颗粒细胞的活跃增殖是确保卵泡正常发育的重要前提之一。然而,随着卵泡发育的进行,壁层颗粒细胞的G0/G1比例增多,颗粒细胞增殖能力逐渐降低[1]。另一方面,由于卵泡中毛细血管的分布局限于卵泡表面膜层,卵泡发育过程必然会导致卵泡内部缺血缺氧状态的加剧[2-4]。进一步研究证实,卵泡内部低氧是造成颗粒细胞增殖活性下降(G0/G1阻滞)的重要原因[1]。前期在猪卵泡中收集G0/G1阻滞高组和低组的颗粒细胞进行转录组测序,发现了多个差异性表达的基因[5]。功能富集分析发现,这些差异表达的基因绝大多数聚集在DNA损伤相关功能及通路上。然而,目前尚未见报道DNA氧化损伤是否参与低氧引起的颗粒细胞增殖阻滞。
本研究利用氯化钴(CoCl2)诱导猪卵泡颗粒细胞产生化学性缺氧,并在此基础上添加抗氧化剂GSH,比较缺氧组与抗氧化剂处理组颗粒细胞增殖活性、ROS水平、DNA氧化损伤(Oxo-8-G水平)、γH2AX蛋白活性变化,研究化学低氧诱导的DNA氧化损伤对猪卵泡颗粒细胞增殖的影响。
1 材料与方法 1.1 主要试剂胰蛋白酶、FBS胎牛血清(美国Gibco公司),谷胱甘肽(GSH)、CoCl2(美国Sigma公司)、CCK-8试剂盒(日本同仁化学)、DCFA-DC探针(碧云天生物技术)、FITC荧光小鼠单克隆抗体(Abcam公司)。
1.2 细胞培养及试验分组按实验室前期建立的方法分离和培养猪卵泡颗粒细胞[6]。简要步骤为:从屠宰场采集商品肉猪的卵巢,放在装有37 ℃无菌生理盐水的保温桶中2 h内运到实验室。使用含有庆大霉素的37 ℃生理盐水和37 ℃的75% 乙醇交替洗涤卵巢,然后用10 mL注射器抽取卵泡液及颗粒细胞,1 200×g离心5 min去除卵泡液,用PBS洗涤颗粒细胞两次,1 000×g离心5 min去除PBS后,用完全培养基在37 ℃、5%、CO2条件下培养于含15%胎牛血清的培养基中,培养24 h左右进行第一次消化传代。正常对照组为完全培养基培养12 h; CoCl2诱导缺氧处理组给予终浓度200 μmol·L-1 CoCl2的培养基进行模拟低氧培养12 h; 单独GSH处理组加入浓度为2 mmol·L-1的GSH进行处理12 h; GSH+CoCl2联合处理组,加入200 μmol·L-1 CoCl2和2 mmol·L-1的GSH联合处理细胞后培养12 h。
1.3 CCK-8法检测细胞增殖活力48孔板每孔加入50 μL CCK-8溶液,并置于37 ℃的5% CO2的培养箱中孵育12 h,再将48孔板置于酶标仪中,测定波长为450 nm时各孔液体的吸光度值,计算细胞的增殖活力。
1.4 细胞内ROS水平的测定用PBS洗涤细胞1次,12孔板中每孔加1 μL DCFH-DA探针溶于培养基,培养箱中孵育20 min,加入PBS洗3次,除去多余探针,加入DAPI染色细胞核10 min,加入1 mL PBS洗5遍,每次5 min,共聚焦显微镜下观察并拍照。
1.5 ELISA法检测Oxo-8-G水平用冰冷甲醇固定5 min,PBS洗涤3次; 用0.1% TritonX-100透化细胞30 min,PBS洗涤3次,每次5 min; 室温用1% BSA封闭1 h,PBS洗3次,每次5 min; 室温用DNA/RNA抗体染色细胞核10 min,PBS洗3次,每次5 min; 共聚焦显微镜下观察并拍照[7]。
1.6 蛋白免疫印迹法检测γH2AX水平用RIPA细胞裂解液提取猪卵泡颗粒细胞总蛋白,蛋白定量后取25 μg总蛋白进行SDS-PAGE凝胶电泳,转膜并封闭,用稀释后的兔一抗4 ℃孵育过夜,TBST洗3次后,放入二抗在室温下摇床孵育1 h,再次TBST洗3次后,添加显色液,于ChemiDoc XRS+系统成像,并用ImageJ软件对蛋白条带进行灰度分析,TUBA1A作为内参蛋白。
1.7 数据统计分析试验数据使用GraphPad PRISM 9.0软件分析,当2个试验组进行比较时使用t检验进行分析,当3个或者更多试验组进行比较时使用ANOVA进行分析。
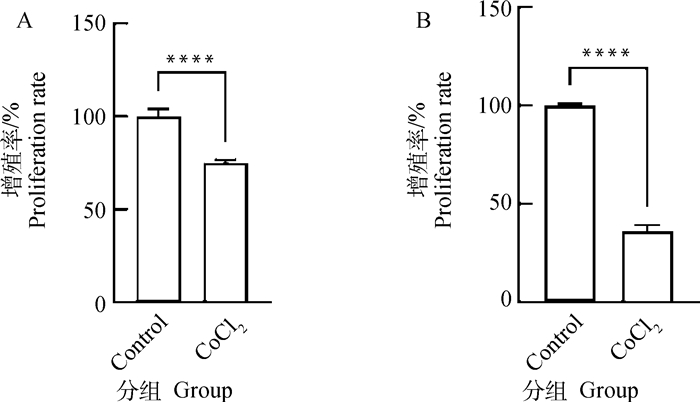
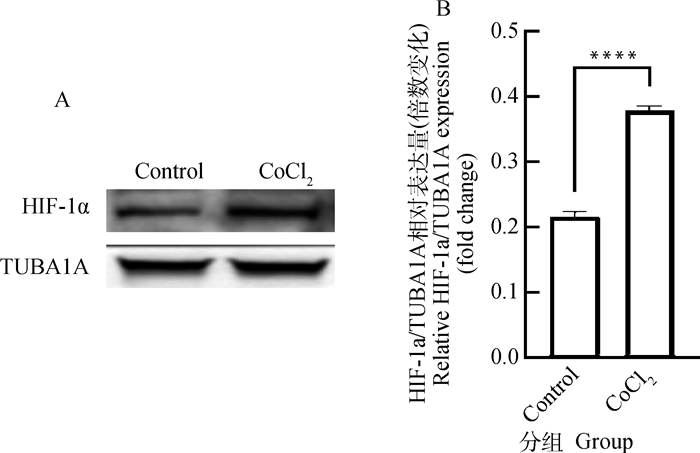
2 结果 2.1 CoCl2对猪卵泡颗粒细胞增殖活力的影响在体内和体外研究中,CoCl2作为一种低氧模拟剂被广泛使用[8-10]。本研究采用200 μmol·L-1 CoCl2分别培养猪卵泡颗粒细胞12和24 h。图 1显示了CCK-8检测猪卵泡颗粒细胞增殖活力的变化。与对照组相比,CoCl2处理12 h组和24 h组细胞增殖活力均极显著下降(P < 0.000 1)。结果表明,CoCl2介导的化学低氧可抑制猪卵泡颗粒细胞增殖。根据图 1显著性水平,选取200 μmol·L-1 CoCl2处理12 h这一试验条件用于后续试验。采用Western blot进一步检测CoCl2处理后低氧标记物-低氧诱导因子HIF-1α的蛋白水平,发现CoCl2能极显著提高颗粒细胞HIF-1α蛋白累积(P < 0.000 1,图 2)。
|
