肠道微生物群作为动物最大的微生态系统,将动物消化道无法消化的饲粮成分降解成可吸收的成分和一些具有生理活性的小分子化合物。肠道菌群(如乳酸菌、链球菌、肠杆菌、梭杆菌)及其代谢产物(短链脂肪酸(short-chain fatty acids,SCFA)、胆汁酸、内毒素等)对宿主营养素消化吸收、免疫系统发育、肠道健康有重要作用[1]。越来越多研究发现,肠道微生物及其代谢产物在调节线粒体功能方面产生影响[2-3],进而影响畜禽机体的抗氧化能力、生长发育、营养代谢周转速度及饲料转化效率等[4]。短链脂肪酸(乙酸、丙酸和丁酸等)可提高宿主细胞氧气消耗和烟酰胺腺嘌呤二核苷酸(NADH)、烟酰胺腺嘌呤二核苷酸磷酸(NADPH)水平,改善线粒体氧化磷酸化(oxidative phosphorylation,OXPHOS)水平和抗氧化性能[5];肠道微生物有害代谢产物(如毒胆酸)可增加活性氧(reactive oxygen species,ROS)含量、抑制线粒体呼吸链复合体的活性[6]。肠道微生物中间产物吲哚丙酸(Indole propionic acid,IPA)诱导线粒体Ca2+超载以及O2过度消耗,从而导致线粒体功能障碍[7]。此外,肠道微生物区系及其代谢产物类型与饲粮营养水平密切相关。研究表明,日粮纤维增加了肠道菌群多样性,并显著升高毛螺菌科丰度以及丁酸浓度[8]。因此,期望通过饲粮营养手段靶向调控肠道微生物菌群及其代谢物来调节畜禽线粒体功能,改善其生长与健康水平。
1 线粒体的生物学功能线粒体是真核细胞中的一种重要的细胞器,能够产生三磷酸腺苷(ATP)、维持细胞Ca2+稳态和调节ROS产生[9]。细胞线粒体产生的ATP,参与糖酵解中底物的氧化、三羧酸循环(TCA)、丙酮酸脱羧[10-11]。其中,氧化磷酸化系统由4个多蛋白复合物(Ⅰ~Ⅳ)和ATP合酶(复合物Ⅴ)组成,OXPHOS是需氧产生ATP的主要来源,特别对神经元[12]等需要高能量的细胞至关重要。作为电子传递链(ETC)的起始位点,复合物Ⅰ、Ⅱ促进电子的传递并与电子受体O2结合,从而驱动ATP合成。钙是线粒体功能的调节因子,在细胞器内的多水平上促进ATP合成,而氧化应激下,细胞中钙相关通道功能障碍,大量Ca2+聚集于线粒体,从而导致线粒体损伤和细胞调亡[13]。当细胞内Ca2+超载时,线粒体内膜上的电子传递链产生ROS,而线粒体内ROS的产生是细胞氧化应激的主要来源[14]。畜禽机体内ROS生成与清除的动态平衡被打破对畜禽健康有害,当ROS的浓度超过抗氧化剂的缓冲能力时,ROS可导致关键生物分子(如脂类、蛋白质或DNA)过氧化损伤。机体线粒体ROS生成的增加可引起热休克反应、生长缓慢、免疫失调等代谢疾病[15-17]。
2 肠道微生物及其代谢产物对线粒体功能的影响胃肠道菌群是动物体内最复杂的共生微生物生态系统,微生物既可以通过代谢宿主的饲粮成分产生氨基酸、SCFA等,也可以通过自身的生物合成基因簇,产生独特结构和功能的代谢产物影响动物生长发育。近年来的大量研究发现,肠道菌群参与调节宿主的神经生理功能,并提出“微生物-肠-脑轴”[18]概念。但这些研究多侧重于肠道微生物及其代谢产物对宿主组织和细胞的影响及其作用机制,对细胞器功能(如线粒体功能)调节作用的研究报道偏少。Saihara等[19]研究发现,小鼠肠道菌群可介导过氧化物酶体增殖物激活受体γ辅激活因子1α(peroxisome proliferator-activated receptor γ coactivator-1α,PGC-1α)通路促进动物线粒体生物合成。线粒体功能方面,益生菌鼠李糖乳杆菌激活过氧化物酶增殖物激活受体α(peroxidase proliferators activate receptors-α,PPAR-α)上调脂肪细胞因子表达,提高线粒体OXPHOS水平[20]。此外,小鼠巨噬细胞中结核分枝杆菌抑制内毒素(LPS)介导的Toll样受体(Toll-like receptors,TLRs)信号通路,从而减少线粒体ROS的产生[21]。上述研究提示,肠道微生物菌群及其代谢产物可维持肠道稳态或介导调节线粒体生物合成代谢途径, 对宿主线粒体功能产生影响。
2.1 肠道微生物及其代谢产物调节线粒体能量代谢SCFA是膳食纤维在动物肠道中的乳酸菌和双歧杆菌等介导下发酵产生的代谢产物[22],包括乙酸、丙酸和丁酸等。短链脂肪酸是结肠上皮细胞的主要能量来源[23],其中丁酸作为首要能源物质,对维持肠道功能稳态以及调节细胞能量代谢具有重要作用。丁酸盐和乙酸盐可通过激活小鼠结肠细胞腺苷酸活化蛋白激酶(AMP-activated protein kinase,AMPK)通路调控线粒体功能,AMPK可作为调节线粒体OXPHOS的能量感受器[24];Mollica等[25]发现,N-丁酸盐通过激活AMPK-乙酰辅酶A羧化酶(acetyl-CoA carboxylaseα,ACC)通路改善了线粒体呼吸能力,从而促进了肝中的线粒体能量代谢。目前,大量畜禽试验已证实丁酸及其衍生物在畜禽生长发育和免疫健康及细胞能量与组织稳态等方面具有显著改善作用。同时,丁酸也可以通过能量底物这一作用,提高结肠上皮细胞线粒体功能,进而调节肠上皮细胞功能。Donohoe等[26]的体外试验证实,丁酸盐进入线粒体,经过β氧化、TCA循环后有助于提高NADH呼吸链酶活性及ATP合成,并促进肠细胞氧化代谢及抑制细胞自噬,当添加丁酸盐到无菌小鼠的结肠细胞中时,OXPHOS水平增加,自噬情况得到改善。体内试验同样证实,饲粮中添加丁酸钠可显著提高饲料转化效率[27]和抗氧化能力[28],一方面,丁酸激活PGC-1α通路并增强线粒体功能来加快能量转化效率;另一方面,丁酸作为信号分子,可以通过抑制组蛋白去乙酰化酶(histone deacetylase,HDAC)参与线粒体能量代谢酶活性的调控。肝组织中产生的初级胆汁酸[29],经过部分肠道厌氧菌的降解成为次级胆汁酸,如去氧胆酸和石胆酸。次级胆汁酸是脂质和能量代谢调节剂,可激活核法呢素X受体(farnesoid X receptor,FXR)和G蛋白偶联胆汁酸受体1(G protein-coupled bile acid receptor 5,TGR5)信号通路与线粒体互作调控[30]。胆汁酸具有促进脂肪消化吸收、抑制肠道有害菌群的生长繁殖以及提高能量代谢水平的作用[31]。肠道中的胆汁酸与FXR受体结合产生成纤维因子19/15(fibroblast growth factor 19/15,FGF19/15)通过脑-肠轴到达下丘脑调节葡萄糖代谢,进一步研究发现,下丘脑中FGF19具有调节能量代谢的作用[32]。
2.2 肠道微生物及其代谢产物调节线粒体ROS的产生OXPHOS产生ATP的过程中,线粒体内膜上的电子传递链产生ROS,畜禽机体ROS的产生随着能量需要的增加呈上升趋势,其原因是线粒体ETC泄漏导致的[33]。复合物Ⅰ是ROS产生的主要来源,其不稳定的性质,将导致蛋白质、脂质和DNA发生氧化反应[34]。肠道微生物及其代谢产物通过调节ROS的产生靶向调控线粒体,动物机体ROS的大量增加主要源于胆汁酸引起的线粒体膜通透性改变。体外试验证实,毒胆酸可增加人和小鼠肝细胞中ROS含量、破坏线粒体膜电位(mitochondrial membrane potential,MMP)和抑制线粒体呼吸链复合体的活性[6]。研究表明脱氧熊胆酸(UDAC)浓度低于50 μmol·L-1时可使线粒体膜通透性增加[35]。饲粮添加高浓度脱氧胆酸钠(NaDOC)可能造成线粒体氧化损伤,NaDOC作为氧化应激诱导剂在结肠以及肝细胞上得到广泛运用[36],NaDOC抑制肠道Ca2+吸收以及增加ROS的产生和线粒体膜通透性的改变,导致线粒体氧化应激以及功能障碍[37]。Xavier等[38]研究表明,一定浓度的UDAC可以降低神经干细胞线粒体ROS产量并提高细胞色素含量,进而保护线粒体的完整性和功能,可作为一种靶向线粒体的抗凋亡和抗氧化物质。虽然在畜禽生产上相关的研究较少,但小鼠模型研究表明,丙酸可干扰线粒体代谢,导致线粒体ROS的产生。