退化。对于子宫肌层退化机制比较公认的说法是子宫平滑肌细胞的自噬[3]。另外,基质金属蛋白酶(matrix metalloproteinase system,MMPs)、胰岛素样生长因子(insulin-like growth factor,IGF)、胰岛素样生长因子结合蛋白(insulin-like growth factor binding protein,IGFBP)、非编码RNA以及雌激素与孕激素也能显著调控子宫组织重塑与修复过程。胰岛素样生长因子系统包括IGFs和IGFBPs,可以帮助子宫复旧[1, 4]。基质金属蛋白酶也能在产后子宫恢复过程中广泛调控细胞外基质重塑[5]。雌激素与孕激素对于子宫炎症的调节、肌层的收缩以及内膜的再生也具有重要调控作用。因此,探明子宫复旧机制对于阐释人类生殖疾病及哺乳动物产后繁殖机能恢复非常必要。此外,加快产后子宫复旧进程,缩短产犊间隔,提高繁殖效率,促进动物产后子宫复旧的技术与方法也已成为繁殖生物学领域的研究热点。目前促进子宫复旧的方法主要集中在防治子宫炎诱发的子宫复旧延迟,维生素与葡萄糖类物质、中草药、激素以及益生菌处理等方面。本文主要综述了动物子宫复旧的调控机制以及促进子宫复旧方法的最新进展,以期为减少人类产后疾病的治疗及促进哺乳动物产后子宫复旧提供一定参考。
1 子宫复旧的调控机制 1.1 干细胞促进复旧期子宫组织的修复在女性生育期内,人类子宫内膜经历了大约450次增殖、分化、脱落和再生周期,子宫内膜这种强大的再生能力归因于位于组织基底层的干细胞[6]。干细胞是未分化的细胞,能够在适当的刺激下同时自我更新和分化成多种组织特异性细胞类型[7]。研究表明,子宫内膜组织中的子宫内膜干细胞(endometrial stem cells,EndoSCs)可以参与子宫内膜的再生和修复[8-10]。目前,研究人员已在子宫内膜组织中鉴定出少数具有集落形成能力、自我更新和分化潜能的EndoSCs,如子宫内膜上皮祖细胞(epithelial progenitor cells,EEPCs)、子宫内膜间充质干细胞(endometrial mesenchymal stem cells,eMSCs)和侧群(side population,SP)细胞[10]。尽管在动物上缺少产后子宫内膜再生相关的模型研究,但是在人上EEPCs已在子宫内膜基底层的腺体中发现,它们在月经开始后48 h内开始重新上皮化[11]。这表明了EEPCs具有促进子宫内膜再生的作用。eMSCs位于子宫内膜层和基底层的血管周围,也在月经血中找到[12]。有研究表明,MSCs具有促进皮肤伤口愈合的能力[13],eMSCs能够向周围受损组织迁移,同样可以促进组织修复和愈合[14]。这些均表明eMSCs在参与子宫内膜修复的调控中发挥着重要作用。目前认为,子宫eMSCs细胞活性的调节主要基于WNT/β-catenin信号通路。Nusse和Clevers[15]发现,WNT/β-catenin信号通路在多种哺乳动物器官中成体干细胞的种群扩增和自我更新中发挥作用。同时,Cao等[16]证实,子宫肌细胞分泌的细胞因子可通过WNT/β-catenin信号通路促进人子宫内膜细胞增殖与再生。在猪上也发现WNT/β-catenin通路可以调节子宫内膜的再生和发育[17]。另外,WNT5A(Wnt family member 5A)在FZD4受体(Frizzled 4)和低密度脂蛋白受体相关蛋白5(low-density lipoprotein receptor-related protein 5,LRP5)存在的情况下可激活WNT/β-catenin通路[18],而FZD4和LRP5均在eMSCs中表达[16]。由此表明,WNT/β-catenin信号通路在调节eMSCs促进产后子宫内膜再生的过程中发挥重要作用。SP细胞具有成体干细胞的基因型、表型和功能特征,属于子宫内膜的干细胞或祖细胞,具有促进子宫内膜再生与修复的能力[10, 19]。Lv等[10]认为,SP细胞在体外能够分化为子宫内膜细胞、子宫基质细胞、上皮细胞,并且将SP细胞分化的子宫内膜细胞移植到小鼠肾实质后能够形成成熟的血管组织,反映了SP细胞具有促进子宫内膜再生和血管组织生成的能力。此外,骨髓间充质干细胞(bone marrow mesenchymal derived stem cells,BMSCs)迁移到子宫内膜,并通过释放细胞因子等方式也能间接促进子宫重塑和修复[20]。
血管生成对子宫复旧同样重要。在子宫内膜表面重新上皮化后,子宫内膜腺体、基质和脉管系统都快速再生[21]。干细胞在子宫内膜血管生成方面也发挥着巨大的作用。在子宫内膜血管周细胞包括毛细血管和微血管周围的周细胞以及位于大血管最外层的外膜细胞,这些细胞已经显示出间充质干细胞(mesenchymal stem cells,MSCs)的表型和再生能力[22]。外膜细胞则被认为是周细胞的前体,也具备克隆形成、自我更新和多系中胚层分化的能力[23]。因此,血管周细胞与外膜细胞具备MSCs的能力进而参与血管的再生修复。有研究表明,来自脐带的MSCs通过宫内注射应用到大鼠模型中,被证明可诱导子宫内膜增殖以及促进血管生成[24]。
因此,在复旧期间干细胞能分化参与子宫内膜再生,并且促进子宫血管生长,为上皮化后基质的扩增提供营养,从而促进产后子宫组织的修复。
1.2 胰岛素样生长因子(insulin-like growth factor,IGF)对子宫复旧的调控IGF在产后子宫组织修复过程中发挥重要作用。IGF与靶组织细胞表面的IGF1受体α亚基结合导致β亚基构象变化,从而激活受体酪氨酸激酶活性[25],活化的受体磷酸化几种底物,包括胰岛素受体底物(insulin receptor substrates,IRS)和Src同源胶原蛋白(Src homology collagen,SHC),并且这些底物中的磷酸酪氨酸残基被某些含有Src同源性2 (Src homology 2,SH2)结构域的信号分子识别,其中有磷脂酰肌醇3-激酶、生长因子受体和含SH2的蛋白酪氨酸磷酸酶2的85 ku调节亚基(p85)[26],它们的结合会进一步激活下游PI3-激酶信号通路[26]。而PI3K/AKT/mTOR是细胞存活、增殖、侵袭、凋亡和细胞周期的关键信号通路[27]。有研究表明,IGFs参与猫子宫细胞的增殖与内膜的生长发育[28]。在奶牛产后,IGFs通过PI3K/AKT/mTOR途径可以促进子宫内膜细胞的增殖[29],并且血浆中低浓度的IGF-Ⅰ会延迟子宫复旧[30]。因此,IGF通过调节PI3K信号通路参与产后子宫内膜细胞的增殖与分化进而促进子宫复旧。
另外,尽管IGFBPs在多数情况下通过阻止IGF与IGF受体结合来抑制IGF的调节作用,阻碍产后子宫内膜再生,但其仍可通过其他方式促进子宫复旧[29]。IGFBP-2通过提高IGF-Ⅰ和IGF-Ⅱ的利用率以及这些配体与其受体的相互作用间接增强子宫收缩[4],促进产后子宫恶露排出与形态恢复。另外,IGFBP-2还通过反式激活血管内皮生长因子基因表达促进产后子宫内膜血管生成,进而促进子宫复旧[31]。
1.3 基质金属蛋白酶(matrix metalloproteinase system,MMPs)对子宫复旧的调控MMPs是含锌的内肽酶,按底物或其结构域的组织结构划分为胶原酶(MMP-1,MMP-8和MMP-13)、明胶酶(MMP-2和MMP-9)、基质分解素(MMP-3,MMP-10和MMP-11)、基质溶素(MMP-7)和金属弹性蛋白酶(MMP-12)[5]。MMPs参与子宫肌层细胞外基质(myometrial extracellular matrix,ECM)中胶原蛋白的降解,进而促进子宫肌层的退化[32]。MMP是主要的ECM重塑蛋白酶[33],胶原酶(MMP-8和MMP-13)会将纤维状胶原切割成碎片,然后变性成明胶,明胶酶(MMP-2和MMP-9)随后会将明胶降解为小肽片段,最后小肽片段通过浸润免疫细胞快速被去除[5]。当子宫肌层ECM降解后,子宫迅速收缩进而促进子宫复旧。此外,MMPs可以调节IGFBPs的活性,IGFBPs抑制IGF与其受体结合,而MMPs可通过切割IGFBPs/IGF复合物,释放IGF与IGF受体结合,进而通过PI3K/AKT/mTOR通路促进子宫内膜细胞增殖与分化(图 1)。MMP-7通过切割IGFBP-5中NH2-末端结构域中与IGF结合的位点进而促进IGF-Ⅱ的释放[34];MMP-1切割IGF-Ⅱ/IGFBP-2复合物,导致游离IGF-Ⅱ的释放[35]。因此,MMPs可通过降低IGFBPs与IGFs的亲和力,促进IGFs与IGF受体结合进而间接促进子宫复旧。其他种类MMPs也在产后子宫复旧的调控中发挥重要作用(表 1)。
