在高度集约化、规模化养殖模式下,热应激是影响动物生产的重要因素之一,特别是在我国南方地区,热应激可导致动物采食量与免疫力下降,严重影响动物生长发育和繁殖性能[1-2]。热应激的作用机制与氧化应激密切相关,它诱导机体组织中活性氧(ROS)过量聚积,导致氧化与抗氧化状态失衡,脂质、蛋白质和核酸氧化,进而引起组织损伤,影响动物健康与生长[3]。当机体组织中核因子E2相关因子2(Nrf2)被激活后可上调下游抗氧化酶基因的表达,提高抗氧化酶活性,有助于提高机体抗氧化能力,缓解机体热应激[4]。热应激诱发氧化应激是造成畜禽机体损伤的关键因素,氧化应激是由于机体抗氧化调节失衡,导致氧化中间产物过多,体内自由基物质大量堆积从而引起机体氧化损伤[5-6]。在畜牧生产中,热应激对雄性动物生殖系统的影响更加突出,有研究指出,热应激可引起小鼠睾丸组织损伤,造成生精细胞凋亡[7]。因此,热应激已成为动物生产过程中所面临的重要挑战之一[8-9],寻找缓解动物热应激的新型安全高效抗氧化剂已迫在眉睫。芦丁(rutin)又称芸香甙,是主要来源于芸香叶的一种黄酮类化合物,广泛存在于多种药用植物和食物中,为淡黄色或淡绿色针状结晶或结晶性粉末[10],它具有抗氧化、抗炎、抗病毒等多种生物学功能[11-13]。研究证明,日粮添加芦丁可改善肥胖诱导的小鼠睾丸细胞结构异常、精子数量下降现象,缓解小鼠睾丸损伤[14-15],同时还具有提高睾丸生精功能的潜力[16]。因此推测,日粮添加芦丁是一种潜在的缓解由热应激诱导的睾丸组织功能障碍的营养调控方式。所以,本试验在构建了热应激小鼠模型基础上,进一步从组织形态、抗氧化、炎症、增殖凋亡等方面系统评估日粮添加芦丁对热应激小鼠睾丸组织的影响,并初步探讨其潜在的作用机制。
1 材料与方法 1.1 实验动物及设计将30只5周龄体重相近ICR系雄性小鼠(20~22 g,购于苏州大学)随机分为5组,各组小鼠分别饲喂基础日粮添加0(CON组)、0(HS组)、250(HS+ R250)、500(HS+R500)和1 000 mg·kg-1(HS+R1000)芦丁的日粮(芦丁购自上海阿拉丁生物科技股份有限公司,纯度95%),于清洁级动物房(温度控制在(22±2)℃,相对湿度为55%~60%)饲养10 d后,除对照组外,所有小鼠在每天10:00至14:00之间禁食并置于42 ℃恒温箱中连续处理8 d,具体方法参照以往报道[17-18]进行。饲养试验结束后称重、腹腔注射5%水合氯醛(按体重计算用量,每10 g体重0.1 mL)麻醉后,眼球采血,脱颈致死,取睾丸称重,并将左侧睾丸置于4%多聚甲醛中固定,右侧睾丸置冻存管内,液氮迅速冷冻后置于-80 ℃低温冰柜储存备用。
1.2 测定指标与方法1.2.1 睾丸指数 睾丸指数=睾丸重(g)/空腹活体重(kg)
1.2.2 睾丸形态组织观察 睾丸组织于4%多聚甲醛中固定48 h后,通过酒精、二甲苯处理,石蜡包埋、切片、伊红和苏木精(HE)染色后置于显微镜(ECLIPSE 80i型,尼康日本)下观察,采用Image J软件参照Li等[18-19]的研究报道测定生精小管直径、横截面积以及生精细胞脱落比率。