丙酸与辅酶A结合后生成重要的中间代谢产物丙酰辅酶A,进一步转化后以琥珀酰辅酶A的形式进入柠檬酸循环[39]。琥珀酰辅酶A在正常生理浓度下可以提高线粒体基质功能水平,但高浓度的琥珀酰辅酶A抑制柠檬酸合成酶,直接导致线粒体ROS产生。在丙酸存在的情况下,柠檬酸循环的第一个步骤被抑制,NADH的产量减少导致线粒体呼吸链复合物Ⅰ效率下降,线粒体功能受到影响。有研究表明,添加较高浓度的丙酸,可加剧ROS的产生[40]。其原因是ROS的存在导致细胞内产生活性氮,活性氮可与丙酸反应生成一种能有效抑制线粒体功能的化合物3-硝基丙酸[41]。饲粮中1%~2%的色氨酸可被一些带有色氨酸酶的梭状芽孢杆菌、大肠杆菌代谢成五羟色胺和褪黑素[23]。褪黑素的实质是一种内源性的吲哚胺,吲哚胺是强大的抗氧化剂和自由基清除剂,可以保护细胞膜、ETC和线粒体DNA免受氧化损伤。在心血管相关研究中发现,褪黑素可通过AMPK-PGC-1α-SIRT3信号通路,提高核因子(NRF)和线粒体转录因子A(mitochondrial transcription factor A,TFAM)的表达,改善呼吸链功能,减少ROS产生,保护心血管。且相比于传统的抗氧化剂,褪黑素对线粒体的保护作用更具有靶向性[42]。
2.3 肠道微生物及其代谢产物调节线粒体Ca2+稳态线粒体Ca2+稳态与线粒体的能量代谢显著相关。当线粒体对Ca2+转运能力降低时,可导致MMP降低、线粒体Ca2+摄取减少,从而影响线粒体呼吸链的完整性[43]。近年来,研究者们发现了线粒体和细胞钙稳态新的生理作用,即线粒体Ca2+的摄取通过调节细胞质Ca2+稳态影响细胞外Ca2+进入,因此可能影响肌肉收缩、神经元兴奋性和细胞迁移功能[44]。线粒体钙单向转运体蛋白(mitochondrial calcium uniporter,MCU)介导的线粒体Ca2+摄取,是由ETC产生的内膜电位驱动的。添加SCFA能够上调牛瘤胃上皮细胞MCU基因表达水平,从而促进线粒体Ca2+摄取以及ATP产生[45]。研究表明,饲粮添加丁酸钠通过调控瞬时受体电位亚家族V成员6 (transient receptor potential channel subfamily V member 6, TRPV6)通路增加了小鼠肠道Ca2+水平[46]。肠道菌群在催化精氨酸的过程中,可以产生鸟氨酸和一氧化氮(NO)等物质[47]。鸟氨酸循环能够促进线粒体能量代谢,鸟氨酸脱羧酶作为鸟氨酸循环、多胺合成代谢途中的第一个限速酶[48],增加了多胺、NO的生成。多胺通过调控细胞Ca2+稳态以及E-钙黏蛋白(E-cadherin)的基因表达,维持肠上皮屏障功能以及细胞膜通透性[49]。当肠道中革兰阴性菌数量增加或肠道屏障受损时,肠道微生物会产生LPS,胡骁飞等[50]通过给肉仔鸡腹腔注射500 μg·kg-1(BW) LPS,发现其对生长性能并无显著作用,但是对胴体品质和胸肌肉品质产生了不良影响,其原因是LPS诱导线粒体功能损伤、线粒体Ca2+-ATP酶活性下降,从而影响肌纤维钙离子转移系统功能[51]。
综上所述,肠道微生物定植后调控机体生长发育,同时,产生大量代谢产物,在维持肠道稳态、辅助动物抵御有害环境和调控线粒体功能方面具有重要作用。肠道微生物及其代谢产物具有很好地调节线粒体状态的作用,通过影响微生物之间的群体响应和调控、线粒体功能相关蛋白表达等途径来发挥作用(图 1)。
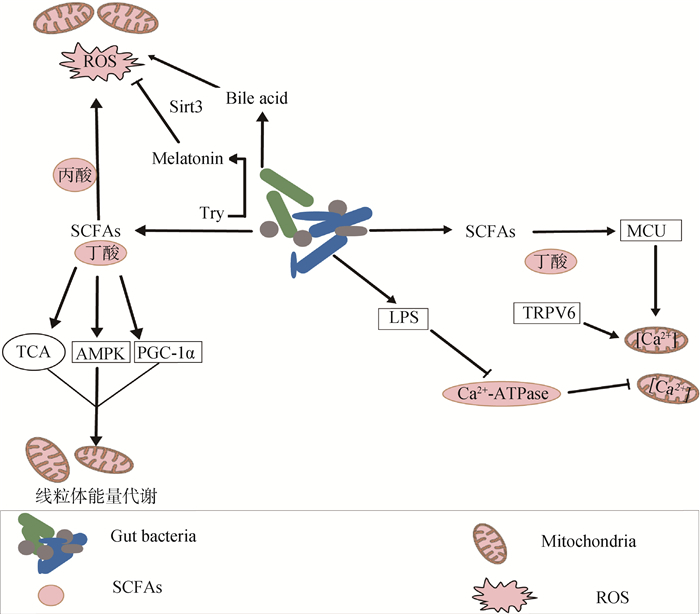
|
图 1 肠道微生物及其代谢产物对线粒体功能的调节途径 Fig. 1 Regulation of mitochondrial function by intestinal microbes and their metabolites |
饲粮种类、水平和结构是影响动物肠道微生物组成和代谢的重要因素。有研究表明,低蛋白质水平(13%)饲粮显著提高了育肥猪回肠中的消化链球菌与梭菌科相对丰度,有助于维持肠道微生物稳态和乙酸的产生,这提示肠道微生物对蛋白质营养的利用与饲粮蛋白质水平相关[52-53];而高蛋白质水平(23%)饲粮增加仔猪肠道内pH,抑制肠杆菌与乳酸杆菌属的生成,促进致病微生物的增殖[54]。Ijaz等[55]研究发现,饲喂高脂饲粮显著降低小鼠肠道中的阿克曼氏菌、乳酸杆菌、双歧杆菌属丰度以及PGC-1α蛋白水平,从而抑制线粒体生物合成。Regmi等[56]用不同直链淀粉含量(<5%、20%、28%和63%)饲粮饲喂仔猪,发现饲喂63%直链淀粉显著提高仔猪粪中的乳酸杆菌、双歧杆菌丰度以及短链脂肪酸与丁酸含量。与2.5%粗纤维饲粮水平相比,7.5%粗纤维饲粮水平显著提高了母猪纤维降解菌丰度和SCFA浓度[57]。Zeitz等[58]发现,饲喂发酵木质纤维素粗纤维饲粮可降低肉鸡肠道厚壁菌门和乳杆菌属丰度和微生物代谢产物浓度[59]。添加剂方面,白藜芦醇、姜黄素等植物提取物具有抗氧化、抗炎等生物功能。给饲喂高脂日粮的大鼠添加白藜芦醇显著提高了肠道丁酸产生菌的比例以及SCFA浓度,从而促进线粒体能量代谢水平[60]。姜黄素通过丝裂原活化蛋白激酶(mitogen activated protein kinase,MAPK)、核因子E2相关因子2(nuclear factor erythroid 2related factor,Nrf2)信号通路降低ROS的水平,缓解热应激诱导的线粒体氧化应激损伤[61]。此外,姜黄素能够降低仔猪大肠杆菌相对丰度以及Toll样受体4 (Toll-like receptors 4,TLR4)蛋白表达,从而抑制ROS的产生,最终缓解线粒体功能障碍[62]。
3.2 饲料转化效率饲料转化效率与线粒体功能有着密切关系,相同的环境和饲粮条件下,与高饲料转化率肉鸡相比,低饲料转化率肉鸡肝线粒体功能和呼吸链复合物(Ⅰ、Ⅱ、Ⅲ和Ⅳ)的活性下降及ROS和蛋白质羰基含量升高[63-64]。同样,与低饲料转化率羔羊相比,高饲料转化率羔羊相关的呼吸链复合物酶活性显著下降[65]。转录组学分析表明,低剩余采食量猪肌肉中的超氧化物歧化酶(superoxide dismutase,SOD)、谷胱甘肽过氧化物酶3(glutathione peroxidase 3,GPX3)、过氧化氧化还原蛋白2(peroxiredoxins 6,PRDX6)mRNA水平显著下降,从而抑制ROS的产生[66]。上述研究表明,动物饲料转化效率与其线粒体功能密切相关。肠道菌群方面,不同饲料转化效率肉鸡的回肠、盲肠微生群多样性差异不显著,但低剩余采食量肉鸡盲肠中Oscillibacter、梭状芽孢杆菌科丰度显著升高[67]。高饲料转化率猪盲肠乳杆菌属和拟杆菌纲中的普雷沃菌属的相对丰度显著高于低饲料转化率猪[68-69]。因此,不同饲料转化效率畜禽肠道微生物的改变是否参与调节机体线粒体功能值得进一步探究。
3.3 饲养阶段不同生长阶段动物肠道微生物的组成和代谢水平对生长发育具有重要影响。Liu等[70]研究表明,哺乳期间仔猪空肠中较高的乳酸杆菌和拟杆菌属丰度,能够促进线粒体生物合成相关基因的表达[71]。也有研究发现,仔猪断奶后其结肠中Alloprevotella和Oscillibacter相对丰度降低,且肝线粒体功能相关基因泛素氧化酶A2亚基(ubiquinone oxidoreductase subunit A2,NDUFA2)和泛素氧化酶A5亚基(ubiquinone oxidoreductase subunit A5,NDUFA5)mRNA表达水平以及ATP含量显著降低,表明仔猪断奶诱导了线粒体氧化应激和功能障碍[72-73]。与妊娠后期相比,哺乳期母猪粪便中的拟杆菌属丰度显著增加,这意味着不可消化的饲粮成分可被发酵并产生SCFA,从而促进线粒体功能相关基因的表达水平[74]。Qi等[75]比较了不同生长阶段对猪肠道菌群的影响,发现6月龄猪肠道中瘤胃球菌科以及有益细菌Oscillospira丰度增加,可促进机体丁酸盐的产生[76]。肉鸭方面,相比生长后期(6~10周龄),2~4周龄肉鸭十二指肠与空肠中乳酸乳球菌丰度相对较高,乳酸乳球菌与线粒体转运蛋白表达水平密切相关,从而为早期生长发育提供能量[77-78]。总之,不同生长阶段动物肠道微生物的组成和代谢产物的变化可参与线粒体功能调节,进而影响宿主生长发育。
