2. 中国科学院微生物研究所, 北京 100080;
3. 中国农业科学院生物技术研究所,北京 100081
2. Institute of Microbiology, Chinese Academy of Sciences, Beijing 100080, China;
3. Biotechnology Research Institute, Chinese Academy of Agricultural Sciences, Beijing 100081, China
非洲猪瘟(African swine fever,ASF)是由非洲猪瘟病毒(African swine fever virus,ASFV)引起的一种猪的急性、烈性、高度接触性传染病[1]。ASFV是一种核质巨DNA病毒,基因组全长170~193 kb,编码150~167个开放阅读框[2]。自2018年传入我国以来,非洲猪瘟疫情在全国范围内迅速扩散,给中国以及全球造成巨大的经济损失。目前,我国出现了基因Ⅰ型ASFV,临床表现为亚急性型或慢性型,严重增加了ASF防控难度[3]。ASFV编码多种蛋白质,通过干扰宿主的天然免疫系统,抑制和逃避宿主的免疫应答反应,为自身的增殖、扩散创造有利条件。例如,MGF505-7R抑制p65的磷酸化和核转移,与STING、TBK1和IKKα互作,抑制cGAS-STING通路介导的Ⅰ型干扰素的产生[4-7];MGF505-11R能够与STING互作,通过泛素化途径降解STING,从而抑制Ⅰ型干扰素的产生[8];E120R能够与IRF3互作,抑制TBK1对IRF3的招募和IRF3的磷酸化,从而抑制Ⅰ型干扰素的产生[9]。
线粒体抗病毒信号蛋白(mitochondrial antiviral signaling, MAVS)是抗RNA病毒信号通路中的关键接头蛋白。在病毒入侵机体时,RIG-Ⅰ样受体(RIG-Ⅰ-like receptor, RLR)识别病原相关分子模式(pathogen-associated molecular patterns, PAMPs),RIG-Ⅰ与MAVS相互作用激活MAVS,进而活化下游NF-κB和IRF3的信号通路,诱导干扰素的表达[10]。然而,在病毒进化过程中也具备了拮抗MAVS的策略,以此逃避先天性免疫系统。例如,日本脑炎病毒(Japanese encephalitis virus,JEV)NS1蛋白通过抑制MAVS的表达,抑制Ⅰ型干扰素的产生[11];口蹄疫病毒(foot-and-mouth disease virus, FMDV)VP1蛋白与MAVS结合,竞争性抑制MAVS与TRAF3的结合,从而抑制Ⅰ型干扰素的产生[12];登革热病毒(dengue virus, DENV)NS4A蛋白与MAVS互作,抑制MAVS与RIG-Ⅰ的结合,从而抑制IRF3的活化[13]。
在研究ASFV多基因家族(multigene families,MGF)成员MGF360-14L对Ⅰ型干扰素通路影响的过程中,作者发现MGF360-14L能够与MAVS相互作用,并且能够抑制MAVS介导的Ⅰ型干扰素的产生,从而逃避宿主先天性免疫反应。
1 材料与方法 1.1 材料1.1.1 细胞 人胚肾细胞HEK293T和猪肾细胞PK-15采用含有10%胎牛血清、1%双抗DMEM培养基在5% CO2和37 ℃的培养箱中培养。
1.1.2 质粒 合成ASFV-18毒株MGF360-14L全长基因(GenBank No.MH 766894),然后亚克隆入p3×Flag-CMV-7.1质粒和pCMV-N-eGFP质粒;TRIM21和MAVS全长基因从PK-15细胞的cDNA中扩增,然后分别亚克隆入pcDNA3.1-Myc和pcDNA3.1-HA载体;cGAS、STING、TBK1、IRF3、IFN-β-luc和pRL-TK等表达质粒由本实验室保存。
1.1.3 主要试剂 兔抗TBK1/NAK、P-TBK1、IRF3、P-IRF3、GAPDH、eGFP-Tag、HA-Tag抗体和HRP标记的羊抗兔IgG抗体以及鼠抗Myc和Flag标签抗体购自Cell Signaling Technology公司;转染试剂(JetPRIME Kit)购自Polyplus Transfection公司。双荧光素酶检测试剂盒购自北京全式金生物技术股份有限公司;HRP标记羊抗鼠IgG抗体、蛋白酶抑制剂和磷酸酶抑制剂购自康为世纪生物科技股份有限公司;免疫共沉淀试剂盒(Pierce Crosslink Magnetic IP/Co-IP Kit)购自Thermo公司;羊抗鼠IgG H&L(Alexa Fluor® 488)和羊抗兔IgG H&L(Alexa Fluor® 594)荧光二抗购自Abcam公司;RNA提取试剂盒(TaKaRa MiniBEST Universal RNA Extraction Kit)和反转录试剂盒(PrimeScriptTM RT Master Mix)购自宝生物(TaKaRa)公司。
