2. 扬州大学兽医学院,扬州 225009;
3. 江苏省动物重要疫病与人兽共患病防控协同创新中心,扬州 225009
2. College of Veterinary Medicine, Yangzhou University, Yangzhou 225009, China;
3. Jiangsu Co-innovation Center for Prevention and Control of Important Animal Infectious Diseases and Zoonoses, Yangzhou 225009, China
镉(cadmium,Cd)是一种环境重金属污染物,半衰期长,具有多器官毒性,可引起肺、肾、肝、骨骼、心血管和生殖系统等器官或组织的损伤。Cd可通过饮水、食物和空气等途径进入动物机体,直接刺激或间接诱发肺相关疾病。体内研究表明,Cd可通过消化道途径引起SD大鼠肺组织的病理性改变,导致局灶性肺组织纤维增生、肺间质血管闭塞与肺泡腔变小[8]。氯化镉(cadmium chloride,CdCl2)溶液腹腔注射SD大鼠,能够引起Cd在肺组织中高度蓄积,导致肺泡Ⅱ型上皮细胞及毛细血管内皮细胞受损、肺泡间隔增厚及间质纤维增生[9]。此外,Cd可显著上调肺细胞凋亡蛋白Bax、capase-3和细胞色素c(cytochrome-c,cyto-c)的表达,对肺产生损伤[10]。体外研究发现,Cd可显著诱导人肺上皮细胞A549细胞中活性氧(reactive oxygen species,ROS)的产生,进一步诱导细胞凋亡。Cd(5 μmol·L-1) 能够显著抑制人肺上皮细胞Beas-2B细胞的生长,降低胶原蛋白-Ⅰ(collagen-Ⅰ,Col-Ⅰ)、Col-Ⅳ及Col-Ⅴ的表达,促进ROS的产生及细胞凋亡。
N-乙酰半胱氨酸(N-acetyl-L-cysteine,NAC)是一种含巯基的抗氧化剂,可清除已生成的自由基离子,抑制细胞凋亡。NAC显著缓解Cd诱导的多器官或组织来源的细胞氧化应激或细胞凋亡。NAC能够刺激肺泡表面活性物质的分泌,亦可促进肺泡Ⅱ型上皮细胞的生成,明显改善肺的功能[20]。然而,NAC如何缓解Cd暴露诱导大鼠肺组织损伤的作用机制尚未阐明。因此,本试验以去卵巢SD大鼠作为动物模型,通过测定肺组织中Cd含量、观察肺组织病理变化、检测肺组织Col-Ⅰ和Col-Ⅲ mRNA的转录水平及凋亡蛋白的表达,旨在探讨NAC通过抑制大鼠肺细胞凋亡缓解Cd致肺组织损伤的作用机理,为临床应用NAC防治Cd暴露引起的肺损伤提供理论依据。
1 材料与方法 1.1 实验动物3月龄雌性SD大鼠40只,体重240 g±10 g,购自江苏大学实验动物中心。
1.2 主要试剂与仪器NAC和CdCl2购自美国Sigma-Aldrich公司。RIPA裂解液、BCA蛋白浓度测定试剂盒、5×SDS-PAGE蛋白上样缓冲液、苏木素伊红(HE)染色试剂盒和Masson染色试剂盒购自翌圣生物科技(上海)股份有限公司。HiScript Q RT SuperMix for qPCR (+gDNA wiper)、ChamQ Universal SYBR qPCR Master Mix和无RNA酶水购自南京诺唯赞生物科技有限公司。Bcl-2、Bax、cleaved caspase- 3(Asp175)、p-Akt、Akt、p53、β-actin抗体、辣根过氧化物酶(HRP)标记的羊抗兔二抗和HRP标记的兔抗小鼠二抗购自美国Cell Signaling technology公司。Trizol Reagent购自美国Invitrogen公司。其他试剂由实验室提供。
Milli-Q Biocel型超纯水系统购自美国Merck Millipore公司。5810R低温高速冷冻离心机购自德国Eppendorf公司。微波消解仪购自安东帕(上海)商贸有限公司。Nanodrop 2000型超微量分光光度计购自美国Thermo公司。Epoch型超微量微孔板分光光度计购自美国BioTek公司。电泳仪和电泳槽购自美国BIO-RAD公司。Tanon 5200型全自动化学发光成像分析系统购自上海天能科技有限公司。PinAAcle 900F火焰原子吸收光谱仪购自美国PerkinElmer公司。ABI7500型荧光定量PCR仪购自美国ABI公司。
1.3 试验分组与处理40只雌性SD大鼠通过卵巢摘除术去除卵巢,饲养室控制昼夜时间均等(12 h/12 h),室温饲养,饲养环境清洁和安静,许可证号:SCXK(苏)2016-0020。40只雌性SD大鼠随机分为假手术对照(Control)组、Cd组、NAC组和NAC + Cd组,每组10只。各组按以下方法处理:对照组大鼠正常饲喂,自由饮用超纯水;NAC组与NAC + Cd组大鼠自由饮用NAC水(浓度为100 mg·L-1),持续1个月;预处理结束,Cd组与NAC+Cd组大鼠自由饮用镉水(浓度为50 mg·L-1)。18个月后,所有大鼠肌内注射2 %戊巴比妥钠(剂量60 mg·kg-1,根据体重计算用量),断颈处死大鼠,完整分离肺。分块装入离心管中,置于-80 ℃保存备用。
1.4 Cd含量测定使用微波消解仪对大鼠肺组织进行消解。方法:将各组大鼠肺组织置于80 ℃烘箱内烘干、研磨粉碎,精确称取0.2 mg组织粉末置于消解管,添加4 mL硝酸(分析纯)。按照梯度温度(90 ℃、120 ℃至170 ℃)的方法进行加热。冷却后,转移至离心管,每管定容至10 mL,置于室温。使用PinAAcle 900F火焰原子吸收光谱仪进行Cd含量测定。