3.4 其他因素动物机体各种应激可介导肠道微生物菌群及其代谢产物变化进而影响线粒体能量代谢水平。在小鼠上,高温急性热应激和中风应激显著降低肠道乳酸杆菌丰度,并抑制抗氧化活性,从而导致ROS产量的增加以及线粒体氧化损伤[79-80]。高脂日粮诱导的小鼠慢性应激可降低其厚壁菌门与拟杆菌门比例及其代谢产物丁酸和SCFA浓度,从而影响线粒体生物合成[81]。Koncz等[82]发现,紫外线诱导小鼠肾细胞超氧化物的增加,从而降低线粒体NADH以及Ca2+水平。日龄方面,研究发现5月龄猪肠道菌群多样性随着月龄增加呈上升趋势,5月龄后保持稳定,但其乳酸杆菌、双歧杆菌的相对丰度呈降低趋势[83]。Amit-Romach等[84]采用16S rRNA基因靶向分析4、14、25日龄肉鸡肠道微生物多样性,发现4日龄肉鸡十二指肠和盲肠乳酸菌属是主要优势菌属,且其比例随着日龄增加而增多。已有研究发现,乳酸杆菌增加可显著提高肠道短链脂肪酸浓度和抗氧化酶活性,并抑制ROS产生,从而影响宿主线粒体功能[85]。
4 小结研究肠道微生物与线粒体功能的关系对动物健康有重要意义。目前,研究多侧重于肠道微生物及其代谢产物对宿主组织和细胞的影响及其作用机制,需进一步加强对细胞器功能(如线粒体功能)的研究。肠道微生物组成和水平通过何种途径影响线粒体功能,以及线粒体与肠道微生物对话机制如何实现尚不清楚。因此,今后应加强研究肠道微生物及其代谢产物对宿主线粒体的调节作用,为饲粮营养手段靶向线粒体功能调控动物生长发育提供新思路和新方法。
| [1] |
ZHAO Q, ELSON C O. Adaptive immune education by gut microbiota antigens[J]. Immunology, 2018, 154(1): 28-37. DOI:10.1111/imm.12896 |
| [2] |
SAHURI-ARISOYLU M, MOULD R R, SHINJYO N, et al. Acetate induces growth arrest in colon cancer cells through modulation of mitochondrial function[J]. Front Nutr, 2021, 8: 588466. DOI:10.3389/fnut.2021.588466 |
| [3] |
SCHÖNFELD P, WOJTCZAK L. Short- and medium-chain fatty acids in energy metabolism: the cellular perspective[J]. J Lipid Res, 2016, 57(6): 943-954. DOI:10.1194/jlr.R067629 |
| [4] |
GONZÁLEZ-BOSCH C, BOORMAN E, ZUNSZAIN P A, et al. Short-chain fatty acids as modulators of redox signaling in health and disease[J]. Redox Biol, 2021, 47: 102165. DOI:10.1016/j.redox.2021.102165 |
| [5] |
陈永标. 线粒体转录因子A对肝外胆汁淤积肝细胞mtDNA氧化损伤的保护作用及机制研究[D]. 重庆: 第三军医大学, 2012. CHEN Y B. Damage to mtDNA in liver injury of patients with extrahepatic cholestasis: the protective effects of mitochondrial transcription factor A[D]. Chongqing: Third Military Medical University of Chinese P.L.A., 2012. (in Chinese) |
| [6] |
ROLO A P, PALMEIRA C M, WALLACE K B. Mitochondrially mediated synergistic cell killing by bile acids[J]. Biochim Biophys Acta, 2003, 1637(1): 127-132. DOI:10.1016/S0925-4439(02)00224-7 |
| [7] |
HAN G, LEE D G. Indole propionic acid induced Ca2+-dependent apoptosis in Candida albicans[J]. IUBMB Life, 2022, 74(3): 235-244. DOI:10.1002/iub.2579 |
| [8] |
SHANG Q H, LIU H S, WU D, et al. Source of fiber influences growth, immune responses, gut barrier function and microbiota in weaned piglets fed antibiotic-free diets[J]. Anim Nutr, 2021, 7(2): 315-325. DOI:10.1016/j.aninu.2020.12.008 |
| [9] |
BEAL M F. Mitochondrial dysfunction in neurodegenerative diseases[J]. Biochim Biophys Acta-Bioenerg, 1998, 1366(1-2): 211-223. DOI:10.1016/S0005-2728(98)00114-5 |
| [10] |
DAVIS R E, WILLIAMS M. Mitochondrial function and dysfunction: an update[J]. J Pharmacol Exp Ther, 2012, 342(3): 598-607. DOI:10.1124/jpet.112.192104 |
| [11] |
YOSHIDA G J. Beyond the warburg effect: N-Myc contributes to metabolic reprogramming in cancer cells[J]. Front Oncol, 2020, 10: 791. DOI:10.3389/fonc.2020.00791 |
| [12] |
SEAGER R, LEE L, HENLEY J M, et al. Mechanisms and roles of mitochondrial localisation and dynamics in neuronal function[J]. Neuronal Signal, 2020, 4(2): NS20200008. DOI:10.1042/NS20200008 |
| [13] |
BROOKES P S, YOON Y, ROBOTHAM J L, et al. Calcium, ATP, and ROS: a mitochondrial love-hate triangle[J]. Am J Physiol Cell Physiol, 2004, 287(4): C817-C833. DOI:10.1152/ajpcell.00139.2004 |
| [14] |