1.2 方法1.2.1 实时荧光定量PCR(RT-qPCR) 收集细胞提取总RNA,然后将RNA反转录为cDNA,具体操作步骤参见RNA提取试剂盒(TaKaRa)和反转录试剂盒(TaKaRa)。使用SYBR荧光染料和ABI7900HT荧光定量PCR仪进行样品的检测。PCR体系:95 ℃ 1 min;95 ℃ 15 s,60 ℃ 15 s和72 ℃ 45 s,40个循环,每个样本进行3次重复检测。RT-qPCR的引物序列: pig-GAPDH-F:5′-CGTCCCTGAGACACGATGGT-3′,pig-GAPDH-R:5′-GGAACATGTAGACCATGTAG-3′;pig-IFN-β-F:5′-GTGGAACTTGATGGGCAGAT-3′,pig-IFN-β-R:5′-TTCCTCCTCCATGATTTCCTC-3′。
1.2.2 双荧光素酶检测 首先将HEK293T细胞传代培养,然后铺48孔细胞培养板,待细胞密度达到70%左右时转染质粒。将IFN-β-luc和pRL-TK与cGAS、STNG、TBK1、MGF360-14L、TRIM21、MAVS或空载共转染。转染24 h后收集细胞,进行双荧光素酶检测,具体操作步骤参见双荧光素酶说明书(北京全式金生物技术股份有限公司)。
1.2.3 免疫共沉淀试验(Co-IP) HEK293T细胞传代培养于6孔细胞培养板中,待细胞密度达到70%左右,依据具体试验转染p3×Flag-MGF360-14L、p-CMV-eGFP-MGF360-14L、pcDNA3.1-Myc-TRIM21、pcDNA3.1-HA-MAVS、p3×Flag-MAVS、pCDEF-HA-Ub或空载质粒转。转染后24 h,细胞用预冷的PBS洗3次,然后用含有蛋白酶和磷酸酶抑制剂的IP裂解液裂解细胞,在4 ℃条件下,12 000×g离心10 min,取上清,4 ℃保存备用。将磁珠与标签抗体Flag或HA在旋转器上室温孵育1 h,然后清洗结合了抗体的磁珠,再将制备好的细胞上清与磁珠4 ℃过夜孵育,清洗磁珠后用洗脱液洗脱,洗脱下来的样品加入loading buffer 100 ℃煮10 min,进行SDS-PAGE分析。
1.2.4 间接免疫荧光试验(IFA) HEK293T细胞铺于激光共聚焦培养皿上,待细胞密度达到70%左右,转染p3×Flag-MGF360-14L和pcDNA3.1-HA-MAVS质粒。转染24 h后,用4%的多聚甲醛固定细胞20 min,然后0.1% Triton X-100透膜15 min,5%的BSA封闭1 h,一抗分别采用鼠抗Flag和兔抗HA标签抗体4 ℃过夜孵育,二抗分别采用羊抗鼠IgG H&L(Alexa Fluor® 488)和羊抗兔IgG H&L(Alexa Fluor® 594)抗体37 ℃孵育1 h,最后用DAPI着染细胞核,置荧光显微镜下观察。
1.2.5 Western blot分析 转染了相应质粒的HEK293T细胞被含有蛋白酶和磷酸酶抑制剂的细胞裂解液裂解后,在4 ℃ 12 000×g离心10 min,收集裂解液上清。采用BCA蛋白定量试剂盒(Thermo)对收集的裂解液进行蛋白定量。裂解液加入SDS-PAGE loading buffer后100 ℃煮沸10 min,上样进行SDS-PAGE,并进行转膜。用5%脱脂乳封闭后,分别用对应蛋白或标签的抗体孵育,然后进行二抗孵育,最后进行ECL显色。
1.2.6 数据分析 本研究中的试验均进行3次独立的重复,使用GraphPad Prism 8软件对试验数据进行统计分析分析方法采用Student’s t-test(*.P < 0.05;**.P < 0.01;***.P < 0.001)。