1.2.3 睾丸组织抗氧化酶活测定 取睾丸组织样本(约100 mg),按1∶9 (质量g/体积mL)的比例加入预冷的生理盐水,冰水浴条件下匀浆,3 000 r·min-1离心15 min,取上清采用南京建成生物工程研究所(中国,南京)提供的试剂盒测定总蛋白(A045-3-1)、还原型谷胱甘肽(GSH,A006-1-1)与丙二醛(MDA,A003-1-1)含量,同时测定总超氧化物歧化酶(T-SOD,A001-1-1)活性和总抗氧化能力(T-AOC,A015-1-2)。
1.2.4 mRNA相对表达量测定 从-80 ℃冰箱中取0.1 g左右的睾丸组织样品,使用TRIzol试剂(TaKaRa,大连)提取总RNA,通过qRT-PCR检测目的基因mRNA表达,具体操作参照Zhang等[20]进行。以β-actin作为内参基因,使用2-ΔΔCT方法计算各基因mRNA的相对表达量[21],引物序列见表 1。
|
|
表 1 qRT-PCR引物序列 Table 1 Primer sequences used for quantitative real-time PCR |
试验数据经Excel 2019初步整理后,使用IBM SPSS Statistics 22.0软件进行统计分析。采用单因素方差分析处理组间差异,并用Tukey法进行多重比较, 采用多项式中的线性和二次分析对不同浓度芦丁的效果进行分析。结果以“平均值±标准误”表示,P<0.05表示差异显著。
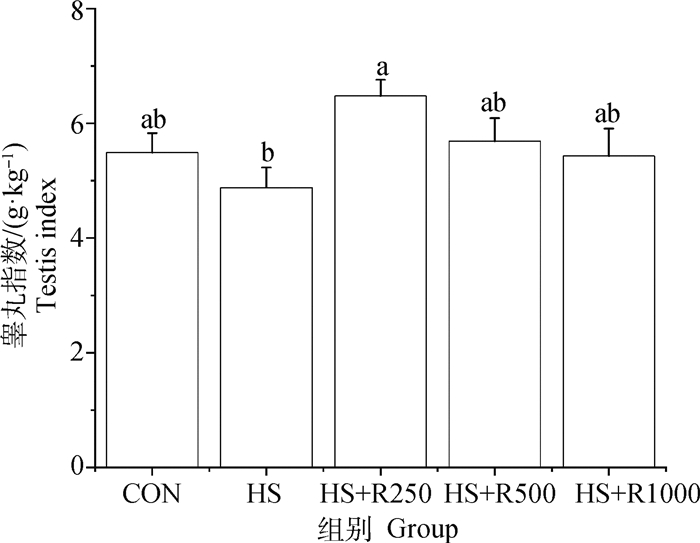
2 结果 2.1 日粮添加不同剂量芦丁对热应激小鼠睾丸指数的影响如图 1所示,HS组小鼠睾丸指数与CON组无显著差异(P>0.05),与HS组相比,HS+R250组小鼠睾丸指数显著增加(P<0.05),添加芦丁组与CON组相比小鼠睾丸指数无显著差异(P>0.05)。
|
CON. 未经高温处理的对照组;HS. 热应激组;HS+R250. 日粮添加250 mg·kg-1芦丁的热应激处理组;HS+R500. 日粮添加500 mg·kg-1芦丁的热应激处理组;HS+R1000. 日粮添加1 000 mg·kg-1芦丁的热应激处理组。柱上不同小写字母表示差异显著(P<0.05),下同 CON. Control group without high temperature treatment; HS. Heat stress group; HS+R250. Heat stress treatment group supplemented with 250 mg·kg-1 rutin; HS+R500. Heat stress treatment group supplemented with 500 mg·kg-1 rutin; HS+R1000. Heat stress treatment group supplemented with 1 000 mg·kg-1 rutin. Different lowercase letters on the column indicate significant difference (P < 0.05), the same as below 图 1 不同剂量芦丁对热应激小鼠睾丸指数的影响 Fig. 1 Effects of different doses of rutin on testis index of heat-stressed mice |