MAILLOUX R J. An update on mitochondrial reactive oxygen species production[J]. Antioxidants, 2020, 9(6): 472. DOI:10.3390/antiox9060472 |
| [15] |
AZAD M A K, KIKUSATO M, ZULKIFLI I, et al. Electrolysed reduced water decreases reactive oxygen species-induced oxidative damage to skeletal muscle and improves performance in broiler chickens exposed to medium-term chronic heat stress[J]. Br Poult Sci, 2013, 54(4): 503-509. DOI:10.1080/00071668.2013.801067 |
| [16] |
CHI Q R, HU X Y, LIU Z Y, et al. H2S exposure induces cell death in the broiler thymus via the ROS-initiated JNK/MST1/FOXO1 pathway[J]. Ecotoxicol Environ Saf, 2021, 222: 112488. DOI:10.1016/j.ecoenv.2021.112488 |
| [17] |
LI L Q. The relevance of mammalian peroxiredoxins to the gametogenesis, embryogenesis, and pregnancy outcomes[J]. Reprod Sci, 2017, 24(6): 812-817. DOI:10.1177/1933719116667217 |
| [18] |
COLLINS S M, SURETTE M, BERCIK P. The interplay between the intestinal microbiota and the brain[J]. Nat Rev Microbiol, 2012, 10(11): 735-742. DOI:10.1038/nrmicro2876 |
| [19] |
SAIHARA K, KAMIKUBO R, IKEMOTO K, et al. Pyrroloquinoline Quinone, a redox-active o-Quinone, stimulates mitochondrial biogenesis by activating the SIRT1/PGC-1α signaling pathway[J]. Biochemistry, 2017, 56(50): 6615-6625. DOI:10.1021/acs.biochem.7b01185 |
| [20] |
JACOUTON E, MACH N, CADIOU J, et al. Lactobacillus rhamnosus CNCMI-4317 modulates Fiaf/Angptl4 in intestinal epithelial cells and circulating level in mice[J]. PLoS One, 2015, 10(10): e0138880. DOI:10.1371/journal.pone.0138880 |
| [21] |
SAINT-GEORGES-CHAUMET Y, EDEAS M. Microbiota-mitochondria inter-talk: consequence for microbiota-host interaction[J]. Pathog Dis, 2016, 74(1): ftv096. DOI:10.1093/femspd/ftv096 |
| [22] |
陈福, 何邵平, 田科雄, 等. 短链脂肪酸的生理功能及其在畜禽生产中的应用[J]. 动物营养学报, 2019, 31(7): 3039-3048. CHEN F, HE S P, TIAN K X, et al. Physiological function of short-chain fatty acids and their application in livestock and poultry production[J]. Chinese Journal of Animal Nutrition, 2019, 31(7): 3039-3048. DOI:10.3969/j.issn.1006-267x.2019.07.014 (in Chinese) |
| [23] |
朱翠, 王少磊, 蒋宗勇, 等. 仔猪肠道微生物代谢产物及益生菌的调控作用[J]. 动物营养学报, 2019, 31(4): 1478-1484. ZHU C, WANG S L, JIANG Z Y, et al. Intestinal microbial metabolites and probiotic regulation in piglets[J]. Chinese Journal of Animal Nutrition, 2019, 31(4): 1478-1484. (in Chinese) |
| [24] |
LANTIER L, FENTZ J, MOUNIER R, et al. AMPK controls exercise endurance, mitochondrial oxidative capacity, and skeletal muscle integrity[J]. FASEB J, 2014, 28(7): 3211-3224. DOI:10.1096/fj.14-250449 |
| [25] |
MOLLICA M P, RASO G M, CAVALIERE G, et al. Butyrate regulates liver mitochondrial function, efficiency, and dynamics in insulin-resistant obese mice[J]. Diabetes, 2017, 66(5): 1405-1418. DOI:10.2337/db16-0924 |
| [26] |
DONOHOE D R, GARGE N, ZHANG X X, et al. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon[J]. Cell Metab, 2011, 13(5): 517-526. DOI:10.1016/j.cmet.2011.02.018 |
| [27] |
AKBAR M A, TEWATIA B S, KUMAR S. Effect of dietary supplementation of salts of organic acids on gut morphology and meat quality of broilers[J]. Indian J Anim Res, 2018, 52(12): 1727-1731. |
| [28] |
WU Y Q, WANG Y L, YIN D F, et al. Effect of supplementation of nicotinamide and sodium butyrate on the growth performance, liver mitochondrial function and gut microbiota of broilers at high stocking density[J]. Food Funct, 2019, 10(11): 7081-7090. DOI:10.1039/C9FO00904C |
| [29] |