2 结果 2.1 ASFV MGF360-14L抑制MAVS介导的Ⅰ型干扰素通路已有研究表明,ASFV MGF的一些成员能够抑制Ⅰ型干扰素的产生。为了确定MGF360-14L对Ⅰ型干扰素的影响,将MGF360-14L-Flag质粒转染HEK293T细胞,并用5 μg·mL-1的poly(I:C)诱导干扰素的产生。RT-qPCR检测结果显示,MGF360-14L能够明显抑制poly(I:C)诱导的IFN-β mRNA的产生(图 1A),荧光素酶试验结果显示MGF360-14L能够抑制MAVS诱导的IFN-β启动子活性(图 1B)。
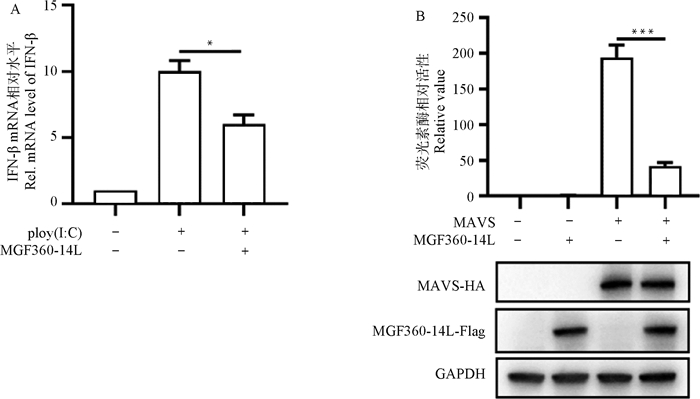
|
A.HEK293T细胞转染MGF360-14L-Flag蛋白表达质粒和poly(I:C)后24 h收集细胞,用RT-qPCR检测IFN-β mRNA;B. 双荧光素酶检测MAVS诱导的IFN-β启动子活性,并进行相对应的Western blot检测 A. HEK293T cells were co-transfected with the MGF360-14L-expressing plasmid and poly(I:C) for 24 h and then harvested for RT-qPCR assay to determine IFN-β mRNA levels; B. Double luciferase was used to detect IFN-β promoter activity induced by MAVS, expression of MAVS-HA and MGF360-14L-Flag was analyzed by Western blot 图 1 ASFV MGF360-14L蛋白基因对MAVS信号通路的抑制 Fig. 1 MGF360-14L inhibited IFN-β mRNA production and IFN-β promoter activity |
由于MGF360-14L能够抑制MAVS介导的IFN-β的产生,为确定其具体作机制,作者将MGF360-14L-Flag和MAVS-HA质粒共转染HEK293T细胞,进行Co-IP试验,结果表明,MGF360-14L能够与MAVS结合(图 2A),反过来MAVS同样能够与MGF360-14L结合(图 2B)。并且共定位试验表明,MGF360-14和MAVS存在胞质内共定位(图 2C)。
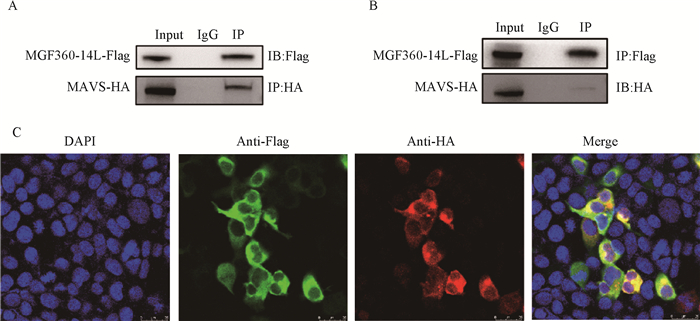
|
A、B. MGF360-14L-Flag和MAVS-HA共转染HEK293T细胞中,转染后24 h收集细胞进行Co-IP试验;C.将MGF360-14L-Flag和MAVS-HA共转染铺有HEK293T细胞进行IFA试验,标尺为25 μm A, B. HEK293T cells were co-transfected with MGF360-14L-Flag and MAVS-HA plasmids, the cells were collected 24 h after transfection for Co-IP assay; C. HEK293T cells were transfected with MGF360-14L-Flag and MAVS-HA plasmids for laser confocal test. The scale is 25 μm 图 2 ASFV MGF360-14L蛋白与MAVS互作 Fig. 2 MGF360-14L interacts with MAVS |
三重基序蛋白21(tripartite motif protein 21, TRIM21)能够促进线粒体抗病毒信号蛋白(MAVS)的K27多聚泛素化从而增强TBK1的招募和活化IRF3,从而启动Ⅰ型干扰素的表达[14]。为了确定MGF360-14L是否能够抑制TRIM21和MAVS引起的Ⅰ型干扰素的产生,作者将不同浓度的MGF360-14L、TRIM21、MAVS以及启动子报告基因IFN-β-luc和内参报告基因pRL-TK共转染HEK293T细胞,进行双荧光素酶检测。结果显示,MGF360-14L能够抑制TRIM21和MAVS共同诱导的IFN-β启动子活性,且呈剂量依赖性(图 3A)。已有研究表明,TRIM21通过与MAVS作用,促进IRF3的磷酸化,促进Ⅰ型干扰素的产生[14]。作者将MGF360-14L-Flag、TRIM21-Myc和MAVS-HA共转染HEK293T细胞后,进行Western blot分析,结果显示,MGF360-14L对MAVS和TRIM21诱导的TBK1和IRF3磷酸化具有抑制作用(图 3B)。