1.5 HE染色与Masson染色将新鲜肺组织置于4%多聚甲醛溶液,固定24 h。通过梯度乙醇脱水、二甲苯透明及石蜡包埋。利用切片机进行组织切片,漂浮于40 ℃温水,使切片铺开,将组织切片置于载玻片,置于60 ℃烘箱内烤片。经HE染色和Masson染色,封片,光学显微镜观察,并拍照。
1.6 qRT-PCR检测肺组织Col-Ⅰ和Col-Ⅲ mRNA的变化利用Trizol试剂提取肺组织总RNA。Nanodrop 2000型超微量分光光度计对样品RNA进行纯度检测,即A260 nm/A280 nm比值。HiScript Q RT SuperMix for qPCR (+gDNA wiper)试剂反转录获取样品cDNA模板,以ChamQ Universal SYBR qPCR Master Mix试剂进行荧光定量PCR,检测肺组织Col-Ⅰ和Col-Ⅲ mRNA的表达。引物序列如表 1,其中,β-actin为内参基因。20 μL反应体系:2×ChamQ Universal SYBR qPCR Master Mix 10 μL,上、下游引物各0.4 μL,cDNA模板2 μL,补充无RNA酶水至20 μL。PCR反应条件:预变性95 ℃ 30 s;循环反应95 ℃ 10 s和60 ℃ 30 s, 共40个循环。采用ABI7500型荧光定量PCR仪进行检测,采用2-ΔΔCt法对试验结果进行数据分析。
|
|
表 1 目的基因引物序列 Table 1 The primers of sequence for target genes |
RIPA裂解液对组织样品进行裂解,BCA蛋白浓度测定试剂盒检测各组样品蛋白浓度,并将各组浓度调整一致。将5×SDS-PAGE蛋白上样缓冲液加入各组样品,沸水煮10 min。电泳条件为110 V,90 min;转膜条件为260 mA,90 min;5%脱脂乳封闭,室温2 h;加入相应一抗(Bcl-2、Bax、cleaved caspase-3、p-Akt、Akt、p53、β-actin抗体),4 ℃过夜;收集一抗抗体,加入二抗(HRP标记羊抗兔抗体、HRP标记兔抗小鼠抗体),室温孵育2 h。收集二抗抗体,通过Tanon 5200型全自动化学发光成像分析系统进行摄片。
1.8 统计分析所有试验数值均用“平均值(x)±标准差(s)”表示。利用SPSS 25.0软件进行单因素方差(ANOVE)分析数值的差异。P < 0.05与P < 0.01分别表示结果的差异性显著和差异性极显著。
2 结果 2.1 大鼠肺组织中Cd含量的测定如图 1所示。与Control组相比,Cd组大鼠肺组织中Cd的含量极显著(P < 0.01)增加,而NAC组无差异性(P>0.05)。与Cd组相比,NAC+Cd组大鼠肺组织中Cd的含量极显著(P < 0.01)减少。
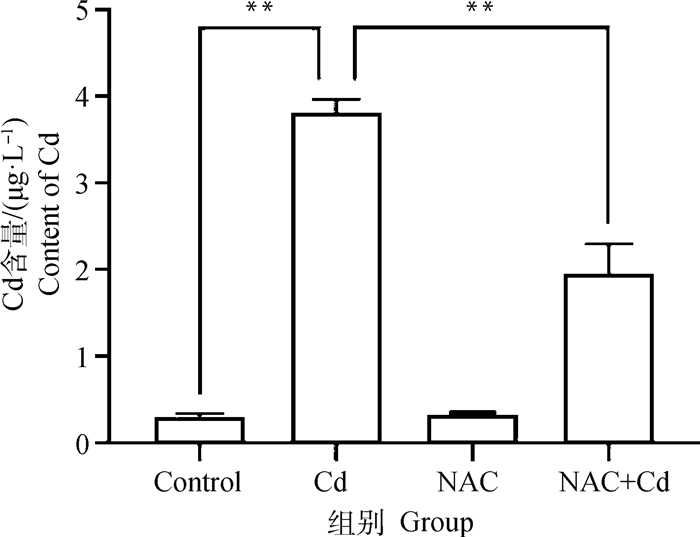
|
**.P < 0.01;*.P < 0.05,下同 **.P < 0.01;*.P < 0.05, the same as below 图 1 大鼠肺组织中Cd含量变化 Fig. 1 The changes of cadmium (Cd) content of lung tissue in rats |
如图 2所示。对大鼠肺组织进行HE染色与Masson染色,与Control组相比,Cd组可见大鼠肺泡结构破坏,肺纤维组织增生。与Cd组相比,NAC+Cd组大鼠肺泡结构明显恢复,并缓解肺组织损伤。
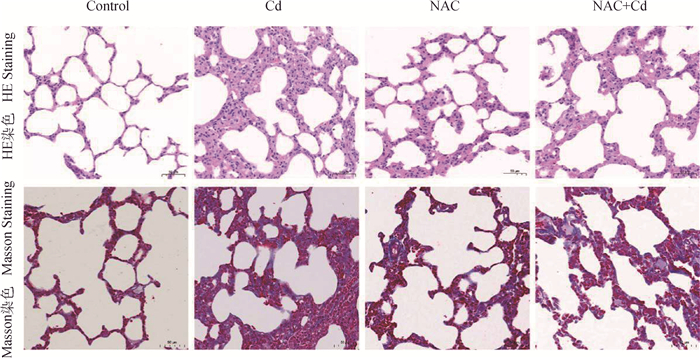
|
图 2 大鼠肺组织HE染色与Masson染色(200×) Fig. 2 The observation of HE staining and Masson staining of lung tissue in rats (200×) |
如图 3所示。与Control相比,Cd组大鼠肺组织中Col-Ⅰ和Col-Ⅲ mRNA表达极显著(P < 0.01)升高,而NAC组无差异性(P>0.05)。与Cd组相比,NAC+Cd组大鼠肺组织中Col-Ⅰ和Col-Ⅲ mRNA的表达极显著(P < 0.01)下降。
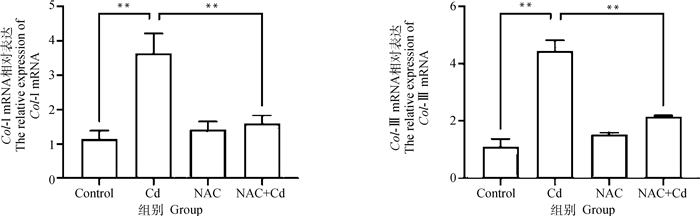
|
图 3 NAC对Cd处理的大鼠肺组织纤维化关键蛋白Col-Ⅰ和Col-Ⅲ mRNA的影响 Fig. 3 The effect of NAC on fibrosis protein Col-Ⅰ和Col-Ⅲ mRNA of lung tissue treated by Cd in rats |
如图 4所示。与Control相比,Cd组大鼠肺组织中Bax/Bcl-2的比值和cleaved caspase-3的表达极显著(P < 0.01)升高,而NAC组无差异性(P>0.05)。与Cd组相比,NAC+Cd组大鼠肺组织中Bax/Bcl-2的比值和cleaved caspase-3的表达显著(P < 0.01或P < 0.05)下降。
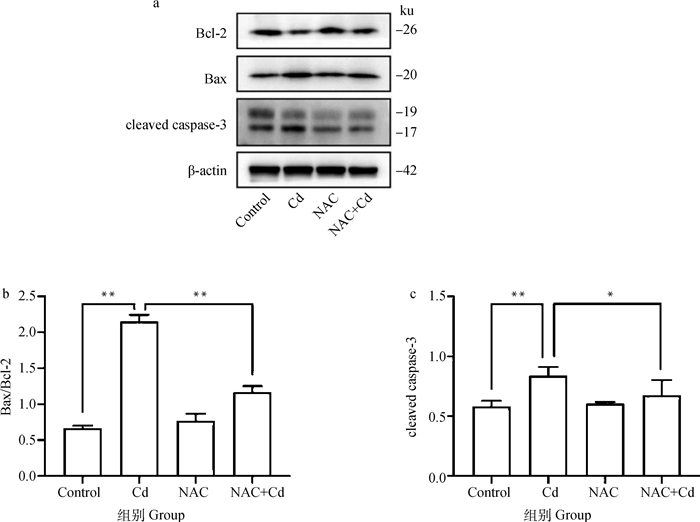
|
图 4 NAC对Cd处理的大鼠肺组织凋亡蛋白(Bcl-2、Bax及cleaved caspase-3)表达的影响 Fig. 4 The effect of NAC on apoptosis-related protein (Bcl-2, Bax and cleaved caspase-3) expression of lung tissue treated by Cd in rats |