NIE Y F, HU J, YAN X H. Cross-talk between bile acids and intestinal microbiota in host metabolism and health[J]. J Zhejiang Univ Sci B, 2015, 16(6): 436-446. DOI:10.1631/jzus.B1400327 |
| [30] |
THOMAS C, GIOIELLO A, NORIEGA L, et al. TGR5-mediated bile acid sensing controls glucose homeostasis[J]. Cell Metab, 2009, 10(3): 167-177. DOI:10.1016/j.cmet.2009.08.001 |
| [31] |
刘敬盛, 杨玉芝, 王君荣, 等. 胆汁酸的营养特性及其在家禽生产中应用的研究进展[J]. 中国畜牧兽医, 2010, 37(1): 13-16. LIU J S, YANG Y Z, WANG J R, et al. Nutritive peculiarity of bile acid and its application in poultry production[J]. China Animal Husbandry & Veterinary Medicine, 2010, 37(1): 13-16. (in Chinese) |
| [32] |
RYAN K K, KOHLI R, GUTIERREZ-AGUILAR R, et al. Fibroblast growth factor-19 action in the brain reduces food intake and body weight and improves glucose tolerance in male rats[J]. Endocrinology, 2013, 154(1): 9-15. DOI:10.1210/en.2012-1891 |
| [33] |
RADAK Z, ZHAO Z F, KOLTAI E, et al. Oxygen consumption and usage during physical exercise: the balance between oxidative stress and ROS-dependent adaptive signaling[J]. Antioxid Redox Sign, 2013, 18(10): 1208-1246. DOI:10.1089/ars.2011.4498 |
| [34] |
HOOD D A, UGUCCIONI G, VAINSHTEIN A, et al. Mechanisms of exercise-induced mitochondrial biogenesis in skeletal muscle: implications for health and disease[J]. Compr Physiol, 2011, 1(3): 1119-1134. |
| [35] |
陆伦根, 曾民德. 胆汁酸对线粒体的毒性作用[J]. 肝脏, 2008, 13(4): 343-346. LU L G, ZENG M D. Toxicity of bile acid on mitochondrial[J]. Chinese Hepatology, 2008, 13(4): 343-346. DOI:10.3969/j.issn.1008-1704.2008.04.023 (in Chinese) |
| [36] |
JEAN-LOUIS S, AKARE S, ALI M A, et al. Deoxycholic acid induces intracellular signaling through membrane perturbations[J]. J Biol Chem, 2006, 281(21): 14948-14960. DOI:10.1074/jbc.M506710200 |
| [37] |
RIVOIRA M A, MARCHIONATTI A M, CENTENO V A, et al. Sodium deoxycholate inhibits chick duodenal calcium absorption through oxidative stress and apoptosis[J]. Comp Biochem Physiol A Mol Integr Physiol, 2012, 162(4): 397-405. DOI:10.1016/j.cbpa.2012.04.016 |
| [38] |
XAVIER J M, MORGADO A L, RODRIGUES C M P, et al. Tauroursodeoxycholic acid increases neural stem cell pool and neuronal conversion by regulating mitochondria-cell cycle retrograde signaling[J]. Cell Cycle, 2014, 13(22): 3576-3589. DOI:10.4161/15384101.2014.962951 |
| [39] |
KIM S A, JANG E H, YOUNG M J, et al. Propionic acid induces mitochondrial dysfunction and affects gene expression for mitochondria biogenesis and neuronal differentiation in SH-SY5Y cell line[J]. NeuroToxicology, 2019, 75: 116-122. DOI:10.1016/j.neuro.2019.09.009 |
| [40] |
FRYE R E, ROSE S, CHACKO J, et al. Modulation of mitochondrial function by the microbiome metabolite propionic acid in autism and control cell lines[J]. Transl Psychiatry, 2016, 6(10): e927. DOI:10.1038/tp.2016.189 |
| [41] |
MACFABE D F, CAIN D P, RODRIGUEZ-CAPOTE K, et al. Neurobiological effects of intraventricular propionic acid in rats: Possible role of short chain fatty acids on the pathogenesis and characteristics of autism spectrum disorders[J]. Behav Brain Res, 2007, 176(1): 149-169. DOI:10.1016/j.bbr.2006.07.025 |
| [42] |
RAMIS M R, ESTEBAN S, MIRALLES A, et al. Protective effects of melatonin and mitochondria-targeted antioxidants against oxidative stress: a review[J]. Curr Med Chem, 2015, 22(22): 2690-2711. DOI:10.2174/0929867322666150619104143 |
| [43] |