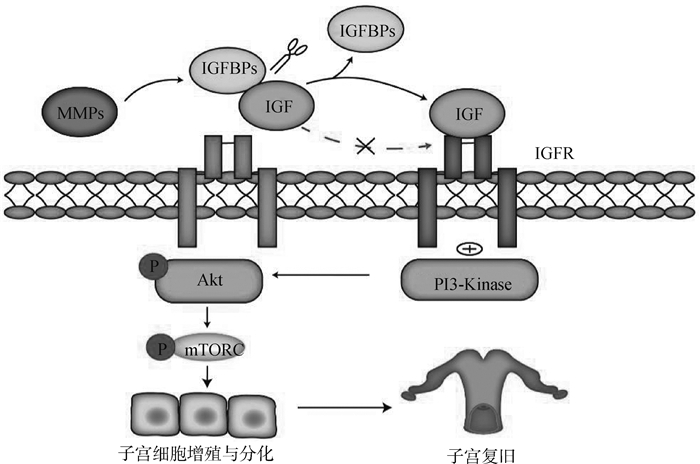
|
MMPs通过切割IGFBPs与IGF复合体释放IGF,释放的IGF通过与其受体IGFR结合通过PI3K/AKT/mTOR促进子宫复旧 MMPs releases IGF by cutting IGFBPs and IGF complex, and the released IGF promotes uterine involution through PI3K/AKT/mTOR by binding with its receptor IGFR 图 1 MMPs通过IGF激活PI3K/AKT/mTOR信号通路促进子宫复旧 Fig. 1 MMPs activates PI3K/AKT/mTOR signaling pathway through IGF to promote uterine involution |
|
|
表 1 其他MMPs在子宫复旧中的功能及调控作用 Table 1 The function and regulation of other MMPs in uterine involution |
miRNAs广泛表达于哺乳动物不同组织中,并且子宫组织中的miRNA与对应的靶基因结合后调节子宫细胞增殖[38]。miR-145/miR-143可以靶向cdc2抑制宫颈上皮细胞增殖[39]。进一步研究发现,miR-145/miR-143可以与靶基因cdc2 mRNA 30UTR 4004-4026和1095-1112序列位点进行特异性结合,下调cdc2的表达,从而将细胞周期阻滞在G1-S期[40]。An等[41]研究表明,miR-449a通过下调几种细胞周期调节因子,如细胞周期蛋白依赖性激酶(CDKs)CDK6和CDK4、细胞周期蛋白(cyclin D1、Cyclin E2),阻止G1期细胞进入S期,进而抑制子宫复旧。Yang等[38]研究也表明,miRNA-200A-PTEN和miRNA-133-FGFR1均可通过PI3K/AKT信号通路参与调控产后子宫复旧。PTEN是一种多功能磷酸酶,通过其C-末端磷酸化调节自身活性,并参与促凋亡或凋亡过程,从而调节下游PI3K/AKT信号通路。成纤维细胞生长因子受体1(fibroblastic growth factor receptor 1,FGFR-1)属于FGF家族,人类子宫组织中的FGFR1参与调控月经后子宫内膜的再生与修复[42]。尽管研究者已经发现miRNAs在调控动物产后子宫复旧过程中扮演重要角色,但是到目前为止相关报道仍然较少,关于miRNAs对子宫复旧的调控机制仍需要做进一步深入研究。
1.5 孕酮(progesterone,P4)和雌二醇(estrogen,E2)对产后子宫复旧的调控P4和E2对子宫肌层的静息与收缩具有反向调节作用。P4水平的升高介导PR诱导IκBα抑制NF-κB进而抑制收缩相关基因(环氧合酶2和前列腺素F2α受体)的表达,维持子宫肌层静止[43]。雌激素受体(ERα)激活增强了OXTR、CX43和环氧合酶2的转录,催化前列腺素的产生,进而促进子宫肌层收缩[44]。产后子宫肌层的收缩对于促进恶露的排除、凋亡上皮的脱落以及后续子宫内膜的再生具有重要意义。
另外,P4与E2可以通过调控细胞的增殖与凋亡促进子宫内膜再生。E2可通过增加子宫内膜细胞中PKB/AKT的磷酸化直接快速影响PI3K相关信号通路促进子宫内膜再生[45],而P4则会抵消E2作用[46]。
2 促进产后子宫复旧的方法 2.1 防治子宫炎促进子宫复旧2.1.1 生物制剂防治子宫炎 产后子宫的微生物污染在产后第一周普遍存在[47-48]。当病原菌未被清除时,子宫会受到感染和发炎延迟子宫复旧[47, 49]。产褥期发生子宫炎与子宫内膜炎阻碍产后子宫内膜的再生、降低子宫肌层的收缩性,是导致子宫复旧不全的主要原因之一[49-51]。自身免疫机能与奶牛产后子宫炎的发生密切相关。白细胞介素-8(IL-8)是一种促炎细胞因子,也是中性粒细胞的主要趋化因子。IL-8与中性粒细胞表面受体CXCR1和CXCR2结合可诱导中性粒细胞活化,刺激趋化作用,增强吞噬和杀伤能力。因此,像IL-8这样的效应分子的存在对于吸引中性粒细胞进入子宫并维持子宫健康至关重要。Zinicola等[52]研究表明,重组牛白细胞介素-8(rbIL-8)处理降低了经产奶牛子宫炎的发生率,进而加快了产后子宫复旧进程;并且认为,rbIL-8能够以接近绿色的方式在体外和体内(阴道内和宫内给药后)产生预期的生物学反应,促进奶牛产后子宫复旧。另外,噬菌体可以控制细菌生长,是防治子宫细菌感染的潜在替代方法[53]。然而,Machado等[54]研究表明,在奶牛分娩后(2±1)d子宫内注入约107 PFU剂量的噬菌体混合物不会影响子宫健康、生殖性能,对预防子宫炎和子宫内膜炎无效。Meira等[55]也在奶牛妊娠第230、260和275天子宫内注入约109 PFU噬菌体混合物,但对防治子宫炎效果甚微。然而,关于噬菌体对奶牛子宫内膜炎的防治尚处于研究的起始阶段,噬菌体是否能够用于产后奶牛子宫炎的有效防治还需进一步研究。除此之外,还有其他一些生物制剂通过防治子宫炎间接促进奶牛产后子宫复旧(表 2)。
|
|
表 2 生物制剂防治子宫炎促进子宫复旧的方法及途径 Table 2 Method and approach for preventing and treating metritis and promoting uterine involution by biological preparation |
2.1.2 非甾体抗炎药促进子宫复旧 非甾体抗炎药(NSAID)具有镇痛、卵巢功能恢复、预防和治疗子宫炎症等作用[59],因此,在一定程度上可以通过防治子宫炎促进子宫复旧。NSAID主要通过抑制环加氧酶(COX)-1和COX-2进而抑制前列腺素合成来减少动物感染时的疼痛、炎症反应和疾病行为[60]。美洛昔康、氟尼辛葡甲胺与卡洛芬都属于NSAID,已被应用于缓解患子宫炎和子宫内膜炎奶牛的疼痛和炎症控制。Pascottini等[61]研究表明,奶牛产后10~13 d每天皮下注射美洛昔康(0.5 mg·kg-1 BW),与对照组相比改善了中性粒细胞(PMN)功能,提高了血液中葡萄糖和IGF-Ⅰ浓度,但对防治子宫炎效果甚微。Amiridis等[62]研究表明,给产后患有子宫炎的奶牛静脉注射氟尼辛葡甲胺,剂量为2.2 mg·kg-1,共6次(前2 d每天2次,随后2 d每天1次),处理组奶牛的子宫复旧速度比对照组快,产后首次发情早于对照组。Rodríguez等[63]研究表明,奶牛在产后12~48 h皮下注射卡洛芬(1.4 mg·kg-1 BW),降低了患临床子宫炎的发生率和风险。关于非甾体抗炎药在防治子宫炎方面的应用报道相对来说较少,其有效性的证据是有限的,因此有待进一步研究。
2.1.3 抗生素防治子宫炎促进子宫复旧 奶牛产后早期使用抗生素可以防治子宫炎,减少产后子宫疾病的发生率,进而有助于子宫复旧。Witte等[64]研究表明,在奶牛产后12~24 h,皮下注射头孢噻呋结晶游离酸(6.6 mg·kg-1 BW),在其血清、子宫内膜组织和恶露中头孢噻呋衍生物的药物浓度均达到抑制相关病原体(如大肠杆菌和化脓杆菌)所需的最低药物浓度。因此,在奶牛产后早期应用头孢噻呋结晶游离酸可以防治产后子宫感染和子宫炎等相关疾病。Credille等[65]研究表明,奶牛产后早期肌肉注射氨苄青霉素三水合物(11 mg·kg-1 BW),每24 h(共3剂)或每12 h(共5剂)1剂持续3 d,在其牛奶、恶露液和子宫内膜组织中均可达到治疗浓度。Risco和Hernandez[66]研究表明,在奶牛产后1~5 d,每天静脉注射盐酸头孢噻呋(2.2 mg·kg-1 BW),也能够抑制子宫病原菌生长。综上所述,头孢噻呋结晶游离酸、氨苄青霉素与盐酸头孢噻呋等抗生素在奶牛产后早期的应用可以抑制引发子宫炎症的相关菌群生长,具有防治子宫炎进而促进子宫复旧的作用。
2.2 维生素、脂肪酸与葡萄糖类物质促进子宫复旧子宫复旧的过程中,胶原被降解,血液中出现游离甘氨酸和羟脯氨酸。血液中的羟脯氨酸和甘氨酸的浓度与子宫复旧有关,可作为评价子宫复旧速度和完成度的指标[67]。奶牛产犊前日粮中添加β-胡萝卜素对繁殖和免疫力有积极作用。Kaewlamun等[67]研究表明,产犊前60 d奶牛饲粮中添加1 g·d-1 β-胡萝卜素,可增加奶牛血液中羟脯氨酸浓度(子宫复旧的指标)与子宫中多形核白细胞的百分比,进而促进产后奶牛子宫复旧。