A. CoCl2处理12 h细胞增殖活力检测; B. CoCl2处理24 h细胞增殖活力检测。****. P < 0.000 1,下同 A.Detection of proliferation activity in granulosa cells treated with cobalt chloride for 12 h; B. Detection of proliferation activity in granulosa cells treated with cobalt chloride for 24 h. ****. P < 0.000 1, the same as below 图 1 CoCl2处理对猪卵泡颗粒细胞增殖活力的影响 Fig. 1 Effects of cobalt chloride treatment on proliferation activity of porcine follicular granulosa cells |
|
A.CoCl2处理12 h后颗粒细胞中HIF-1α蛋白表达的Western blot检测图; B.HIF-1α的相对表达量,HIF-1α相对表达水平采用ImageJ软件的灰度分析功能进行量化 A. The Western blot analysis of HIF-1α protein levels in granulosa cells treated with cobalt chloride for 12 h; B. The relative expression of HIF-1α quantified by densitometric analysis using ImageJ software 图 2 CoCl2处理对猪卵泡颗粒细胞内HIF-1α蛋白表达的影响 Fig. 2 Effects of cobalt chloride treatment on expression of HIF-1α protein in porcine follicular granulosa cells |
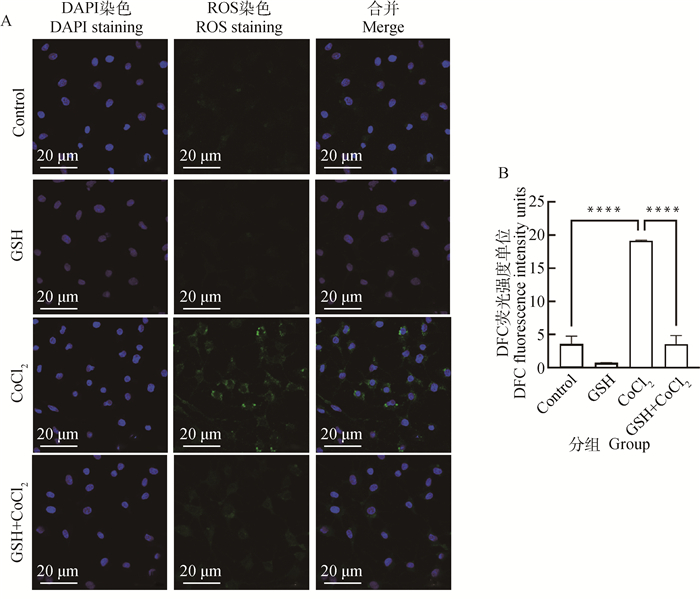
图 3显示了ROS荧光探针检测200 μmol·L-1的CoCl2处理12 h下猪卵泡颗粒细胞ROS水平变化。与对照组相比,CoCl2处理组ROS水平极显著上升(P < 0.000 1)。检测结果表明,CoCl2诱导的化学性缺氧可导致猪卵泡颗粒细胞内ROS水平升高。

|
A.CoCl2处理12 h后荧光检测ROS水平; B.CoCl2处理12 h后各处理组平均荧光强度 A. The fluorescent imaging of ROS detection in granulosa cells after 12 h of CoCl2 treatment; B. The ROS level was assessed by analyzing the average fluorescence intensity in each treatment group after 12 h of CoCl2 treatment 图 3 CoCl2处理对猪卵泡颗粒细胞内ROS水平的影响 Fig. 3 Effects of cobalt chloride treatment on ROS levels in porcine follicular granulosa cells |
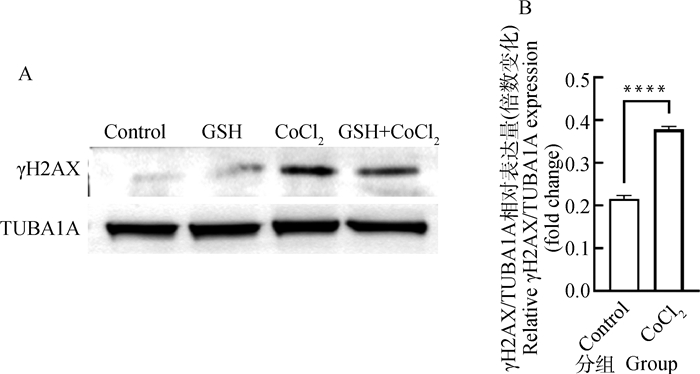
组蛋白2A变异体H2AX是细胞中感应DNA损伤最敏感的分子,损伤发生后可以迅速磷酸化产生γH2AX,因此可用来反映DNA损伤断裂程度。Western blot检测结果(图 4A)显示,与对照组相比,CoCl2处理组γH2AX表达极显著升高(P < 0.000 1)。检测结果表明,CoCl2诱导的化学性缺氧可促进猪卵泡颗粒细胞γH2AX蛋白的表达。
|
A. CoCl2处理12 h后颗粒细胞中γH2AX蛋白表达的Western blot检测图; B. γH2AX的相对表达量,γH2AX相对表达水平采用ImageJ软件的灰度分析功能进行量化 A. The Western blot analysis of γH2AX protein levels in granulosa cells treated with cobalt chloride for 12 h; B. The relative expression of γH2AX quantified by densitometric analysis using ImageJ software 图 4 CoCl2处理对猪卵泡颗粒细胞内γH2AX蛋白表达的影响 Fig. 4 Effects of cobalt chloride treatment on expression of γH2AX protein in porcine follicular granulosa cells |
图 5显示了FITC荧光Anti-DNA/RNA Damage抗体检测200 μmol·L-1的CoCl2处理12 h猪卵泡颗粒细胞Oxo-8-G水平变化,通过检测细胞质RNA中的Oxo-8-G水平来反映DNA氧化损伤情况[11]。与对照组相比,缺氧12 h后Oxo-8-G水平极显著上升(P < 0.000 1)。检测结果表明,CoCl2诱导的化学性缺氧可导致猪卵泡颗粒细胞DNA损伤(Oxo-8-G和DNA断裂的积累)。

|