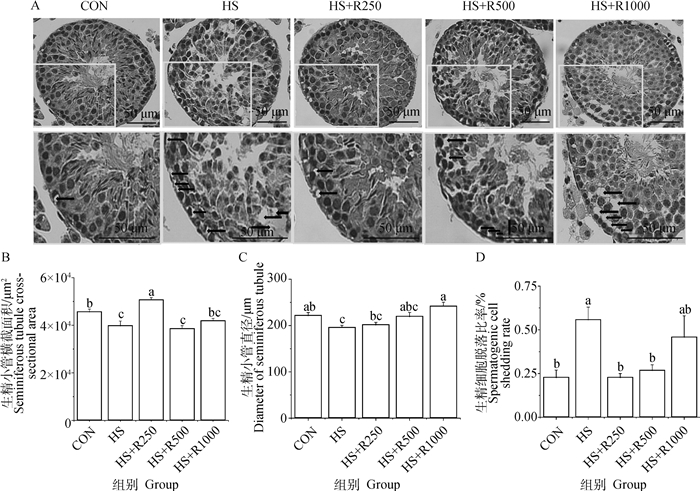
由图 2可知,与CON组相比,HS组小鼠生精小管横截面积和生精小管直径显著降低(P<0.05),生精细胞脱落比率显著增加(P<0.05);与HS组相比,HS+R250组小鼠生精小管横截面积显著增加(P<0.05),生精细胞脱落比率显著降低(P<0.05),生精小管直径无显著性差异(P>0.05),而HS+R500组生精细胞脱落比率显著降低(P<0.05),HS+R1000组小鼠生精小管直径显著增加(P<0.05);芦丁处理组小鼠睾丸生精小管直径及生精小管脱落比率与CON组无显著差异(P>0.05),但HS+R250组小鼠睾丸生精小管横截面积显著高于CON组(P<0.05)。
|
A. HE染色的小鼠睾丸组织,下排图为相应上图矩形框内结构的放大,黑色箭头指示的是生精细胞脱落情况;B. 生精小管横截面积; C. 生精小管直径; D. 生精细胞脱落比率 A. HE stained mouse testicular tissue, the lower line of pictures are the enlargement of the structure in the corresponding rectangular box above, and the black arrow indicates the spermatogenic cells shed; B. Spermatogenic tubule cross-sectional area; C. Diameter of spermatogenic tubule; D. Spermatogenic cell shedding rate 图 2 不同剂量芦丁对热应激小鼠睾丸组织形态的影响(HE染色) Fig. 2 The effect of different doses of rutin on the morphology of heat-stressed mice testis (HE staining) |
如表 2所示,HS组小鼠睾丸中MDA含量较CON组显著增加(P<0.05),T-AOC活性与GSH含量显著降低(P<0.05),T-SOD活性无显著性差异(P>0.05);与HS组相比,HS+R250组MDA含量显著降低(P<0.05),T-AOC与GSH含量显著增加(P<0.05),HS+R500和HS+R1000组小鼠睾丸组织GSH含量显著高于HS组(P<0.05),而芦丁处理热应激小鼠睾丸组织中MDA、GSH含量以及T-AOC和T-SOD活性与CON组相比均无显著性差异(P>0.05)。