如图 5所示。与Control相比,Cd组大鼠肺组织中p-Akt/Akt的比值极显著(P < 0.01)降低,p53的表达极显著(P < 0.01)升高,而NAC组无差异性(P>0.05)。与Cd组相比,NAC+Cd组大鼠肺组织中p-Akt/Akt的比值极显著(P < 0.01)增加,而p53的表达极显著(P < 0.01)下降。
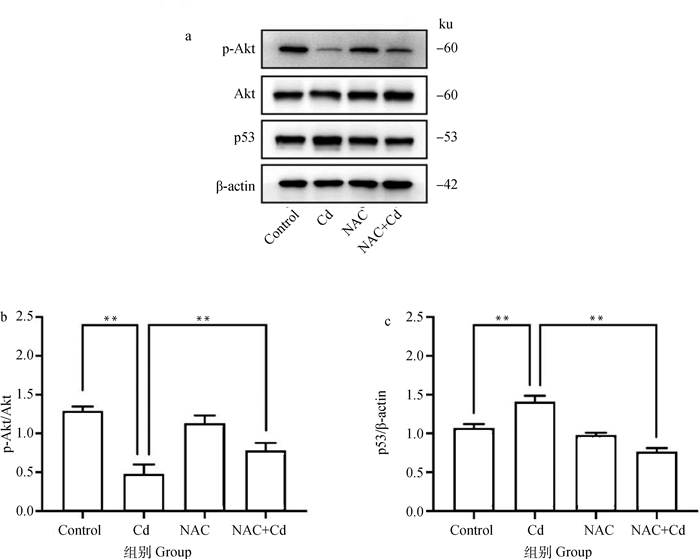
|
图 5 NAC对Cd处理的大鼠肺组织Akt磷酸化及p53表达的影响 Fig. 5 The effect of NAC on Akt phosphorylation and p53 expression of lung tissue treated by Cd in rats |
环境中的重金属主要来源于工业废弃物的泄漏,并对动物的健康构成潜在的威胁[21]。Cd作为一种无生理功能的重金属,被认为是损伤动物机体的有毒有害物质之一。在世界范围内,Cd及其化合物在环境中的生产和释放显著增加,但无有效的回收方式。Cd可在机体内长期积累,半衰期长[23]。Cd作为一种环境性雌激素,通过“模仿”雌激素的功能,以高亲和力在受体激素结合域活化雌激素受体(estrogen receptor,ER),发挥内分泌干扰物的作用。然而,这种作用可被抗雌激素药物阻断[26]。此外,动物的性别可影响肺相关疾病的患病率,一方面,与性激素是否直接相关尚不清楚;另一方面,性激素可调节肺的基本生理状态及其发育过程[27]。因此,本试验以去卵巢SD大鼠作为动物模型,减少或排除雌激素的作用。
Cd及其化合物可引起水、土壤和空气的污染,最终进入植物、动物或人机体内。已知Cd是动物体内最易积累的毒性重金属之一,机体组织半衰期可长达30年,血液中半衰期为3~4个月[21]。Cd可与机体内的氨基、巯基、硫蛋白等结合,损害酶防御系统,破坏肺、肝、肾等器官的正常结构,引发机体组织内Cd的蓄积。本试验结果证明Cd组大鼠肺组织中Cd含量显著增加,血清中Cd的含量也显著增加(数据未发表)。此外,Cd与特发性肺纤维化的发生密切相关,其通过触发波形蛋白瓜氨酸化,活化成纤维细胞,上调纤维化蛋白的表达,导致肺纤维化[31]。成纤维细胞可活化细胞外基质Col-Ⅰ和Col-Ⅲ,后两者的过度沉积导致组织的纤维化[32]。本试验结果显示,Cd组大鼠肺泡结构损伤、萎缩或消失,肺纤维组织增生,显著升高Col-Ⅰ和Col-Ⅲ mRNA的表达。这表明Cd暴露可增加大鼠肺组织中Cd含量,诱导肺组织纤维化胶原酶Col-Ⅰ和Col-Ⅲ mRNA表达的上调。
动物试验及临床数据分析表明,Cd可引起肺损伤,诱导肺细胞凋亡。Cd能够破坏动物机体的抗氧化系统,活化肺细胞线粒体凋亡途径,上调凋亡蛋白Bax、caspase-3、细胞色素C和p53 mRNA的表达,诱导细胞凋亡。低浓度(1~10 μmol·L-1)醋酸镉(CdAc2)处理大鼠克拉拉细胞(Clara细胞)与肺泡Ⅱ型细胞,能够以Bax与p53依赖的方式诱导细胞凋亡。Cd诱导人肺成纤维细胞MRC-5细胞的多种类型死亡,通过线粒体膜电位的“崩溃”,以caspase-3依赖的方式诱导细胞凋亡[36]。如前所述,Cd作为一种环境性雌激素,可结合雌激素受体,后者始终存在于肺细胞与支气管上皮细胞中。其中,雌激素受体β能够活化PI3K/Akt/Bcl-XL信号通路,调节细胞增殖、侵袭、转移、线粒体合成及抗细胞凋亡的作用。然而,Cd可通过激活大鼠神经元细胞中Akt/哺乳动物雷帕霉素靶蛋白(mammalian target of rapamycin,mTOR)信号通路,并诱导ROS的产生。Cd也可诱导氧化应激,通过活化猪淋巴结或鸡肾组织中PI3K/Akt信号通路,进一步诱导凋亡。本试验结果显示,Cd组大鼠肺组织中细胞凋亡蛋白Bax、cleaved caspase-3和p53的表达显著增加,Akt磷酸化受抑制(这与其他研究结果不太一致。分析认为本研究中动物模型为去卵巢SD大鼠,可能是雌激素受体在该过程中发挥了比较重要的调节作用,但仍需进一步研究证实)。这表明Cd暴露可诱导大鼠肺组织细胞凋亡蛋白Bax和cleaved caspase-3的表达,并通过p53途径进一步诱导凋亡。
NAC作为一种常用的小分子高效抗氧化剂,可通过增加细胞内谷胱甘肽-S-基转移酶的活性诱导还原型谷胱甘肽(GSH)的合成,清除氧自由基离子,发挥抗氧化的作用。本试验结果显示,NAC可显著降低大鼠肺组织中Cd含量,缓解肺组织病理变化,表明NAC对肺组织损伤有一定的缓解作用。此外,NAC能够缓解Cd诱导的大鼠肾上腺嗜铬细胞瘤细胞PC12细胞的损伤,上调凋亡蛋白Bcl-2/Bax的比值与cleaved caspase-3蛋白的表达,下调细胞凋亡率。郑嘉铭[46]的研究也表明,NAC可显著下调Cd诱导的大鼠成骨细胞凋亡蛋白Bax/Bcl-2的比值与p53及cleaved caspase-3蛋白的表达。最近研究表明,NAC通过降低外周血结缔组织生长因子(connective tissue growth factor,CTGF)与纤维连接蛋白(fibronectin,FN)的表达,发挥抗肺纤维化的作用。本试验结果显示,NAC可显著下调Cd组大鼠肺组织中细胞凋亡蛋白Bax/Bcl-2的比值与cleaved caspase-3及p53蛋白的表达。这表明NAC可降低Cd致大鼠肺组织凋亡蛋白Bax和cleaved caspase-3的表达,进一步缓解Cd诱导的肺组织凋亡。