|
A. 双荧光素酶检测不同浓度的ASFV MGF360-14L蛋白对MAVS/TRIM21诱导的IFN-β启动子活性的抑制作用;B. Western blot检测MGF360-14L蛋白对MAVS诱导的TBK和IRF3的抑制作用 A. Dual luciferase assay was used to detect the inhibitory effect of different concentrations of ASFV MGF360-14L on MAVS/ TRIM21-induced IFN-β promoter activity; B. TRIM21-Myc, TBK1, P-TBK1, IRF3, P-IRF3, MAVS-HA and MGF360-14L-Flag were analyzed by Western blot 图 3 ASFV MGF360-14L抑制MAVS诱导的TBK1和IRF3的磷酸化 Fig. 3 MGF360-14L inhibits the phosphorylation of TBK1 and IRF3 |
前期研究结果表明,MGF360-14L和TRIM21互作[15],并且TRIM21与MAVS也具有相互作用[14]。为了探究MGF360-14L蛋白、TRIM21和MAVS三者之间的相互作用,将TRIM21-Myc和MAVS-Flag质粒共转染HEK293T细胞,或者不同浓度的MGF360-14L-eGFP与TRIM21-Myc和MAVS-Flag表达质粒共转染HEK293T细胞,Co-IP竞争试验结果显示,MAVS与TRIM21结合,在转染MGF360-14L质粒后,MAVS能与MGF360-14L互作,且MGF360-14L存在时MAVS与TRIM21的互作被减弱(图 4A)。将TRIM21-Myc、MAVS-Flag和Ub-HA共转染HEK293T细胞或者MGF360-14L-eGFP、TRIM21-Myc、MAVS-Flag和Ub-HA共转染HEK293T细胞,Western blot检测结果表明,TRIM21能够促进MAVS的泛素化,MGF360-14L对TRIM21促进MAVS的泛素化有抑制作用(图 4B)。综上所述,作者推测MGF360-14L通过与TRIM21竞争结合MAVS,抑制了TRIM21诱导的MAVS的泛素化,从而抑制Ⅰ型干扰素的产生。
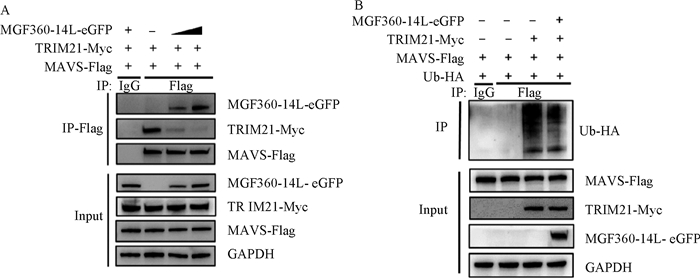
|
A. 将ASFV MGF360-14L-eGFP、MAVS-Flag和TRIM21-Myc蛋白表达质粒共转染HEK293T细胞,进行Co-IP和Western blot检测;B. 将MGF360-14L-eGFP、MAVS-Flag、RIM21-Myc和Ub-HA蛋白表达质粒共转染HEK293T细胞,进行Co-IP试验以及Western blot检测 A. HEK293T cells were transfected with MGF360-14L-eGFP, TRIM21-Myc, MAVS-Flag. Co-IP and Western blot were performed 24 h after transfection; B. HEK293T cells transfected with MGF360-14L-eGFP, TRIM21-Myc, MAVS-Flag and Ub-HA, Co-IP and Western blot were performed 24 h after transfection 图 4 ASFV MGF360-14L抑制MAVS的泛素化 Fig. 4 MGF360-14L inhibits the ubiquitination of MAVS |
MGF360-14L为ASFV的一种非结构蛋白,位于ASFV基因组左侧可变区。已有文献报道,当缺失强毒株Georgia/2007的6个MGF基因(包括MGF360-14L)[16],以及缺失强毒株ASFV HLJ/18的7个基因(包括MGF360-14L)[17]后,缺失株ASFV-G-MGF和HLJ/18-7GD免疫猪均能完全抵抗强毒株的攻击。但仅缺失强毒株Georgia/2007的MGF360-13L和MGF360-14L并未影响ASFV在细胞上的复制水平和对猪的致病性[18],提示MGF360-14L可能不是ASFV的毒力基因。但我们的前期研究结果表明,MGF360-14L能够与IRF3互作,并通过招募TRIM21介导IRF3的K63位多聚泛素化,从而抑制IFN-β的产生[15],由此推测MGF360-14L可能参与ASFV逃避宿主先天性免疫系统,促进ASFV的感染。