|
|
表 2 不同剂量芦丁对热应激小鼠睾丸抗氧化功能的影响 Table 2 Effects of different doses of rutin on the anti-oxidant function in the testis of heat-stressed mice |
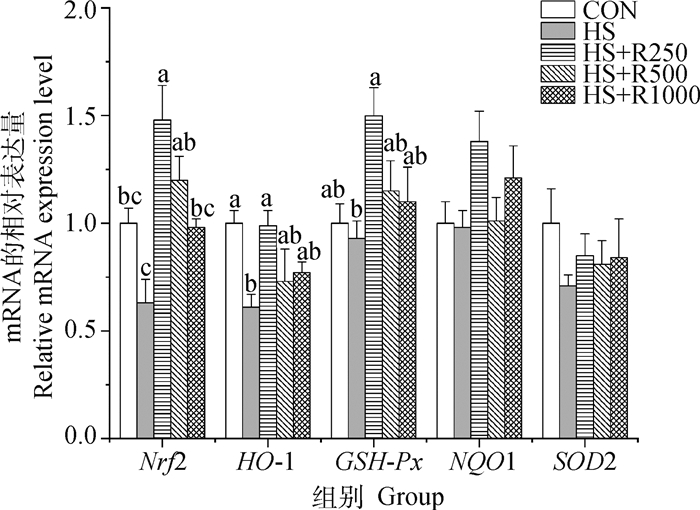
如图 3可知,HS组小鼠睾丸中HO-1 mRNA的表达量与CON组相比显著降低(P<0.05);与HS组相比,HS+R250组Nrf2、HO-1、GSH-Px基因的相对表达量显著增加(P<0.05),HS+R500组Nrf2表达量显著升高(P<0.05),HS+R1000组Nrf2表达量无显著性差异(P<0.05)。与CON组相比,HS+R250组Nrf2 mRNA表达量显著增加(P<0.05),SOD2与NQO1 mRNA表达量在各组之间均无显著性差异(P>0.05)。
|
图 3 不同剂量芦丁对热应激小鼠睾丸抗氧化相关基因表达的影响 Fig. 3 The effect of different doses of rutin on the expression of antioxidant-related genes in the testis of heat-stressed mice |
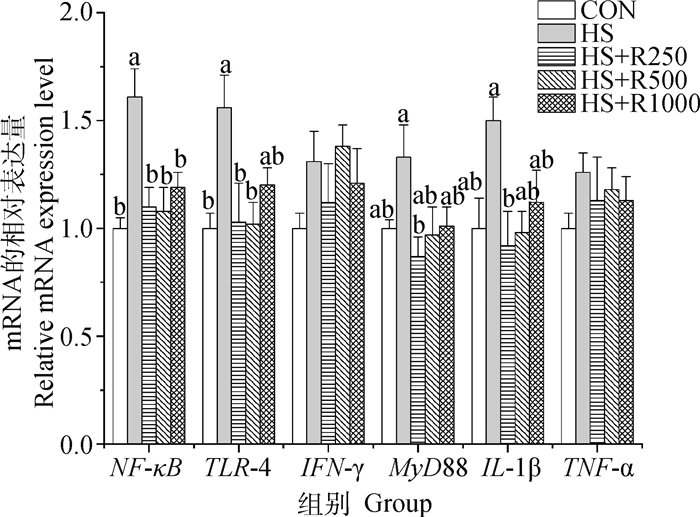
如图 4可知,与CON组相比,HS组小鼠睾丸NF-κB和TLR-4表达量显著增加(P<0.05),MyD88、IL-1β、TNF-α和IFN-γ mRNA表达量无显著性差异(P>0.05);与HS组相比,HS+R250组NF-κB、TLR-4、MyD88与IL-1β mRNA表达量显著降低(P<0.05),HS+R500组NF-κB和TLR-4的mRNA表达量显著下降(P<0.05),HS+R1000组中NF-κB mRNA的表达量也显著低于HS组(P>0.05);日粮添加250~1 000 mg·kg-1芦丁处理热应激小鼠睾丸组织中炎症相关基因表达量与CON组之间未见显著差异(P>0.05)。
|
图 4 不同剂量芦丁对热应激小鼠睾丸炎症相关基因表达的影响 Fig. 4 Effects of different doses of rutin on the expression of inflammation-related genes in the testis of heat-stressed mice |
由图 5可知,与CON组相比,HS组Bax基因的相对表达量显著增加(P<0.05),Bcl-2表达量显著降低(P<0.05);与HS组相比,HS+R250组Bax mRNA表达量显著降低(P<0.05),Bcl-2表达量显著升高(P<0.05),HS+R1000组Bcl-2 mRNA表达量也显著高于HS组(P<0.05),HS+R500组无显著性变化(P>0.05);日粮添加250~1 000 mg·kg-1芦丁处理热应激小鼠睾丸组织中增殖与凋亡相关基因表达量与CON组之间未见显著差异(P>0.05)。