4 结论本研究从肺细胞凋亡的角度探讨了NAC对Cd致大鼠肺组织损伤的缓解作用,并通过p53途径减轻肺组织病理变化及下调肺细胞凋亡蛋白的表达。这为NAC缓解Cd诱导肺损伤相关的机理研究提供新的理论依据。
| [1] |
GENCHI G, SINICROPI M S, LAURIA G, et al. The effects of cadmium toxicity[J]. Int J Environ Res Public Health, 2020, 17(11): 3782. DOI:10.3390/ijerph17113782 |
| [2] |
SATARUG S. Dietary cadmium intake and its effects on kidneys[J]. Toxics, 2018, 6(1): 15. DOI:10.3390/toxics6010015 |
| [3] |
KUMAR S, SHARMA A. Cadmium toxicity: effects on human reproduction and fertility[J]. Rev Environ Health, 2019, 34(4): 327-338. DOI:10.1515/reveh-2019-0016 |
| [4] |
SÖDERHOLM M, BORNÉ Y, HEDBLAD B, et al. Blood cadmium concentration and risk of subarachnoid haemorrhage[J]. Environ Res, 2020, 180: 108826. DOI:10.1016/j.envres.2019.108826 |
| [5] |
VENDITTI M, BEN RHOUMA M, ROMANO M Z, et al. Evidence of melatonin ameliorative effects on the blood-testis barrier and sperm quality alterations induced by cadmium in the rat testis[J]. Ecotoxicol Environ Saf, 2021, 226: 112878. DOI:10.1016/j.ecoenv.2021.112878 |
| [6] |
TAHA M M, MAHDY-ABDALLAH H, SHAHY E M, et al. Impact of occupational cadmium exposure on bone in sewage workers[J]. Int J Occup Environ Health, 2018, 24(3-4): 101-108. DOI:10.1080/10773525.2018.1518745 |
| [7] |
SIROT V, SAMIERI C, VOLATIER J L, et al. Cadmium dietary intake and biomarker data in French high seafood consumers[J]. J Expo Sci Environ Epidemiol, 2008, 18(4): 400-409. DOI:10.1038/sj.jes.7500615 |
| [8] |
李有幸, 陆钦晨, 庞雅琴, 等. 消化道途径亚慢性镉暴露致大鼠肺损伤的实验研究[J]. 预防医学论坛, 2018, 24(11): 807-809. LI Y X, LU Q C, PANG Y Q, et al. Experimental study on lung injury induced by subchronic cadmium exposure in digestive tract among rats[J]. Preventive Medicine Tribune, 2018, 24(11): 807-809. DOI:10.16406/j.pmt.issn.1672-9153.2018.11.004 (in Chinese) |
| [9] |
周桂凤, 贺全仁. 镉中毒致肺损伤的病理形态学观察[J]. 职业与健康, 2004, 20(1): 4-5. ZHOU G F, HE Q R. Pathologic survey on lung impairment induced by cadmium poisoning[J]. Occupation and Health, 2004, 20(1): 4-5. DOI:10.3969/j.issn.1004-1257.2004.01.002 (in Chinese) |
| [10] |
YESILDAG K, GUR C, ILERITURK M, et al. Evaluation of oxidative stress, inflammation, apoptosis, oxidative DNA damage and metalloproteinases in the lungs of rats treated with cadmium and carvacrol[J]. Mol Biol Rep, 2022, 49(2): 1201-1211. DOI:10.1007/s11033-021-06948-z |