Omega-3脂肪酸可以调节类花生酸(前列腺素和白三烯)的合成[68],参与促炎或抗炎刺激调节子宫免疫[69]。子宫内膜中的PGF2α和白三烯可以激活中性粒细胞,并增加其不依赖于抗体的细胞介导的趋化性、迁移性、吞噬作用和细胞毒性能力[69],这对于产后预防子宫感染至关重要。Ulfina等[70]研究发现,与对照组相比,产犊前3周到产后3周日粮中添加750 g·d-1碎亚麻籽(含omega-3脂肪酸)的奶牛能够显著增加产后30 d内子宫复旧率。
另外,日粮中添加过瘤胃葡萄糖(rumen-protected glucose,RPG)能促进子宫复旧。IGF-Ⅰ和IGF-Ⅱ则被证明可以通过调节PI3K/AKT/mTOR通路对细胞增殖和组织修复的反应[71]。Wang等[29]研究表明,产前7 d直至产后14 d每天2次共喂食200 g RPG的奶牛产后14 d的血液IGF-Ⅰ水平明显更高,与对照组相比,RPG组(处理组)奶牛IGF家族成员(IGF-Ⅰ、IGF-Ⅱ和IGF1受体)和IGF结合蛋白(IGFBP-1、IGFBP-2、IGFBP-4和IGFBP-5) mRNA均表达上调;补充RPG还增加了子宫内膜磷酸化AKT与总AKT的比值和p-mTOR与总mTOR的比值,表明PRG可能通过IGF/IGFR/PI3K/AKT/mTOR信号通路促进奶牛产后子宫复旧。
2.3 中草药促进子宫复旧葫芦巴中的葫芦巴碱被归类为植物雌激素,具有激活雌激素受体的能力[72];孜然、葫芦巴与姜黄可以重建中性粒细胞功能和淋巴细胞增殖能力[73];青蒿素具有抗菌作用与抗炎作用[74-75]。这些草药在预防产后子宫感染与促进产后子宫恢复等方面发挥重要作用。Japheth等[76]研究表明,中草药(青蒿25 g、姜黄25 g、孜然25 g、葫芦巴25 g、小茴香25 g、茴香草25 g和生姜25 g)混合剂和标准浓缩饲料每天1次饲喂产后水牛可以提高其免疫力并促进恶露排出,进而促进水牛产后早期子宫复旧。Lee等[77]研究表明,产后5 d内奶牛每天日粮中添加70 g生化汤草药制剂,在产后第7天子宫内膜的面积和直径明显减小,产后第13天宫腔积液量平均值明显低于对照组的((1.2±0.6) cm3vs. (2.3±0.8) cm3);产后第19天,子宫张力评分平均值也明显低于对照组的(1.0±0.0 vs. 1.5±0.5),最后子宫复旧与卵巢恢复的时间也早于对照组的。以上研究均表明产后使用中药组合制剂可以加速奶牛产后恶露排出,预防子宫感染,促进子宫复旧。
2.4 激素促进子宫复旧子宫内膜持续释放高浓度的PGF2α是子宫复旧过程的必要条件,主要是使子宫平滑肌收缩以改善恶露的排出以及子宫的形态和功能恢复[62, 78]。Yu等[79]研究表明,荷斯坦奶牛在产后早期接受PGF2α处理可以促进子宫健康,同时可以抑制产后感染。Menlendez等[80]发现,产后早期给予外源性PGF2α可以增加子宫肌电活动,促进子宫收缩。此外,马绒毛膜促性腺激素(equine chorionic gonadotropin,eCG)可以促进排卵周期早期恢复,有益于子宫复旧。Canadas等[81]研究表明,产后第8天用eCG处理的奶牛比未处理的平均子宫角直径更小,子宫复旧更好;同时发现eCG能够增加排卵周期中雌二醇的浓度。而雌二醇浓度的升高能够通过PI3K相关信号通路促进子宫内膜再生,进而促进产后子宫复旧[44, 46, 82]。
3 展望子宫复旧是一个复杂的过程,干细胞、金属蛋白酶、miRNAs等诸多因子参与了子宫组织的修复与重塑及子宫内膜的再生。尽管目前人们已在细胞和miRNAs水平对子宫复旧的机制进行了初步的探索,但这方面的研究仍比较少,子宫复旧的分子调控机制仍不清楚。非编码RNA一直是生物学领域研究的热点,除了miRNAs可以作用于子宫复旧以外,cirRNAs也可以发挥作用。已有研究表明,cir8073吸附miR-449a调节CEP55基因表达,通过PI3K/AKT/mTOR通路促进子宫内膜上皮细胞增殖[83]。但是关于cirRNAs调控子宫复旧的机制仅限于上述报道。另外,lncRNA是否也参与调控产后子宫复旧尚不清楚。因此,对于非编码RNA参与调控产后子宫复旧的具体分子机制仍需要做进一步深入和广泛研究。此外,目前已有的研究主要集中在体外单个细胞因子或基因对子宫复旧的调控,这既不能够实现体内子宫复旧机制的精确研究,也不能在系统生物学层面研究子宫复旧的分子网络调控机制。同时,子宫微生物是否在子宫复旧的过程中参与产后子宫复旧也不清楚。因此,将来可借助如转录组、微生物组、代谢组等多组学技术进一步挖掘调控动物产后子宫复旧的分子网络机制。
重组牛白细胞介素-8、中药等能够防治产后奶牛子宫炎,进而促进产后子宫复旧,但其经济成本较高,这限制了其在临床上的应用,如何降低生物制剂与中药的使用成本,这是将来研究促进奶牛子宫复旧的方向之一。日粮中添β-胡萝卜素、脂肪酸与过瘤胃葡萄糖等可促进子宫复旧,其实用性与可行性较高,但尚处于实验室研究阶段。将来需要扩大试验的样本量,对研究结果再进行大规模群体验证以增加临床应用的有效性与可靠性。另外,肠道微生物及生殖道微生物与动物和人的健康息息相关,是目前生命科学领域研究热点。Deng等[84]研究表明,奶牛产后经阴道输注乳酸菌可改变血清前列腺素浓度,加速其产后子宫复旧。然而,微生态制剂在调控产后子宫复旧方面的研究尚处于起始阶段,仍需要做深入细致的研究。此外,干细胞对于组织再生至关重要,在将来,通过刺激体内干细胞再生或外源输入干细胞疗法也可能有助于产后子宫恢复。
| [1] |
SALAMONSEN L A. Tissue injury and repair in the female human reproductive tract[J]. Reproduction, 2003, 125(3): 301-311. DOI:10.1530/rep.0.1250301 |
| [2] |
SPOONER M K, LENIS Y Y, WATSON R, et al. The role of stem cells in uterine involution[J]. Reproduction, 2021, 161(3): R61-R77. DOI:10.1530/REP-20-0425 |
| [3] |
HSU K F, PAN H A, HSU Y Y, et al. Enhanced myometrial autophagy in postpartum uterine involution[J]. Taiwan J Obstet Gynecol, 2014, 53(3): 293-302. DOI:10.1016/j.tjog.2013.01.030 |
| [4] |
LLEWELLYN S, FITZPATRICK R, KENNY D A, et al. Endometrial expression of the insulin-like growth factor system during uterine involution in the postpartum dairy cow[J]. Domest Anim Endocrinol, 2008, 34(4): 391-402. DOI:10.1016/j.domaniend.2007.11.003 |
| [5] |
NGUYEN T T T N, SHYNLOVA O, LYE S J. Matrix metalloproteinase expression in the rat myometrium during pregnancy, term labor, and postpartum[J]. Biol Reprod, 2016, 95(1): 24. DOI:10.1095/biolreprod.115.138248 |
| [6] |
COUSINS F L, PANDOY R, JIN S Y, et al. The elusive endometrial epithelial stem/progenitor cells[J]. Front Cell Dev Biol, 2021, 9: 640319. DOI:10.3389/fcell.2021.640319 |
| [7] |
LEE Y J, YI K W. Bone marrow-derived stem cells contribute to regeneration of the endometrium[J]. Clin Exp Reprod Med, 2018, 45(4): 149-153. DOI:10.5653/cerm.2018.45.4.149 |