A. CoCl2处理12 h后荧光检测Oxo-8-G水平; B. 各处理组Oxo-8-G荧光水平量化统计,采用ImageJ软件分析细胞中Oxo-8-G的平均荧光强度 A. The fluorescence detection of Oxo-8-G level in granulosa cells after 12 h of CoCl2 treatment; B. The average fluorescence intensity in each treatment group was assessed using ImageJ software 图 5 CoCl2处理对猪卵泡颗粒细胞内Oxo-8-G水平的影响 Fig. 5 Effects of cobalt chloride treatment on Oxo-8-G levels in porcine granulosa follicular cells |
为了进一步探究缺氧引起的DNA氧化损伤机制,本研究在CoCl2处理基础上添加抗氧化物GSH处理猪卵泡颗粒细胞。试验组已研究出猪卵泡颗粒细胞的最适GSH浓度为2 mmol·L-1。图 6显示了CCK-8检测猪卵泡颗粒细胞增殖活力的变化,与CoCl2处理组相比,在CoCl2处理基础上添加抗氧化物GSH处理12 h后,细胞增殖水平极显著上升(P < 0.000 1)。结果表明,在模拟缺氧环境的基础上加入抗氧化剂GSH处理后恢复了细胞增殖活力。图 7显示了ROS荧光探针检测猪卵泡颗粒细胞ROS水平变化,与CoCl2处理组相比,在CoCl2处理基础上添加抗氧化物GSH处理12 h后,ROS水平极显著下降(P < 0.000 1)。结果表明,在模拟缺氧环境的基础上加入抗氧化剂GSH处理后可降低ROS表达[12-13]。Western blot检测结果(图 4A,图 8)显示,与CoCl2处理组相比,在CoCl2处理基础上添加抗氧化物GSH处理12 h后γH2AX表达极显著降低(P < 0.000 1)。结果表明,在模拟缺氧环境的基础上加入抗氧化物GSH处理后能够降低γH2AX蛋白的表达。图 9显示了FITC荧光Anti-DNA/RNA Damage抗体检测猪卵泡颗粒细胞Oxo-8-G水平变化,与CoCl2处理组相比,在CoCl2处理基础上添加抗氧化物GSH处理12 h后,Oxo-8-G水平极显著下降(P < 0.000 1),结果表明,在模拟缺氧环境的基础上加入抗氧化物GSH处理后能够挽救DNA损伤。

|
A. CoCl2和GSH处理12 h细胞增殖活力检测; B. CoCl2和GSH处理24 h细胞增殖活力检测 A. Detection of proliferation activity in granulosa cells treated with CoCl2 and/or GSH for 12 h; B. Detection of proliferation activity in granulosa cells treated with CoCl2 and/or GSH for 24 h 图 6 GSH处理对猪卵泡颗粒细胞增殖活力的影响 Fig. 6 Effects of GSH treatment on proliferation activity of porcine follicular granulosa cells |
|
A. CoCl2和GSH处理12 h后荧光检测ROS水平; B. 各处理组ROS荧光水平量化统计,采用ImageJ软件分析细胞中ROS的平均荧光强度 A. The fluorescent imaging of ROS detection in granulosa cells following 12 h of CoCl2 and/or GSH treatment; B. The average fluorescence intensity in each treatment group was calculated using ImageJ software 图 7 GSH处理对猪卵泡颗粒细胞内ROS水平的影响 Fig. 7 Effects of GSH treatment on ROS levels in porcine follicular granulosa cells |

|
图 8 GSH处理对猪卵泡颗粒细胞内γH2AX蛋白表达的影响 Fig. 8 Effects of GSH treatment on expression of γH2AX protein in porcine follicular granulosa cells |

|
A. CoCl2和GSH处理12 h后荧光检测Oxo-8-G水平; B. 处理12 h后各处理组平均荧光强度 A. The fluorescence detection of Oxo-8-G levels after 12 h of CoCl2 and/or GSH treatment; B. The average fluorescence intensity of each treatment group after 12 h of treatment 图 9 GSH处理对猪卵泡颗粒细胞内Oxo-8-G水平的影响 Fig. 9 Effects of GSH treatment on Oxo-8-G levels in porcine granulosa follicular cells |