虽然MAVS作为RNA病毒抑制Ⅰ型干扰素的靶基因,也有相关文献报道某些DNA病毒能与MAVS互作,从而抑制Ⅰ型干扰素的产生。例如,卡波氏肉瘤相关疱疹病毒(Kaposi’s sarcoma-associated herpesvirus,KSHV)的外膜蛋白ORF33与STING和MAVS互作,招募PPM1G促进STING和MAVS的去磷酸化,从而促进KSHV逃避先天性免疫反应[19];Ⅰ型单纯疱疹病毒(herpes simplex virus 1, HSV-1)的US11蛋白能够与RIG-Ⅰ相互作用,抑制RIG-Ⅰ和MAVS的互作,从而下调Ⅰ型干扰素的表达[20];人疱疹病毒6B(human herpesvirus 6B, HHV-6B)的U26蛋白能够降解MAVS,抑制RIG-Ⅰ/MAVS信号通路介导的先天性免疫反应[21]。也有研究表明,在病毒感染过程中,E3泛素连接酶TRIM21能够靶向MAVS的K27多聚泛素化,促进TBK1的招募,从而上调Ⅰ型干扰素的表达,提高机体先天性免疫反应抵抗病毒入侵[14]。
为了探讨MGF360-14L是否通过MAVS途径抑制Ⅰ型干扰素,从而逃避宿主先天性免疫系统,本研究采用双荧光素酶试验检测MGF360-14L对MAVS诱导的IFN-β启动子活性的影响,Co-IP和共定位检测MGF360-14L与MAVS的互作关系,及Western blot分析MGF360-14L对MAVS和TRIM21诱导的TBK1和IRF3磷酸化的影响。研究结果显示,MGF360-14L能抑制MAVS所诱导的IFN-β mRNA水平和启动子活性(图 1A、B)。Co-IP试验表明,MGF360-14L与MAVS具有相互作用,但并未进行内源性的互作研究,而共定位试验显示,二者存在胞质内的共定位(图 2A~C)。进一步的研究表明,MGF360-14L能够抑制TRIM21和MAVS共同诱导的IFN-β启动子活性,并且对MAVS和TRIM21诱导的TBK1和IRF3磷酸化也具有抑制作用(图 3A和B),作者初步推断MGF360-14L与MAVS互作从而抑制IFN-β的产生。此外,MGF360-14L可能通过与TRIM21竞争结合MAVS,抑制TRIM21对MAVS的泛素化作用(图 4A和B)。本研究进一步探讨了MGF360-14L的逃避宿主先天性免疫的作用机制,为ASF疫苗的研制提供线索。
4 结论非洲猪瘟病毒MGF360-14L通过与MAVS互作,抑制了TRIM21诱导的MAVS的泛素化,从而抑制Ⅰ型干扰素的产生。
| [1] |
DIXON L K, SUN H, ROBERTS H. African swine fever[J]. Antiviral Res, 2019, 165: 34-41. DOI:10.1016/j.antiviral.2019.02.018 |
| [2] |
ALONSO C, BORCA M, DIXON L, et al. ICTV virus taxonomy profile: Asfarviridae[J]. J Gen Virol, 2018, 99(5): 613-614. DOI:10.1099/jgv.0.001049 |
| [3] |
SUN E C, HUANG L Y, ZHANG X F, et al. Genotype Ⅰ African swine fever viruses emerged in domestic pigs in China and caused chronic infection[J]. Emerg Microbes Infect, 2021, 10(1): 2183-2193. DOI:10.1080/22221751.2021.1999779 |
| [4] |
LI D, YANG W P, LI L L, et al. African swine fever virus MGF-505-7R negatively regulates cGAS-STING-mediated signaling pathway[J]. J Immunol, 2021, 206(8): 1844-1857. DOI:10.4049/jimmunol.2001110 |