| [11] |
KIRAN KUMAR K M, NAVEEN KUMAR M, PATIL R H, et al. Cadmium induces oxidative stress and apoptosis in lung epithelial cells[J]. Toxicol Mech Methods, 2016, 26(9): 658-666. DOI:10.1080/15376516.2016.1223240 |
| [12] |
储娜, 张璇, 陈思远, 等. 木犀草素对镉诱导的肺上皮Beas-2B细胞损伤具有明显的保护作用[J]. 南方医科大学学报, 2021, 41(5): 729-735. CHU N, ZHANG X, CHEN S Y, et al. Luteolin has a significant protective effect against cadmium-induced injury in lung epithelial Beas-2B cells[J]. Journal of Southern Medical University, 2021, 41(5): 729-735. (in Chinese) |
| [13] |
BARONI T, LILLI C, BELLUCCI C, et al. In vitro cadmium effects on ECM gene expression in human bronchial epithelial cells[J]. Cytokine, 2015, 72(1): 9-16. DOI:10.1016/j.cyto.2014.12.002 |
| [14] |
FAGHFOURI A H, ZAREZADEH M, TAVAKOLI-ROUZBEHANI O M, et al. The effects of N-acetylcysteine on inflammatory and oxidative stress biomarkers: A systematic review and meta-analysis of controlled clinical trials[J]. Eur J Pharmacol, 2020, 884: 173368. DOI:10.1016/j.ejphar.2020.173368 |
| [15] |
LIU F, WANG X Y, ZHOU X P, et al. Cadmium disrupts autophagic flux by inhibiting cytosolic Ca2+-dependent autophagosome-lysosome fusion in primary rat proximal tubular cells[J]. Toxicology, 2017, 383: 13-23. DOI:10.1016/j.tox.2017.03.016 |
| [16] |
LIU F, LI Z F, WANG Z Y, et al. Role of subcellular calcium redistribution in regulating apoptosis and autophagy in cadmium-exposed primary rat proximal tubular cells[J]. J Inorg Biochem, 2016, 164: 99-109. DOI:10.1016/j.jinorgbio.2016.09.005 |
| [17] |
CHEN X M, BI M Y, YANG J, et al. Cadmium exposure triggers oxidative stress, necroptosis, Th1/Th2 imbalance and promotes inflammation through the TNF-α/NF-κB pathway in swine small intestine[J]. J Hazard Mater, 2022, 421: 126704. DOI:10.1016/j.jhazmat.2021.126704 |
| [18] |
SUNDARESAN S, JOHN S, PANEERSELVAM G, et al. Gallic acid attenuates cadmium mediated cardiac hypertrophic remodelling through upregulation of Nrf2 and PECAM-1 signalling in rats[J]. Environ Toxicol Pharmacol, 2021, 87: 103701. DOI:10.1016/j.etap.2021.103701 |
| [19] |
LV W, SUI L, YAN X N, et al. ROS-dependent Atg4 upregulation mediated autophagy plays an important role in Cd-induced proliferation and invasion in A549 cells[J]. Chem Biol Interact, 2018, 279: 136-144. DOI:10.1016/j.cbi.2017.11.013 |