卵泡发育是雌性动物繁殖的基础,而颗粒细胞的活跃增殖是保证卵泡生长发育的前提条件。然而,卵泡发育过程伴随颗粒细胞增殖活力的逐渐降低。揭示颗粒细胞增殖调控规律对于认清卵泡发育的机制具有重要意义。细胞增殖是一个高度耗能的过程,细胞群体的快速增殖会加速氧气消耗,导致低氧环境的出现[14]。另一方面,在哺乳动物卵巢中,卵泡毛细血管分布于卵泡壁外侧的内膜细胞层,而卵泡内部的颗粒细胞和卵母细胞则处于无血管缺氧环境中[15]。随着卵泡发育变大,内层颗粒细胞与卵泡壁的距离越来越远,但卵泡血管系统始终无法穿透基底膜进入颗粒细胞,导致颗粒细胞缺氧问题更加突出[16-17]。有研究表明,卵泡直径越大,卵泡内部O2浓度就越低[18]。这提示,在卵泡发育过程中,颗粒细胞可能面临低氧应激。前期结果已经证实,低氧会造成颗粒细胞周期发生G0/G1阻滞,导致细胞增殖活性下降[1]。然而低氧抑制颗粒细胞增殖的机制尚需进一步阐明[19]。本研究利用CoCl2介导的颗粒细胞化学低氧模型,通过检测抗氧化物GSH处理前后细胞增殖、ROS水平、DNA氧化损伤等相关指标,揭示DNA氧化损伤可能是低氧诱导颗粒细胞增殖阻滞的潜在途径。
3.2 利用CoCl2成功建立了颗粒细胞化学低氧模型物理性缺氧常在充有氮气缺氧装置及常氧装置中完成[20],因需要严格检测氧浓度,技术性要求高,所以其运用受到限制,化学性缺氧则操作方便,CoCl2为常用的化学性低氧模拟剂[12, 21]。其作用机理在于Co2+与O2结合后,可阻断氧感受器中Fe2+与氧结合,使细胞在不缺氧的条件下产生缺氧错觉,最终达到常氧下物理诱导低氧类似的效果[22-24]。CoCl2可诱导多种细胞产生化学性缺氧损伤,但不同细胞对CoCl2诱导缺氧的敏感性有所不同[25-27]。本研究在体外培养的猪卵巢颗粒细胞中添加CoCl2,通过浓度梯度试验,前期筛选到200 μmol·L-1 CoCl2是引起颗粒细胞增殖活力出现极显著抑制的最低浓度。根据文献报道,CoCl2处理体外细胞的浓度一般在50~400 μmol·L-1范围[28-29],200 μmol·L-1属于细胞试验常用的处理浓度。因此采用200 μmol·L-1作为CoCl2的最适浓度处理浓度。为确认CoCl2是否产生低氧效果,对颗粒细胞中的低氧指标进行测定。HIF-1α属于低氧诱导因子(HIF)家族成员,是介导低氧反应的核心调控因子[30]。在常氧条件下,HIF-1α通过泛素-蛋白酶体途径迅速降解; 只有在低氧环境中,HIF-1α蛋白才能维持稳定[31-32]。因此根据HIF-1α蛋白表达水平即可判断组织、细胞是否缺氧[33]。通过测定HIF-1α的蛋白累积情况(图 2),进一步证实CoCl2处理能显著增强颗粒细胞中的低氧应答反应,说明利用CoCl2化学低氧模型能成功模拟颗粒细胞的低氧状态。
3.3 CoCl2会增加颗粒细胞细胞氧化应激水平,诱导DNA氧化损伤研究表明,低氧条件会诱导细胞产生活性氧簇(ROS)[34-36]。当机体细胞ROS产生速度超过其清除速度,过量累积的ROS会攻击DNA、蛋白质、脂质等生物大分子,造成细胞的氧化应激损伤。就DNA而言,氧化应激会导致包括DNA链断裂、DNA位点突变、DNA双链畸变、原癌基因与肿瘤抑制基因突变等形式的DNA损伤。DNA损伤过程中会形成某些特定的损伤产物(如γH2AX、Oxo-8-G等),因此通过测定这些DNA损伤标记物的水平可反映DNA损伤情况。本研究发现,CoCl2处理显著增加了猪卵泡颗粒细胞中ROS水平,并导致γH2AX蛋白的大量累积。而添加抗氧化剂GSH阻断ROS生成,可显著降低γH2AX蛋白水平。同样,GSH能显著抑制CoCl2处理后DNA氧化损伤标记物Oxo-8-G的累积。上述结果表明,CoCl2介导的化学性缺氧能诱导颗粒细胞产生氧化应激状态,继而诱发DNA氧化损伤。
3.4 DNA氧化损伤与颗粒细胞增殖阻滞的关系DNA损伤会激活细胞周期检验点,阻断细胞周期的运行,进而抑制细胞增殖[37-38]。本研究发现,CoCl2诱导的化学性缺氧会导致猪卵泡颗粒细胞增殖活性降低,该过程伴随细胞ROS水平升高,DNA损伤增加及DNA氧化损伤水平的升高。而在CoCl2处理基础上添加GSH以阻断ROS的产生,可显著降低DNA氧化损伤水平,并恢复颗粒细胞增殖活力。该结果表明,CoCl2造成的猪颗粒细胞增殖阻滞与DNA氧化应激损伤密切相关,而其中涉及的信号通路和效应分子仍有待进一步研究。
4 结论本研究证明,DNA氧化损伤可能参与低氧诱导的颗粒细胞增殖阻滞。该发现从DNA氧化损伤的角度解析低氧对颗粒细胞增殖的调控作用及机制,这对于认清低氧影响卵泡发育的基本规律、丰富和完善繁殖学理论具有重要意义。另一方面,研究结果揭示,添加抗氧化剂GSH可以缓解颗粒细胞中的DNA氧化损伤,解除低氧状态下颗粒细胞增殖阻滞状态,这为后续开发促卵泡发育的技术与措施提供了试验证据。
| [1] |
LI C Y, LIU Z J, WU G, et al. FOXO1 mediates hypoxia-induced G0/G1 arrest in ovarian somatic granulosa cells by activating the TP53INP1-p53-CDKN1A pathway[J]. Development, 2021, 148(14): dev199453. DOI:10.1242/dev.199453 |