| [5] |
LIU X L, AO D, JIANG S, et al. African swine fever virus A528R inhibits TLR8 mediated NF-κB activity by targeting p65 activation and nuclear translocation[J]. Viruses, 2021, 13(10): 2046. DOI:10.3390/v13102046 |
| [6] |
LI J N, SONG J, KANG L, et al. pMGF505-7R determines pathogenicity of African swine fever virus infection by inhibiting IL-1β and type Ⅰ IFN production[J]. PLoS Pathog, 2021, 17(7): e1009733. DOI:10.1371/journal.ppat.1009733 |
| [7] |
LI D, ZHANG J, YANG W P, et al. African swine fever virus protein MGF-505-7R promotes virulence and pathogenesis by inhibiting JAK1-and JAK2-mediated signaling[J]. J Biol Chem, 2021, 297(5): 101190. DOI:10.1016/j.jbc.2021.101190 |
| [8] |
YANG K D, HUANG Q T, WANG R Y, et al. African swine fever virus MGF505-11R inhibits type Ⅰ interferon production by negatively regulating the cGAS-STING-mediated signaling pathway[J]. Vet Microbiol, 2021, 263: 109265. DOI:10.1016/j.vetmic.2021.109265 |
| [9] |
LIU H S, ZHU Z X, FENG T, et al. African swine fever virus E120R protein inhibits interferon beta production by interacting with IRF3 to block its activation[J]. J Virol, 2021, 95(18): e00824-21. |
| [10] |
SETH R B, SUN L J, EA C K, et al. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-κB and IRF3[J]. Cell, 2005, 122(5): 669-682. DOI:10.1016/j.cell.2005.08.012 |
| [11] |
ZHOU D Y, LI Q Y, JIA F, et al. The Japanese encephalitis virus NS1' protein inhibits type Ⅰ IFN production by targeting MAVS[J]. J Immunol, 2020, 204(5): 1287-1298. DOI:10.4049/jimmunol.1900946 |
| [12] |
EKANAYAKA P, LEE S Y, HERATH T U B, et al. Foot-and-mouth disease virus VP1 target the MAVS to inhibit type-Ⅰ interferon signaling and VP1 E83K mutation results in virus attenuation[J]. PLoS Pathog, 2020, 16(11): e1009057. DOI:10.1371/journal.ppat.1009057 |
| [13] |