| [8] |
SANTAMARIA X, MAS A, CERVELLÓ I, et al. Uterine stem cells: from basic research to advanced cell therapies[J]. Hum Reprod Update, 2018, 24(6): 673-693. DOI:10.1093/humupd/dmy028 |
| [9] |
YIN M Z, ZHOU H J, LIN C X, et al. CD34+KLF4+ stromal stem cells contribute to endometrial regeneration and repair[J]. Cell Rep, 2019, 27(9): 2709-2724.e3. DOI:10.1016/j.celrep.2019.04.088 |
| [10] |
LV Q Y, WANG L L, LUO X Z, et al. Adult stem cells in endometrial regeneration: molecular insights and clinical applications[J]. Mol Reprod Dev, 2021, 88(6): 379-394. DOI:10.1002/mrd.23476 |
| [11] |
EVANS J, SALAMONSEN L A, WINSHIP A, et al. Fertile ground: human endometrial programming and lessons in health and disease[J]. Nat Rev Endocrinol, 2016, 12(11): 654-667. DOI:10.1038/nrendo.2016.116 |
| [12] |
GARGETT C E, SCHWAB K E, DEANE J A. Endometrial stem/progenitor cells: the first 10 years[J]. Hum Reprod Update, 2016, 22(2): 137-163. |
| [13] |
GUILLAMAT-PRATS R. The role of MSC in wound healing, scarring and regeneration[J]. Cells, 2021, 10(7): 1729. DOI:10.3390/cells10071729 |
| [14] |
LEÑERO C, BOWLES A C, CORREA D, et al. Characterization and response to inflammatory stimulation of human endometrial-derived mesenchymal stem/stromal cells[J]. Cytotherapy, 2022, 24(2): 124-136. DOI:10.1016/j.jcyt.2021.07.005 |
| [15] |
NUSSE R, CLEVERS H. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities[J]. Cell, 2017, 169(6): 985-999. DOI:10.1016/j.cell.2017.05.016 |
| [16] |
CAO M Z, CHAN R W S, CHENG F H C, et al. Myometrial cells stimulate self-renewal of endometrial mesenchymal stem-like cells through WNT5A/β-catenin signaling[J]. Stem Cells, 2019, 37(11): 1455-1466. DOI:10.1002/stem.3070 |
| [17] |
BUKOWSKA J, ZIECIK A J, LAGUNA J, et al. The importance of the canonical wnt signaling pathway in the porcine endometrial stromal stem/progenitor cells: implications for regeneration[J]. Stem Cells Dev, 2015, 24(24): 2873-2885. DOI:10.1089/scd.2015.0078 |
| [18] |
MIKELS A J, NUSSE R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context[J]. PLoS Biol, 2006, 4(4): e115. DOI:10.1371/journal.pbio.0040115 |
| [19] |
TATEBAYASHI R, NAKAMURA S, MINABE S, et al. Gene-expression profile and postpartum transition of bovine endometrial side population cells[J]. Biol Reprod, 2021, 104(4): 850-860. DOI:10.1093/biolre/ioab004 |
| [20] |
GAO L F, HUANG Z W, LIN H Y J, et al. Bone marrow mesenchymal stem cells (BMSCs) restore functional endometrium in the rat model for severe asherman syndrome[J]. Reprod Sci, 2019, 26(3): 436-444. DOI:10.1177/1933719118799201 |
| [21] |
GARGETT C E, NGUYEN H P T, YE L. Endometrial regeneration and endometrial stem/progenitor cells[J]. Rev Endocr Metab Disord, 2012, 13(4): 235-251. DOI:10.1007/s11154-012-9221-9 |
| [22] |
LI S Y, DING L J. Endometrial perivascular progenitor cells and uterus regeneration[J]. J Pers Med, 2021, 11(6): 477. DOI:10.3390/jpm11060477 |
| [23] |
ZHU X X, YU F, YAN G J, et al. Human endometrial perivascular stem cells exhibit a limited potential to regenerate endometrium after xenotransplantation[J]. Hum Reprod, 2021, 36(1): 145-159. |
| [24] |
ZHANG L, LI Y, GUAN C Y, et al. Therapeutic effect of human umbilical cord-derived mesenchymal stem cells on injured rat endometrium during its chronic phase[J]. Stem Cell Res Ther, 2018, 9(1): 36. DOI:10.1186/s13287-018-0777-5 |
| [25] |
KRAEMER W J, RATAMESS N A, HYMER W C, et al. Growth hormone(s), testosterone, insulin-like growth factors, and cortisol: roles and integration for cellular development and growth with exercise[J]. Front Endocrinol (Lausanne), 2020, 11: 33. DOI:10.3389/fendo.2020.00033 |