| [2] |
刘召远, 张正红, 吴佳琦, 等. 组织缺氧在黄体形成过程中的作用[J]. 中国医学科学院学报, 2019, 41(6): 837-841. LIU Z Y, ZHANG Z H, WU J Q, et al. Contribution of tissue hypoxia to corpus luteum formation[J]. Acta Academiae Medicinae Sinicae, 2019, 41(6): 837-841. (in Chinese) |
| [3] |
杜秀雅, 马华刚. 血管新生相关因子对卵泡生长发育影响的研究进展[J]. 中国计划生育学杂志, 2014, 22(7): 502-504. DU X Y, MA H G. Research on the impact of vascular related factors on the growth and development of follicles[J]. Chinese Journal of Family Planning, 2014, 22(7): 502-504. (in Chinese) |
| [4] |
傅夏燕, 陈其臻, 尹喆, 等. 缺氧诱导因子-1调控卵泡发育的研究进展[J]. 同济大学学报: 医学版, 2021, 42(1): 136-141. FU X Y, CHEN Q Z, YIN Z, et al. Research progress of hypoxia inducible factor-1 on follicular development[J]. Journal of Tongji University: Medical Science, 2021, 42(1): 136-141. (in Chinese) |
| [5] |
LI C Y, MENG X Q, LIU S, et al. Oocytes and hypoxanthine orchestrate the G2-M switch mechanism in ovarian granulosa cells[J]. Development, 2020, 147(13): dev184838. DOI:10.1242/dev.184838 |
| [6] |
WU G, LI C Y, TAO J L, et al. FSH mediates estradiol synthesis in hypoxic granulosa cells by activating glycolytic metabolism through the HIF-1α-AMPK-GLUT1 signaling pathway[J]. J Biol Chem, 2022, 298(5): 101830. DOI:10.1016/j.jbc.2022.101830 |
| [7] |
TEMPKA D, TOKARZ P, CHMIELEWSKA K, et al. Downregulation of PARP1 transcription by CDK4/6 inhibitors sensitizes human lung cancer cells to anticancer drug-induced death by impairing OGG1-dependent base excision repair[J]. Redox Biol, 2018, 15: 316-326. DOI:10.1016/j.redox.2017.12.017 |
| [8] |
RANA N K, SINGH P, KOCH B. CoCl2 simulated hypoxia induce cell proliferation and alter the expression pattern of hypoxia associated genes involved in angiogenesis and apoptosis[J]. Biol Res, 2019, 52(1): 12. DOI:10.1186/s40659-019-0221-z |
| [9] |
DAI Z J, GAO J, MA X B, et al. Up-regulation of hypoxia inducible factor-1α by cobalt chloride correlates with proliferation and apoptosis in PC-2 cells[J]. J Exp Clin Cancer Res, 2012, 31(1): 28. DOI:10.1186/1756-9966-31-28 |
| [10] |
CHU C Y, JIN Y T, ZHANG W, et al. CA IX is upregulated in CoCl2-induced hypoxia and associated with cell invasive potential and a poor prognosis of breast cancer[J]. Int J Oncol, 2016, 48(1): 271-280. DOI:10.3892/ijo.2015.3253 |