| [20] |
孙琦玮, 郑燕飞, 庞焕. 乙酰半胱氨酸联合吸入性糖皮质激素治疗婴幼儿支原体肺炎的疗效观察[J]. 中国实用医药, 2021, 16(34): 134-136. SUN Q W, ZHENG Y F, PANG H. Efficacy observation of acetylcysteine combined with inhaled glucocorticoid in the treatment of infantile mycoplasma pneumonia[J]. China Practical Medicine, 2021, 16(34): 134-136. DOI:10.14163/j.cnki.11-5547/r.2021.34.049 (in Chinese) |
| [21] |
RAFATI RAHIMZADEH M, RAFATI RAHIMZADEH M, KAZEMI S, et al. Cadmium toxicity and treatment: An update[J]. Caspian J Intern Med, 2017, 8(3): 135-145. |
| [22] |
GENCHI G, SINICROPI M S, CAROCCI A, et al. Response to comment on Giuseppe Genchi et al. Mercury Exposure and Heart Diseases. Int. J. Environ. Res. Public Health 2017, 14, 74[J]. Int J Environ Res Public Health, 2017, 14(7): 761. DOI:10.3390/ijerph14070761 |
| [23] |
SILVER M K, LOZOFF B, MEEKER J D. Blood cadmium is elevated in iron deficient U. S. children: a cross-sectional study[J]. Environ Health, 2013, 12: 117. DOI:10.1186/1476-069X-12-117 |
| [24] |
AQUINO N B, SEVIGNY M B, SABANGAN J, et al. The role of cadmium and nickel in estrogen receptor signaling and breast cancer: metalloestrogens or not?[J]. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev, 2012, 30(3): 189-224. DOI:10.1080/10590501.2012.705159 |
| [25] |
GARCIA-MORALES P, SACEDA M, KENNEY N, et al. Effect of cadmium on estrogen receptor levels and estrogen-induced responses in human breast cancer cells[J]. J Biol Chem, 1994, 269(24): 16896-16901. DOI:10.1016/S0021-9258(19)89474-7 |
| [26] |
STOICA A, KATZENELLENBOGEN B S, MARTIN M B. Activation of estrogen receptor-α by the heavy metal cadmium[J]. Mol Endocrinol, 2000, 14(4): 545-553. |
| [27] |
CAREY M A, CARD J W, VOLTZ J W, et al. The impact of sex and sex hormones on lung physiology and disease: lessons from animal studies[J]. Am J Physiol Lung Cell Mol Physiol, 2007, 293(2): L272-L278. DOI:10.1152/ajplung.00174.2007 |
| [28] |
CHEN C, XUN P C, NISHIJO M, et al. Cadmium exposure and risk of lung cancer: a meta-analysis of cohort and case-control studies among general and occupational populations[J]. J Expo Sci Environ Epidemiol, 2016, 26(5): 437-444. DOI:10.1038/jes.2016.6 |
| [29] |