PAUPE V, PRUDENT J, DASSA E P, et al. CCDC90A (MCUR1) is a cytochrome c oxidase assembly factor and not a regulator of the mitochondrial calcium uniporter[J]. Cell Metab, 2015, 21(1): 109-116. DOI:10.1016/j.cmet.2014.12.004 |
| [44] |
PAUPE V, PRUDENT J. New insights into the role of mitochondrial calcium homeostasis in cell migration[J]. Biochem Biophys Res Commun, 2018, 500(1): 75-86. DOI:10.1016/j.bbrc.2017.05.039 |
| [45] |
KENT-DENNIS C, PENNER G B. Effects of a proinflammatory response on metabolic function of cultured, primary ruminal epithelial cells[J]. J Dairy Sci, 2021, 104(1): 1002-1017. DOI:10.3168/jds.2020-19092 |
| [46] |
GOMMERS L M M, VAN DER WIJST J, BOS C, et al. Colonic expression of calcium transporter TRPV6 is regulated by dietary sodium butyrate[J]. Pflugers Arch, 2022, 474(3): 293-302. DOI:10.1007/s00424-021-02648-6 |
| [47] |
HE H Y, HENDERSON A C, DU Y L, et al. Two-enzyme pathway links L-arginine to nitric oxide in n-nitroso biosynthesis[J]. J Am Chem Soc, 2019, 141(9): 4026-4033. DOI:10.1021/jacs.8b13049 |
| [48] |
HERBERG L J, ROSE I C, DE BELLEROCHE J S, et al. Ornithine decarboxylase induction and polyamine synthesis in the kindling of seizures: the effect of α-difluoromethylornithine[J]. Epilepsy Res, 1992, 11(1): 3-7. DOI:10.1016/0920-1211(92)90015-L |
| [49] |
WANG J Y. Polyamines regulate expression of E-cadherin and play an important role in control of intestinal epithelial barrier function[J]. Inflammo Pharmacology, 2005, 13(1-3): 91-101. DOI:10.1163/156856005774423890 |
| [50] |
胡骁飞, 魏凤仙, 呙于明. 脂多糖(LPS)刺激对肉仔鸡生产性能及肌肉品质影响[J]. 中国农业大学学报, 2011, 16(1): 60-65. HU X F, WEI F X, GUO Y M. Effect of lipopolysaccharide (LPS) challenge on performance and meat quality of broiler chickens[J]. Journal of China Agricultural University, 2011, 16(1): 60-65. (in Chinese) |
| [51] |
冯京海, 张敏红, 郑姗姗, 等. 日循环高温对肉鸡线粒体活性氧产生量、钙泵活性及胸肌品质的影响[J]. 畜牧兽医学报, 2006, 37(12): 1304-1311. FENG J H, ZHANG M H, ZHENG S S, et al. The effect of cyclic high temperature on mitochondrial ROS production, Ca2+-ATPase activity and breast meat quality of broilers[J]. Acta Veterinaria et Zootechnica Sinica, 2006, 37(12): 1304-1311. DOI:10.3321/j.issn:0366-6964.2006.12.012 (in Chinese) |
| [52] |
FAN P X, LIU P, SONG P X, et al. Moderate dietary protein restriction alters the composition of gut microbiota and improves ileal barrier function in adult pig model[J]. Sci Rep, 2017, 7(1): 43412. DOI:10.1038/srep43412 |
| [53] |
LIU R L, HE J W, JI X, et al. A moderate reduction of dietary crude protein provide comparable growth performance and improve metabolism via changing intestinal microbiota in Sushan nursery pigs[J]. Animals, 2021, 11(4): 1166. DOI:10.3390/ani11041166 |
| [54] |
POLLOCK J, GLENDINNING L, SMITH L A, et al. Temporal and nutritional effects on the weaner pig ileal microbiota[J]. Anim Microbiome, 2021, 3(1): 58. DOI:10.1186/s42523-021-00119-y |
| [55] |
IJAZ M U, AHMAD M I, HUSSAIN M, et al. Meat protein in high-fat diet induces adipogensis and dyslipidemia by altering gut microbiota and endocannabinoid dysregulation in the adipose tissue of mice[J]. J Agric Food Chem, 2020, 68(13): 3933-3946. DOI:10.1021/acs.jafc.0c00017 |
| [56] |
REGMI P R, METZLER-ZEBELI B U, GÄNZLE M G, et al. Starch with high amylose content and low in vitro digestibility increases intestinal nutrient flow and microbial fermentation and selectively promotes bifidobacteria in pigs[J]. J Nutr, 2011, 141(7): 1273-1280. DOI:10.3945/jn.111.140509 |