| [11] |
史华旭, 吴子一, 陈世奇, 等. DNA氧化损伤标志物8-羟基脱氧鸟苷检测方法及其临床意义[J]. 沈阳医学院学报, 2019, 21(1): 79-82. SHI H X, WU Z Y, CHEN S Q, et al. Detection methods of oxidative DNA damage marker 8-hydroxy-2-deoxyguanosine and its clinical significance[J]. Journal of Shenyang Medical College, 2019, 21(1): 79-82. (in Chinese) |
| [12] |
凌学斌, 王军, 綦苗苗, 等. 缺氧、缺氧/复氧对心肌细胞内ROS、MAPKs活性表达的影响[J]. 海南医学院学报, 2021, 27(7): 481-487. LING X B, WANG J, QI M M, et al. Effect of hypoxia and hypoxia/reoxygenation on the viability expression of ROS and MAPKs in myocardial cells[J]. Journal of Hainan Medical University, 2021, 27(7): 481-487. (in Chinese) |
| [13] |
郑坤, 嘎鲁, 马宇衡, 等. 活性氧(ROS)依赖性抗肿瘤药物的研究进展[J]. 广东药科大学学报, 2022, 38(1): 130-136. ZHENG K, GA L, MA Y H, et al. Research progress of reactive oxygen species(ROS)-dependent anticancer drugs[J]. Journal of Guangdong Pharmaceutical University, 2022, 38(1): 130-136. (in Chinese) |
| [14] |
ORTMANN B, DRUKER J, ROCHA S. Cell cycle progression in response to oxygen levels[J]. Cell Mol Life Sci, 2014, 71(18): 3569-3582. DOI:10.1007/s00018-014-1645-9 |
| [15] |
FADHILLAH, YOSHIOKA S, NISHIMURA R, et al. Hypoxia promotes progesterone synthesis during luteinization in bovine granulosa cells[J]. J Reprod Dev, 2014, 60(3): 194-201. DOI:10.1262/jrd.2014-014 |
| [16] |
朱轶轩, 董晓英. 基于氧感知通路探讨早发性卵巢功能不全发生机制的研究进展[J]. 中国医药导报, 2021, 18(22): 55-58. ZHU Y X, DONG X Y. Research progress in the pathogenesis of premature ovarian insufficiency based on oxygen sensing pathway[J]. China Medical Herald, 2021, 18(22): 55-58. (in Chinese) |
| [17] |
REDMER D A, REYNOLDS L P. Angiogenesis in the ovary[J]. Rev Reprod, 1996, 1(3): 182-192. DOI:10.1530/ror.0.0010182 |
| [18] |
BASINI G, BIANCO F, GRASSELLI F, et al. The effects of reduced oxygen tension on swine granulosa cell[J]. Regul Pept, 2004, 120(1-3): 69-75. DOI:10.1016/j.regpep.2004.02.013 |
| [19] |
GARDNER L B, LI Q, PARK M S, et al. Hypoxia inhibits G1/S transition through regulation of p27 expression[J]. J Biol Chem, 2001, 276(11): 7919-7926. DOI:10.1074/jbc.M010189200 |
| [20] |
LIU Y, ZHANG S L, SU D C, et al. Inhibiting (pro)renin receptor-mediated p38 MAPK signaling decreases hypoxia/ reoxygenation-induced apoptosis in H9c2 cells[J]. Mol Cell Biochem, 2015, 403(1-2): 267-276. DOI:10.1007/s11010-015-2356-8 |