GONZÁLEZ A, LAPORTE D, MOENNE A. Cadmium accumulation involves synthesis of glutathione and phytochelatins, and activation of CDPK, CaMK, CBLPK, and MAPK signaling pathways in Ulva compressa[J]. Front Plant Sci, 2021, 12: 669096. DOI:10.3389/fpls.2021.669096 |
| [30] |
FILIPIČ M. Mechanisms of cadmium induced genomic instability[J]. Mutat Res, 2012, 733(1-2): 69-77. DOI:10.1016/j.mrfmmm.2011.09.002 |
| [31] |
LI F J, SUROLIA R, LI H S, et al. Citrullinated vimentin mediates development and progression of lung fibrosis[J]. Sci Transl Med, 2021, 13(585): eaba2927. DOI:10.1126/scitranslmed.aba2927 |
| [32] |
李楠楠. 黄芪甲苷Ⅳ通过PPM1A调控TGF-β1/Smad信号通路减轻矽肺肺损伤和纤维化[D]. 济南: 山东大学, 2021. LI N N. Astragaloside Ⅳ regulates TGF-β/Smad signaling pathway through PPM1A to reduce silicosis injury and fibrosis[D]. Jinan: Shandong University, 2021. (in Chinese) |
| [33] |
LÅG M, WESTLY S, LERSTAD T, et al. Cadmium-induced apoptosis of primary epithelial lung cells: involvement of Bax and p53, but not of oxidative stress[J]. Cell Biol Toxicol, 2002, 18(1): 29-42. DOI:10.1023/A:1014467112463 |
| [34] |
ZHANG J X, ZHANG Y, QI X, et al. TRAF2/ASK1/JNK signaling pathway is involved in the lung apoptosis of swine induced by cadmium exposure[J]. Biol Trace Elem Res, 2022, 200(6): 2758-2766. DOI:10.1007/s12011-021-02860-6 |
| [35] |
WANG C, NIE G H, YANG F, et al. Molybdenum and cadmium co-induce oxidative stress and apoptosis through mitochondria-mediated pathway in duck renal tubular epithelial cells[J]. J Hazard Mater, 2020, 383: 121157. DOI:10.1016/j.jhazmat.2019.121157 |
| [36] |
SHIH C M, WU J S, KO W C, et al. Mitochondria-mediated caspase-independent apoptosis induced by cadmium in normal human lung cells[J]. J Cell Biochem, 2003, 89(2): 335-347. DOI:10.1002/jcb.10488 |
| [37] |
HSU L H, CHU N M, KAO S H. Estrogen, estrogen receptor and lung cancer[J]. Int J Mol Sci, 2017, 18(8): 1713. DOI:10.3390/ijms18081713 |
| [38] |
YUAN Y, WANG Y, HU F F, et al. Cadmium activates reactive oxygen species-dependent AKT/mTOR and mitochondrial apoptotic pathways in neuronal cells[J]. Biomed Environ Sci, 2016, 29(2): 117-126. |
| [39] |
YIMING L, YANFEI H, HANG Y, et al. Cadmium induces apoptosis of pig lymph nodes by regulating the PI3K/AKT/HIF-1α pathway[J]. Toxicology, 2021, 451: 152694. DOI:10.1016/j.tox.2021.152694 |
| [40] |