HE Z J, ZHU X, WEN W T, et al. Dengue virus subverts host innate immunity by targeting adaptor protein MAVS[J]. J Virol, 2016, 90(16): 7219-7230. DOI:10.1128/JVI.00221-16 |
| [14] |
XUE B B, LI H Y, GUO M M, et al. TRIM21 promotes innate immune response to RNA viral infection through Lys27-linked polyubiquitination of MAVS[J]. J Virol, 2018, 92(14): e00321-18. |
| [15] |
WANG Y, CUI S, XIN T, et al. African swine fever virus MGF360-14L negatively regulates type Ⅰ interferon signaling by targeting IRF3[J]. Front Cell Infect Microbiol, 2022, 11: 818969. DOI:10.3389/fcimb.2021.818969 |
| [16] |
O'DONNELL V, HOLINKA L G, GLADUE D P, et al. African swine fever virus Georgia isolate harboring deletions of MGF360 and MGF505 genes is attenuated in swine and confers protection against challenge with virulent parental virus[J]. J Virol, 2015, 89(11): 6048-6056. DOI:10.1128/JVI.00554-15 |
| [17] |
CHEN W Y, ZHAO D M, HE X J, et al. A seven-gene-deleted African swine fever virus is safe and effective as a live attenuated vaccine in pigs[J]. Sci China Life Sci, 2020, 63(5): 623-634. DOI:10.1007/s11427-020-1657-9 |
| [18] |
BORCA M V, O'DONNELL V, HOLINKA L G, et al. Development of a fluorescent ASFV strain that retains the ability to cause disease in swine[J]. Sci Rep, 2017, 7(1): 46747. DOI:10.1038/srep46747 |
| [19] |
YU K, TIAN H B, DENG H Y. PPM1G restricts innate immune signaling mediated by STING and MAVS and is hijacked by KSHV for immune evasion[J]. Sci Adv, 2020, 6(47): eabd0276. DOI:10.1126/sciadv.abd0276 |
| [20] |
XING J J, WANG S, LIN R T, et al. Herpes simplex virus 1 tegument protein US11 downmodulates the RLR signaling pathway via direct interaction with RIG-Ⅰ and MDA-5[J]. J Virol, 2012, 86(7): 3528-3540. DOI:10.1128/JVI.06713-11 |
| [21] |
JIANG X F, TANG T, GUO J F, et al. Human herpesvirus 6B U26 inhibits the activation of the RLR/MAVS signaling pathway[J]. mBio, 2021, 12(1): e03505-20. |
(编辑 孟培)