| [21] |
肖婷婷, 王莉, 曹相玫, 等. 氯化钴对小鼠海马神经元细胞缺氧损伤的影响[J]. 神经解剖学杂志, 2020, 36(4): 439-444. XIAO T T, WANG L, CAO X M, et al. Effects of cobalt chloride on hypoxic injury of hippocampal neurons in mice[J]. Chinese Journal of Neuroanatomy, 2020, 36(4): 439-444. (in Chinese) |
| [22] |
PIRET J P, MOTTET D, RAES M, et al. CoCl2, a chemical inducer of hypoxia-inducible factor-1, and hypoxia reduce apoptotic cell death in hepatoma cell line HepG2[J]. Ann N Y Acad Sci, 2002, 973(1): 443-447. |
| [23] |
CIAFRÈ S A, NIOLA F, GIORDA E, et al. CoCl2-simulated hypoxia in skeletal muscle cell lines: role of free radicals in gene up-regulation and induction of apoptosis[J]. Free Radic Res, 2007, 41(4): 391-401. |
| [24] |
刘菲, 张昊, 刘博, 等. 氯化钴诱导的N2a细胞缺氧损伤模型的机制研究[J]. 广东药科大学学报, 2020, 36(2): 249-253. LIU F, ZHANG H, LIU B, et al. Mechanism of cobalt chloride induced hypoxia on N2a cells[J]. Journal of Guangdong Pharmaceutical University, 2020, 36(2): 249-253. (in Chinese) |
| [25] |
马雪, 张晓馨, 史清海, 等. 氯化钴诱导大鼠原代脑微血管内皮细胞缺氧损伤的实验研究[J]. 新疆医科大学学报, 2017, 40(8): 1051-1055, 1060. MA X, ZHANG X X, SHI Q H, et al. Experimental study on primary rat brain microvascular endothelial cells in hypoxia injury induced by cobalt chloride[J]. Journal of Xinjiang Medical University, 2017, 40(8): 1051-1055, 1060. (in Chinese) |
| [26] |
GAO X X, LIU C H, HU Z L, et al. The biological effect of cobalt chloride mimetic-hypoxia on nucleus pulposus cells and the comparability with physical hypoxia in vitro[J]. Front Biosci (Landmark Ed), 2021, 26(10): 799-812. |
| [27] |
朱梦怡, 柯敏霞, 王皓, 等. 氯化钴诱导hiPSC-CMs体外缺氧模型的建立[J]. 浙江理工大学学报: 自然科学版, 2021, 45(1): 84-93. ZHU M Y, KE M X, WANG H, et al. Establishment of a CoCl2-mediated in vitro hiPSC-CMs hypoxia model[J]. Journal of Zhejiang Sci-Tech University: Natural Sciences Edition, 2021, 45(1): 84-93. (in Chinese) |
| [28] |
黄佳诚. 低氧模拟剂氯化钴对大鼠脂肪来源间充质干细胞体外增殖、成脂分化及迁移的影响的初步研究[D]. 广州: 南方医科大学, 2014. HUANG J C. The effect of hypoxic condition establish by CoCl2 on the proliferation, adipogenic differentiation and migration of Rat adipose-derived stem cells in vitro[D]. Guangzhou: Southern Medical University, 2014. (in Chinese) |
| [29] |