| [26] |
HAKUNO F, TAKAHASHI S I. 40 YEARS OF IGF1:IGF1 receptor signaling pathways[J]. J Mol Endocrinol, 2018, 61(1): T69-T86. DOI:10.1530/JME-17-0311 |
| [27] |
MIRICESCU D, TOTAN A, STANESCU-SPINU I I, et al. PI3K/AKT/mTOR signaling pathway in breast cancer: from molecular landscape to clinical aspects[J]. Int J Mol Sci, 2020, 22(1): 173. DOI:10.3390/ijms22010173 |
| [28] |
AǦAOǦLU Ö K, AǦAOǦLU A R, ÖZMEN O, et al. Expression of the insulin-like growth factor (IGF) gene family in feline uterus during pregnancy[J]. Biotech Histochem, 2021, 96(6): 439-449. DOI:10.1080/10520295.2020.1818285 |
| [29] |
WANG Y, HAN X F, TAN Z L, et al. Rumen-protected glucose stimulates the insulin-like growth factor system and mTOR/AKT pathway in the endometrium of early postpartum dairy cows[J]. Animals, 2020, 10(2): 357. DOI:10.3390/ani10020357 |
| [30] |
VALDMANN M, KURYKIN J, KAART T, et al. Relationships between plasma insulin-like growth factor-1 and insulin concentrations in multiparous dairy cows with cytological endometritis[J]. Vet Rec, 2018, 183(4): 126. DOI:10.1136/vr.104640 |
| [31] |
BACH L A. What happened to the IGF binding proteins?[J]. Endocrinology, 2018, 159(2): 570-578. DOI:10.1210/en.2017-00908 |
| [32] |
GENG J N, HUANG C, JIANG S W. Roles and regulation of the matrix metalloproteinase system in parturition[J]. Mol Reprod Dev, 2016, 83(4): 276-286. DOI:10.1002/mrd.22626 |
| [33] |
GOBIN E, BAGWELL K, WAGNER J, et al. A pan-cancer perspective of matrix metalloproteases (MMP) gene expression profile and their diagnostic/prognostic potential[J]. BMC Cancer, 2019, 19(1): 581. DOI:10.1186/s12885-019-5768-0 |
| [34] |
HEMERS E, DUVAL C, MCCAIG C, et al. Insulin-like growth factor binding protein-5 is a target of matrix metalloproteinase-7:implications for epithelial-mesenchymal signaling[J]. Cancer Res, 2005, 65(16): 7363-7369. DOI:10.1158/0008-5472.CAN-05-0157 |
| [35] |
GUAN S P, LAM A T L, NEWMAN J P, et al. Matrix metalloproteinase-1 facilitates MSC migration via cleavage of IGF-2/IGFBP2 complex[J]. FEBS Open Bio, 2018, 8(1): 15-26. DOI:10.1002/2211-5463.12330 |
| [36] |
SAWICKI G, LEON H, SAWICKA J, et al. Degradation of myosin light chain in isolated rat hearts subjected to ischemia-reperfusion injury: a new intracellular target for matrix metalloproteinase-2[J]. Circulation, 2005, 112(4): 544-552. DOI:10.1161/CIRCULATIONAHA.104.531616 |
| [37] |
LOMBARDI A, MAKIEVA S, RINALDI S F, et al. Expression of matrix metalloproteinases in the mouse uterus and human myometrium during pregnancy, labor, and preterm labor[J]. Reprod Sci, 2018, 25(6): 938-949. DOI:10.1177/1933719117732158 |
| [38] |
YANG H, FU L, LUO Q F, et al. Identification and validation of key miRNAs and miRNA-mRNA regulatory network associated with uterine involution in postpartum Kazakh sheep[J]. Arch Anim Breed, 2021, 64(1): 119-129. DOI:10.5194/aab-64-119-2021 |
| [39] |
ANTON L, DEVINE A, SIERRA L J, et al. miR-143 and miR-145 disrupt the cervical epithelial barrier through dysregulation of cell adhesion, apoptosis and proliferation[J]. Sci Rep, 2017, 7(1): 3020. DOI:10.1038/s41598-017-03217-7 |
| [40] |
YUAN D Z, LEI Y, ZHAO D, et al. Progesterone-induced miR-145/miR-143 inhibits the proliferation of endometrial epithelial cells[J]. Reprod Sci, 2019, 26(2): 233-243. DOI:10.1177/1933719118768687 |
| [41] |
AN X P, LIU X R, ZHANG L, et al. MiR-449a regulates caprine endometrial stromal cell apoptosis and endometrial receptivity[J]. Sci Rep, 2017, 7(1): 12248. DOI:10.1038/s41598-017-12451-y |
| [42] |