GAO D, XU Z, QIAO P P, et al. Cadmium induces liver cell apoptosis through caspase-3A activation in purse red common carp (Cyprinus carpio)[J]. PLoS One, 2013, 8(12): e83423. DOI:10.1371/journal.pone.0083423 |
| [41] |
KASPERCZYK S, DOBRAKOWSKI M, KASPERCZYK A, et al. The administration of N-acetylcysteine reduces oxidative stress and regulates glutathione metabolism in the blood cells of workers exposed to lead[J]. Clin Toxicol (Phila), 2013, 51(6): 480-486. DOI:10.3109/15563650.2013.802797 |
| [42] |
LIU X, WANG L, CAI J, et al. N-acetylcysteine alleviates H2O2-induced damage via regulating the redox status of intracellular antioxidants in H9c2 cells[J]. Int J Mol Med, 2019, 43(1): 199-208. |
| [43] |
ALNAHDI A, JOHN A, RAZA H. N-acetyl cysteine attenuates oxidative stress and glutathione-dependent redox imbalance caused by high glucose/high palmitic acid treatment in pancreatic Rin-5F cells[J]. PLoS One, 2019, 14(12): e0226696. DOI:10.1371/journal.pone.0226696 |
| [44] |
袁燕, 张雅静, 陈洁, 等. Fas/FasL信号通路在镉致PC12细胞凋亡中的作用及NAC的保护效应[J]. 中国兽医科学, 2018, 48(10): 1318-1324. YUAN Y, ZHANG Y J, CHEN J, et al. Role of Fas/FasL signaling pathway in apoptosis of PC12 cells induced by cadmium and protective effect of NAC[J]. Chinese Veterinary Science, 2018, 48(10): 1318-1324. (in Chinese) |
| [45] |
袁燕, 江辰阳, 达剑森, 等. NAC在镉致PC12细胞凋亡线粒体途径中的保护效应[J]. 中国兽医科学, 2018, 48(7): 924-930. YUAN Y, JIANG C Y, DA J S, et al. Protective effect of NAC on Cd-induced mitochondrial apoptotic pathway in PC12 cells[J]. Chinese Veterinary Science, 2018, 48(7): 924-930. (in Chinese) |
| [46] |
郑嘉铭. p53介导的线粒体凋亡通路在镉致大鼠成骨细胞损伤中的作用[D]. 扬州: 扬州大学, 2020. ZHENG J M. Activation of mitochondrial p53 apoptotic pathway is involved in cadmium induced osteoblast injury[D]. Yangzhou: Yangzhou University, 2020. (in Chinese) |
| [47] |
虞乐群, 陆益民, 田高润, 等. NAC对特发性肺纤维化患者血清结缔组织生长因子和纤维连接蛋白水平的影响[J]. 国际免疫学杂志, 2020, 43(3): 270-275. YU L Q, LU Y M, TIAN G R, et al. Effect of N-acetylcysteine on serum connective tissue growth factor and fibronectin levels in patients with idiopathic pulmonary fibrosis[J]. International Journal of Immunology, 2020, 43(3): 270-275. DOI:10.3760/cma.j.issn.1673-4394.2020.03.006 (in Chinese) |
| [48] |
BURNS D P, DRUMMOND S E, BOLGER D, et al. N-acetylcysteine decreases fibrosis and increases force-generating capacity of mdx diaphragm[J]. Antioxidants (Basel), 2019, 8(12): 581. DOI:10.3390/antiox8120581 |
(编辑 白永平)