| [57] |
JIANG X Y, LU N S, XUE Y, et al. Crude fiber modulates the fecal microbiome and steroid hormones in pregnant Meishan sows[J]. Gen Comp Endocrinol, 2019, 277: 141-147. DOI:10.1016/j.ygcen.2019.04.006 |
| [58] |
ZEITZ J O, NEUFELD K, POTTHAST C, et al. Effects of dietary supplementation of the lignocelluloses FibreCell and OptiCell on performance, expression of inflammation-related genes and the gut microbiome of broilers[J]. Poult Sci, 2019, 98(1): 287-297. DOI:10.3382/ps/pey345 |
| [59] |
RÖHE I, METZGER F, VAHJEN W, et al. Effect of feeding different levels of lignocellulose on performance, nutrient digestibility, excreta dry matter, and intestinal microbiota in slow growing broilers[J]. Poult Sci, 2020, 99(10): 5018-5026. DOI:10.1016/j.psj.2020.06.053 |
| [60] |
YANG C, DENG Q C, XU J Q, et al. Sinapic acid and resveratrol alleviate oxidative stress with modulation of gut microbiota in high-fat diet-fed rats[J]. Food Res Int, 2019, 116: 1202-1211. DOI:10.1016/j.foodres.2018.10.003 |
| [61] |
WU J, IBTISHAM F, NIU Y F, et al. Curcumin inhibits heat-induced oxidative stress by activating the MAPK-Nrf2/ARE signaling pathway in chicken fibroblasts cells[J]. J Therm Biol, 2019, 79: 112-119. DOI:10.1016/j.jtherbio.2018.12.004 |
| [62] |
GAN Z D, WEI W Y, LI Y, et al. Curcumin and resveratrol regulate intestinal bacteria and alleviate intestinal inflammation in weaned piglets[J]. Molecules, 2019, 24(7): 1220. DOI:10.3390/molecules24071220 |
| [63] |
IQBAL M, PUMFORD N R, TANG Z X, et al. Compromised liver mitochondrial function and complex activity in low feed efficient broilers are associated with higher oxidative stress and differential protein expression[J]. Poult Sci, 2005, 84(6): 933-941. DOI:10.1093/ps/84.6.933 |
| [64] |
OJANO-DIRAIN C, IQBAL M, WING T, et al. Glutathione and respiratory chain complex activity in duodenal mitochondria of broilers with low and high feed efficiency[J]. Poult Sci, 2005, 84(5): 782-788. DOI:10.1093/ps/84.5.782 |
| [65] |
SHARIFABADI H R, ZAMIRI M J, ROWGHANI E, et al. Relationship between the activity of mitochondrial respiratory chain complexes and feed efficiency in fat-tailed Ghezel lambs[J]. J Anim Sci, 2012, 90(6): 1807-1815. DOI:10.2527/jas.2011-4791 |
| [66] |
VINCENT A, LOUVEAU I, GONDRET F, et al. Divergent selection for residual feed intake affects the transcriptomic and proteomic profiles of pig skeletal muscle[J]. J Anim Sci, 2015, 93(6): 2745-2758. DOI:10.2527/jas.2015-8928 |
| [67] |
LIU J, STEWART S N, ROBINSON K, et al. Linkage between the intestinal microbiota and residual feed intake in broiler chickens[J]. J Anim Sci Biotechnol, 2021, 12(1): 22. DOI:10.1186/s40104-020-00542-2 |
| [68] |
TAN Z, YANG T, WANG Y, et al. Metagenomic analysis of cecal microbiome identified microbiota and functional capacities associated with feed efficiency in landrace finishing pigs[J]. Front Microbiol, 2017, 8: 1546. DOI:10.3389/fmicb.2017.01546 |
| [69] |
WANG Z X, HE Y Z, WANG C D, et al. Variations in microbial diversity and metabolite profiles of female landrace finishing pigs with distinct feed efficiency[J]. Front Vet Sci, 2021, 8: 702931. DOI:10.3389/fvets.2021.702931 |
| [70] |
LIU Y, ZHENG Z J, YU L H, et al. Examination of the temporal and spatial dynamics of the gut microbiome in newborn piglets reveals distinct microbial communities in six intestinal segments[J]. Sci Rep, 2019, 9(1): 3453. DOI:10.1038/s41598-019-40235-z |