CHIUMIA D, SCHULKE K, GROEBNER A E, et al. Initiation of conceptus elongation coincides with an endometrium basic fibroblast growth factor (FGF2) protein increase in heifers[J]. Int J Mol Sci, 2020, 21(5): 1584. DOI:10.3390/ijms21051584 |
| [43] |
HARDY D B, JANOWSKI B A, COREY D R, et al. Progesterone receptor plays a major antiinflammatory role in human myometrial cells by antagonism of nuclear factor-κB activation of cyclooxygenase 2 expression[J]. Mol Endocrinol, 2006, 20(11): 2724-2733. DOI:10.1210/me.2006-0112 |
| [44] |
MENDELSON C R, GAO L, MONTALBANO A P. Multifactorial regulation of myometrial contractility during pregnancy and parturition[J]. Front Endocrinol (Lausanne), 2019, 10: 714. DOI:10.3389/fendo.2019.00714 |
| [45] |
KAYISLI O G, KAYISLI U A, LULECI G, et al. In vivo and in vitro regulation of Akt activation in human endometrial cells is estrogen dependent[J]. Biol Reprod, 2004, 71(3): 714-721. DOI:10.1095/biolreprod.104.027235 |
| [46] |
MARUYAMA T, YOSHIMURA Y. Molecular and cellular mechanisms for differentiation and regeneration of the uterine endometrium[J]. Endocr J, 2008, 55(5): 795-810. DOI:10.1507/endocrj.K08E-067 |
| [47] |
MACHADO V S, SILVA T H. Adaptive immunity in the postpartum uterus: potential use of vaccines to control metritis[J]. Theriogenology, 2020, 150: 201-209. DOI:10.1016/j.theriogenology.2020.01.040 |
| [48] |
GALVÃO K N, BICALHO R C, JEON S J. Symposium review: the uterine microbiome associated with the development of uterine disease in dairy cows[J]. J Dairy Sci, 2019, 102(12): 11786-11797. DOI:10.3168/jds.2019-17106 |
| [49] |
BRODZKI P, BRODZKI A, KUREK Ł, et al. Effect of uterine inflammatory status as well as calcium and magnesium concentrations on the uterine involution process in dairy cows[J]. Ann Anim Sci, 2016, 16(3): 759-768. DOI:10.1515/aoas-2015-0098 |
| [50] |
WANG Y, WANG J F, LI H T, et al. Characterization of the cervical bacterial community in dairy cows with metritis and during different physiological phases[J]. Theriogenology, 2018, 108: 306-313. DOI:10.1016/j.theriogenology.2017.12.028 |
| [51] |
BJÖRKMAN S, OLIVIERO C, KAUFFOLD J, et al. Prolonged parturition and impaired placenta expulsion increase the risk of postpartum metritis and delay uterine involution in sows[J]. Theriogenology, 2018, 106: 87-92. DOI:10.1016/j.theriogenology.2017.10.003 |
| [52] |
ZINICOLA M, BICALHO M L S, SANTIN T, et al. Effects of recombinant bovine interleukin-8 (rbIL-8) treatment on health, metabolism, and lactation performance in Holstein cattle Ⅱ: postpartum uterine health, ketosis, and milk production[J]. J Dairy Sci, 2019, 102(11): 10316-10328. DOI:10.3168/jds.2019-16335 |
| [53] |
ZDUŃCZYK S, JANOWSKI T. Bacteriophages and associated endolysins in therapy and prevention of mastitis and metritis in cows: current knowledge[J]. Anim Reprod Sci, 2020, 218: 106504. DOI:10.1016/j.anireprosci.2020.106504 |
| [54] |
MACHADO V S, BICALHO M L S, PEREIRA R V, et al. The effect of intrauterine administration of mannose or bacteriophage on uterine health and fertility of dairy cows with special focus on Escherichia coli and Arcanobacterium pyogenes[J]. J Dairy Sci, 2012, 95(6): 3100-3109. DOI:10.3168/jds.2011-5063 |
| [55] |
MEIRA E B S JR, ROSSI R S, TEIXEIRA A G, et al. The effect of prepartum intravaginal bacteriophage administration on the incidence of retained placenta and metritis[J]. J Dairy Sci, 2013, 96(12): 7658-7665. DOI:10.3168/jds.2013-6774 |
| [56] |
DAETZ R, CUNHA F, BITTAR J H, et al. Clinical response after chitosan microparticle administration and preliminary assessment of efficacy in preventing metritis in lactating dairy cows[J]. J Dairy Sci, 2016, 99(11): 8946-8955. DOI:10.3168/jds.2016-11400 |