翁苓苓, 高玲, 张闽光. CoCl2化学模拟肝癌体外缺氧模型的建立及对HIF-1α、VEGF基因的影响[J]. 肝脏, 2019, 24(9): 1049-1052. WENG L L, GAO L, ZHANG M G. CoCl2 chemical simulation of the establishment of liver cancer hypoxic models and the impact on the HIF-1α and VEGF genes[J]. Chinese Hepatology, 2019, 24(9): 1049-1052. (in Chinese) |
| [30] |
刘芳. 藏羚羊STAT3、HIF-1α和HIF-2α基因表达的生物学意义[D]. 西宁: 青海大学, 2012. LIU F. Biological Significance of signal transducer and activator of transcription 3, hypoxia inducible factor 1 alpha and hypoxia inducible factor 2 alpha in high altitude hypoxic adaptation species-Tibetan antelope[D]. Xining: Qinghai University, 2012. (in Chinese) |
| [31] |
HUANG Y, TAN F B, ZHOU Y, et al. Hypoxia-preconditioned olfactory mucosa mesenchymal stem cells abolish cerebral ischemia/reperfusion-induced pyroptosis and apoptotic death of microglial cells by activating HIF-1α[J]. Aging (Albany NY), 2020, 12(11): 10931-10950. |
| [32] |
梁楚婷, 郭炜骅, 谭理, 等. 低氧诱导因子-1:细胞适应氧供应改变的关键蛋白[J]. 生物化学与生物物理进展, 2019, 46(11): 1041-1049. LIANG C T, GUO W H, TAN L, et al. Hypoxia-inducible factor-1:a key protein for cells adapting to changes in oxygen supply[J]. Progress in Biochemistry and Biophysics, 2019, 46(11): 1041-1049. (in Chinese) |
| [33] |
LIU Y H, GUO C, SUN Y Q, et al. Polymorphisms in HIF-1a gene are not associated with diabetic retinopathy in China[J]. World J Diabetes, 2021, 12(8): 1304-1311. |
| [34] |
YANG S S, LIAN G J. ROS and diseases: role in metabolism and energy supply[J]. Mol Cell Biochem, 2020, 467(1-2): 1-12. |
| [35] |
XIONG M Q, ZHAO Y, MO H H, et al. Intermittent hypoxia increases ROS/HIF-1α 'related oxidative stress and inflammation and worsens bleomycin-induced pulmonary fibrosis in adult male C57BL/6 J mice[J]. Int Immunopharmacol, 2021, 100: 108165. |
| [36] |
HUANG L, AO Q L, ZHANG Q H, et al. Hypoxia induced paclitaxel resistance in human ovarian cancers via hypoxia-inducible factor 1α[J]. J Cancer Res Clin Oncol, 2010, 136(3): 447-456. |
| [37] |
XU X W, CAO W L, SUN W, et al. Knockdown of CCDC132 attenuates gastric cancer cells proliferation and tumorigenesis by facilitating DNA damage signaling[J]. Cancer Manag Res, 2019, 11: 9585-9597. |
| [38] |
冉茂良, 高环, 尹杰, 等. 氧化应激与DNA损伤[J]. 动物营养学报, 2013, 25(10): 2238-2245. RAN M L, GAO H, YIN J, et al. Oxidative stress and DNA injury[J]. Chinese Journal of Animal Nutrition, 2013, 25(10): 2238-2245. (in Chinese) |
(编辑 郭云雁)