| [71] |
GU Y G, XIAO X, PAN R R, et al. Lactobacillus plantarum dy-1 fermented barley extraction activates white adipocyte browning in high-fat diet-induced obese rats[J]. J Food Biochem, 2021, 45(4): e13680. |
| [72] |
NOVAIS A K, DESCHÊNE K, MARTEL-KENNES Y, et al. Weaning differentially affects mitochondrial function, oxidative stress, inflammation and apoptosis in normal and low birth weight piglets[J]. PLoS One, 2021, 16(2): e0247188. DOI:10.1371/journal.pone.0247188 |
| [73] |
LI Y, GUO Y, WEN Z S, et al. Weaning stress perturbs gut microbiome and its metabolic profile in piglets[J]. Sci Rep, 2018, 8(1): 18068. DOI:10.1038/s41598-018-33649-8 |
| [74] |
FU H, HE M Z, WU J Y, et al. Deep investigating the changes of gut microbiome and its correlation with the shifts of host serum metabolome around parturition in sows[J]. Front Microbiol, 2021, 12: 729039. DOI:10.3389/fmicb.2021.729039 |
| [75] |
QI K K, MEN X M, WU J, et al. Effects of growth stage and rearing pattern on pig gut microbiota[J]. Curr Microbiol, 2022, 79(5): 136. DOI:10.1007/s00284-022-02828-2 |
| [76] |
GOPHNA U, KONIKOFF T, NIELSEN H B. Oscillospira and related bacteria - From metagenomic species to metabolic features[J]. Environ Microbiol, 2017, 19(3): 835-841. DOI:10.1111/1462-2920.13658 |
| [77] |
ZHU C H, SONG W T, TAO Z Y, et al. Analysis of microbial diversity and composition in small intestine during different development times in ducks[J]. Poult Sci, 2020, 99(2): 1096-1106. DOI:10.1016/j.psj.2019.12.030 |
| [78] |
MONNÉ M, CHAN K W, SLOTBOOM D J, et al. Functional expression of eukaryotic membrane proteins in Lactococcus lactis[J]. Protein Sci, 2005, 14(12): 3048-3056. DOI:10.1110/ps.051689905 |
| [79] |
XIN J E, ZENG D, WANG H S, et al. Preventing non-alcoholic fatty liver disease through Lactobacillus johnsonii BS15 by attenuating inflammation and mitochondrial injury and improving gut environment in obese mice[J]. Appl Microbiol Biotechnol, 2014, 98(15): 6817-6829. DOI:10.1007/s00253-014-5752-1 |
| [80] |
DUMITRESCU L, POPESCU-OLARU I, COZMA L, et al. Oxidative stress and the microbiota-gut-brain axis[J]. Oxid Med Cell Longev, 2018, 2018: 2406594. |
| [81] |
ORTEGA-HERNÁNDEZ A, MARTÍNEZ-MARTÍNEZ E, GÓMEZ-GORDO R, et al. The interaction between mitochondrial oxidative stress and gut microbiota in the cardiometabolic consequences in diet-induced obese rats[J]. Antioxidants (Basel), 2020, 9(7): 640. DOI:10.3390/antiox9070640 |
| [82] |
KONCZ P, SZANDA G, RAJKI A, et al. Reactive oxygen species, Ca2+ signaling and mitochondrial NAD(P)H level in adrenal glomerulosa cells[J]. Cell Calcium, 2006, 40(4): 347-357. DOI:10.1016/j.ceca.2006.04.003 |
| [83] |
LIM M Y, SONG E J, KANG K S, et al. Age-related compositional and functional changes in micro-pig gut microbiome[J]. GeroScience, 2019, 41(6): 935-944. DOI:10.1007/s11357-019-00121-y |
| [84] |
AMIT-ROMACH E, SKLAN D, UNI Z. Microflora ecology of the chicken intestine using 16S ribosomal DNA primers[J]. Poult Sci, 2004, 83(7): 1093-1098. DOI:10.1093/ps/83.7.1093 |
| [85] |
NURRAHMA B A, TSAO S P, WU C H, et al. Probiotic supplementation facilitates recovery of 6-OHDA-induced motor deficit via improving mitochondrial function and energy metabolism[J]. Front Aging Neurosci, 2021, 13: 668775. DOI:10.3389/fnagi.2021.668775 |
(编辑 范子娟)