| [57] |
MEIRA E B S JR, ELLINGTON-LAWRENCE R D, SILVA J C C, et al. Recombinant protein subunit vaccine reduces puerperal metritis incidence and modulates the genital tract microbiome[J]. J Dairy Sci, 2020, 103(8): 7364-7376. DOI:10.3168/jds.2019-17006 |
| [58] |
MACHADO V S, DE SOUZA BICALHO M L, DE SOUZA MEIRA JUNIOR E B, et al. Subcutaneous immunization with inactivated bacterial components and purified protein of Escherichia coli, Fusobacterium necrophorum and Trueperella pyogenes prevents puerperal metritis in Holstein dairy cows[J]. PLoS One, 2014, 9(3): e91734. DOI:10.1371/journal.pone.0091734 |
| [59] |
CUI L Y, QU Y, CAI H L, et al. Meloxicam inhibited the proliferation of LPS-stimulated bovine endometrial epithelial cells through Wnt/β-catenin and PI3K/AKT pathways[J]. Front Vet Sci, 2021, 8: 637707. DOI:10.3389/fvets.2021.637707 |
| [60] |
LOMB J, NEAVE H W, WEARY D M, et al. Changes in feeding, social, and lying behaviors in dairy cows with metritis following treatment with a nonsteroidal anti-inflammatory drug as adjunctive treatment to an antimicrobial[J]. J Dairy Sci, 2018, 101(5): 4400-4411. DOI:10.3168/jds.2017-13812 |
| [61] |
PASCOTTINI O B, VAN SCHYNDEL S J, SPRICIGO J F W, et al. Effect of anti-inflammatory treatment on systemic inflammation, immune function, and endometrial health in postpartum dairy cows[J]. Sci Rep, 2020, 10(1): 5236. DOI:10.1038/s41598-020-62103-x |
| [62] |
AMIRIDIS G S, LEONTIDES L, TASSOS E, et al. Flunixin meglumine accelerates uterine involution and shortens the calving-to-first-oestrus interval in cows with puerperal metritis[J]. J Vet Pharmacol Ther, 2001, 24(5): 365-367. DOI:10.1046/j.1365-2885.2001.00358.x |
| [63] |
RODRÍGUEZ A R, PALMA P I, SOLAR M A, et al. Early postpartum treatment with carprofen in a dairy herd with high incidence of clinical metritis-a case study[J]. J Appl Anim Res, 2021, 49(1): 139-146. DOI:10.1080/09712119.2021.1909033 |
| [64] |
WITTE T S, IWERSEN M, KAUFMANN T, et al. Determination of ceftiofur derivatives in serum, endometrial tissue, and lochia in puerperal dairy cows after subcutaneous administration of ceftiofur crystalline free acid[J]. J Dairy Sci, 2011, 94(1): 284-290. DOI:10.3168/jds.2010-3645 |
| [65] |
CREDILLE B C, GIGUÈRE S, VICKROY T W, et al. Disposition of ampicillin trihydrate in plasma, uterine tissue, lochial fluid, and milk of postpartum dairy cattle[J]. J Vet Pharmacol Ther, 2015, 38(4): 330-335. DOI:10.1111/jvp.12178 |
| [66] |
RISCO C A, HERNANDEZ J. Comparison of ceftiofur hydrochloride and estradiol cypionate for metritis prevention and reproductive performance in dairy cows affected with retained fetal membranes[J]. Theriogenology, 2003, 60(1): 47-58. DOI:10.1016/S0093-691X(02)01299-2 |
| [67] |
KAEWLAMUN W, OKOUYI M, HUMBLOT P, et al. Does supplementing dairy cows with β-carotene during the dry period affect postpartum ovarian activity, progesterone, and cervical and uterine involution?[J]. Theriogenology, 2011, 75(6): 1029-1038. DOI:10.1016/j.theriogenology.2010.11.010 |
| [68] |
GULLIVER C E, FRIEND M A, KING B J, et al. The role of omega-3 polyunsaturated fatty acids in reproduction of sheep and cattle[J]. Anim Reprod Sci, 2012, 131(1-2): 9-22. DOI:10.1016/j.anireprosci.2012.02.002 |
| [69] |
DE MEDEIROS FERREIRA J R, VILLELA S B, BIANCONI C, et al. Uterine involution of mares supplemented with dietary algae-derived omega-3 fatty acids during the peripartum period[J]. J Equine Vet Sci, 2021, 106: 103733. DOI:10.1016/j.jevs.2021.103733 |
| [70] |