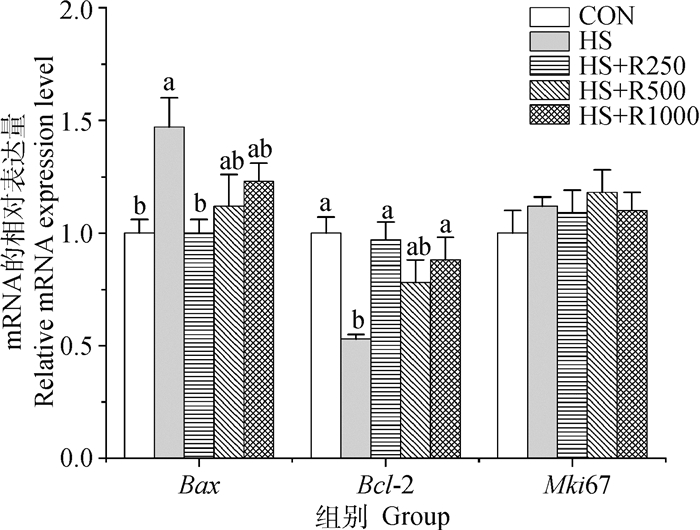
|
图 5 不同剂量芦丁对热应激小鼠睾丸增殖与凋亡基因表达的影响 Fig. 5 Effects of different doses of rutin on testis proliferation and apoptosis genes expression in heat-stressed mice |
睾丸是具有高度增殖能力的组织,大多数哺乳动物的睾丸位于低于体温2~ 8 ℃的阴囊内,对温度非常敏感[22-23]。温度过高(如42 ℃)可使睾丸温度升高,导致睾丸组织形态异常,抑制精子发生,影响精子成熟与存活[24-25]。睾丸组织形态是反映睾丸组织功能与生殖能力的重要指标之一。吴璟等[26]研究发现,将ICR小鼠下腹部连续7 d置于43 ℃恒温水浴锅中热应激处理15 min后可显著降低小鼠睾丸系数,减少生精细胞数量,使小鼠睾丸生精上皮空泡变性及萎缩,从而改变睾丸形态,导致小鼠睾丸组织损伤诱导氧化应激。而本试验结果显示,HS组小鼠置于42 ℃恒温箱中(4 h·d-1)连续热处理8 d后睾丸指数与正常组无明显变化,这可能与热处理的方式和时间有关;但本试验中热处理小鼠睾丸组织生精小管直径和横截面积显著降低,生精细胞脱落比率显著增加,提示高温处理可改变小鼠睾丸形态,引起睾丸组织损伤。Mehfooz等[15]研究发现,日粮添加芦丁对束缚应激引起的小鼠睾丸损伤、细胞凋亡以及精子形态具有一定的保护作用。在本试验中日粮添加250 mg·kg-1芦丁可使热应激小鼠睾丸指数和生精小管横截面积显著提高,添加1 000 mg·kg-1芦丁显著增加了热应激小鼠生精小管直径,且添加250和500 mg·kg-1芦丁均可显著降低热应激小鼠生精细胞脱落比率,提示芦丁可改变小鼠睾丸形态缓解睾丸损伤,这可能与芦丁具有较好的抗氧化能力,缓解动物氧化应激和促进睾丸生精功能密切相关[13, 16],而添加1 000 mg·kg-1芦丁对热应激小鼠生精细胞脱落比率无显著性差异,提示芦丁添加量过高对机体可能会产生一定的负面影响,但长期添加高剂量芦丁对机体的影响及作用机制尚有待进一步研究。
3.2 日粮添加芦丁对热应激小鼠睾丸抗氧化功能的影响抗氧化酶是一种存在于细胞环境中的蛋白质,它能通过调控抗氧化酶活性及数量来清除体内自由基[27]。GSH作为一种内源性活性肽,能够清除或中和体内过氧化物质和自由基,缓解机体氧化应激[28]。研究表明,日粮添加芦丁对大鼠睾丸免受缺血再灌注损伤具有保护作用,这可能与芦丁可提高超氧化物歧化酶和过氧化氢酶的活性[29]并增强GSH含量缓解氧化应激反应有关[16]。与之相似,本试验发现日粮中添加250、500和1 000 mg·kg-1芦丁均能显著提高热应激小鼠睾丸组织中GSH含量,发挥其抗氧化功能。此外,日粮添加250 mg·kg-1的芦丁可显著提高HS组小鼠睾丸组织T-AOC活性,显著降低MDA含量。