ULFINA G G, KIMOTHI S P, OBEROI P S, et al. Modulation of post-partum reproductive performance in dairy cows through supplementation of long- or short-chain fatty acids during transition period[J]. J Anim Physiol Anim Nutr, 2015, 99(6): 1056-1064. DOI:10.1111/jpn.12304 |
| [71] |
FORBES B E, BLYTH A J, WIT J M. Disorders of IGFs and IGF-1R signaling pathways[J]. Mol Cell Endocrinol, 2020, 518: 111035. DOI:10.1016/j.mce.2020.111035 |
| [72] |
VENKATA K C N, SWAROOP A, BAGCHI D, et al. A small plant with big benefits: fenugreek (Trigonella foenum-graecum Linn.) for disease prevention and health promotion[J]. Mol Nutr Food Res, 2017, 61(6): 1600950. DOI:10.1002/mnfr.201600950 |
| [73] |
BERNHARDT V, D'SOUZA J R T. Immunomodulatory potential of herbal medicine in type 2 DM patients as evaluvated by neutrophil phagocytic index, serum opsonisation and lymphocyte proliferation rate[J]. Asian J Pharm Clin Res, 2012, 5(2): 36-41. |
| [74] |
黄梅, 沈建英, 杜成成, 等. 青蒿素及其衍生物的抗菌活性初步研究[J]. 中国中药杂志, 2019, 44(9): 1946-1952. HUANG M, SHEN J Y, DU C C, et al. Preliminary study on antibacterial activity of artemisinin and its derivatives[J]. China Journal of Chinese Materia Medica, 2019, 44(9): 1946-1952. DOI:10.19540/j.cnki.cjcmm.20190131.001 (in Chinese) |
| [75] |
BAHUGUNA A, RAMALINGAM S, ARUMUGAM A, et al. Molecular and in silico evidences explain the anti-inflammatory effect of Trachyspermum ammi essential oil in lipopolysaccharide induced macrophages[J]. Process Biochem, 2020, 96: 138-145. DOI:10.1016/j.procbio.2020.06.006 |
| [76] |
JAPHETH K P, KUMARESAN A, PATBANDHA T K, et al. Supplementation of a combination of herbs improves immunity, uterine cleansing and facilitate early resumption of ovarian cyclicity: a study on post-partum dairy buffaloes[J]. J Ethnopharmacol, 2021, 272: 113931. DOI:10.1016/j.jep.2021.113931 |
| [77] |
LEE K H, LEE Y T, CHEN T C, et al. Effects of Sheng Hua Tang on uterine involution and ovarian activity in postpartum dairy cows[J]. Asian-Australas J Anim Sci, 2013, 26(9): 1247-1254. DOI:10.5713/ajas.2013.13042 |
| [78] |
STEPHEN C P, JOHNSON W H, LEBLANC S J, et al. The impact of ecbolic therapy in the early postpartum period on uterine involution and reproductive health in dairy cows[J]. J Vet Med Sci, 2019, 81(3): 491-498. DOI:10.1292/jvms.18-0617 |
| [79] |
YU G M, BAI J H, LIU Y, et al. A weekly postpartum PGF2αprotocol enhances uterine health in dairy cows[J]. Reprod Biol, 2016, 16(4): 295-299. DOI:10.1016/j.repbio.2016.10.006 |
| [80] |
MELENDEZ P, MCHALE J, BARTOLOME J, et al. Uterine involution and fertility of holstein cows subsequent to early postpartum PGF2α treatment for acute puerperal metritis[J]. J Dairy Sci, 2004, 87(10): 3238-3246. DOI:10.3168/jds.S0022-0302(04)73460-8 |
| [81] |
CANADAS E R, LONERGAN P, BUTLER S T. Effect of equine chorionic gonadotropin administration on day 8 post-partum on ovarian follicular development, uterine health and uterine involution in lactating dairy cows[J]. Theriogenology, 2019, 123: 54-61. DOI:10.1016/j.theriogenology.2018.09.022 |
| [82] |
WANG X R, BAO H C, LIU X M, et al. Effects of endometrial stem cell transplantation combined with estrogen in the repair of endometrial injury[J]. Oncol Lett, 2018, 16(1): 1115-1122. |
| [83] |
LIU X R, ZHANG L, LIU Y X, et al. Circ-8073 regulates CEP55 by sponging miR-449a to promote caprine endometrial epithelial cells proliferation via the PI3K/AKT/mTOR pathway[J]. Biochim Biophys Acta Mol Cell Res, 2018, 1865(8): 1130-1147. DOI:10.1016/j.bbamcr.2018.05.011 |
| [84] |
DENG Q, ODHIAMBO J F, FAROOQ U, et al. Intravaginally administered lactic acid bacteria expedited uterine involution and modulated hormonal profiles of transition dairy cows[J]. J Dairy Sci, 2015, 98(9): 6018-6028. DOI:10.3168/jds.2014-8559 |
(编辑 范子娟)