MDA是机体内评价脂质氧化损伤的重要指标[30],故本试验结果提示,日粮添加250 mg·kg-1能够有效抑制小鼠睾丸脂质过氧化,缓解氧化应激。在本试验中日粮添加250、500和1 000 mg·kg-1芦丁均未对睾丸组织中T-SOD活性产生显著性影响,但韩雨薇等[31]研究发现,对蛛网膜下腔出血(SAH)大鼠给药50 mg·kg-1芦丁后可通过增加T-SOD与GSH-Px活性缓解SAH大鼠氧化应激反应,此两者结果不同的原因可能与芦丁处理的剂量、方式或检测组织不同有关。
Nrf2是一个与抗氧化功能密切相关的重要转录因子,可通过调节相关抗氧化基因表达影响抗氧化防御系统,如HO-1[32]。HO-1作为一种抗氧化酶在应激状态下能够保持机体细胞稳定性[33],表达上调时可有助于增强机体抗氧化功能,缓解组织氧化损伤[34]。本研究发现,热应激小鼠睾丸组织中HO-1 mRNA的表达量显著降低,而日粮添加250 mg·kg-1芦丁可提高热应激小鼠睾丸Nrf2 mRNA表达量,上调HO-1和GSH-Px mRNA的表达,且HO-1表达水平与对照组小鼠无显著性差异。因此推测,日粮添加芦丁缓解热应激诱导的小鼠睾丸氧化损伤功能可能与Nrf2/HO-1信号通路有关,这与王斌等[35]研究发现灌胃芦丁(每天100 mg·kg-1)可通过Nrf2/HO-1信号通路缓解小鼠肾氧化应激损伤的结果一致。芦丁是从植物中提取的一种天然黄酮类物质,多被认为是一种安全性物质,但天然类物质也可对机体造成不良影响[36]。Féres等[37]发现,添加2 000 mg·kg-1芸香苷(dimorphandra mollis,芦丁含量为(76±3)%)可对机体产生一定毒副作用。本研究也发现与250 mg·kg-1芦丁组相比,日粮添加1 000 mg·kg-1芦丁显著降低了睾丸组织中Nrf2 mRNA表达量,提示日粮添加高剂量芦丁并未线性提高其抗氧化功能,这可能与高剂量芦丁可对机体产生一定副作用有关[37-38],但其具体机制尚需要进一步研究。
3.3 日粮添加芦丁对热应激小鼠睾丸炎症因子相关基因表达的影响热应激可以导致炎症因子增加、抗炎因子下降,从而诱导炎症反应发生[39]。NF-κB介导的信号通路参与体内多种反应,TLR-4可激活NF-κB通路介导下游炎症因子表达[40]。有研究表明,TLR-4/NF-κB信号通路与抗炎免疫机制密切相关,被认为是经典的炎症相关信号通路[41]。MyD88是一种髓样蛋白分子,处于TLR-4信号通路的下游,参与TLR-4基因所介导的炎症信号转导[42],TLR-4通过MyD88途径使NF-κB信号通路被激活, 合成并释放TNF-α、IL-1β等相关促炎症因子,完成炎症反应信号的传递[43]。本试验结果表明,热应激导致小鼠睾丸组织中TLR-4/NF-κB信号通路被激活,MyD88和IL-1β基因表达显著增强,这与巩栋梁[44]研究发现, 热应激状态下猪肠道组织中TLR-4/NF-κB信号通路被激活后上调IL-6等相关促炎症因子表达,从而导致机体炎症反应的结果一致。大量研究表明,芦丁在增强机体免疫的同时也可抑制多个促炎因子基因表达[45-46],并对大鼠肾组织具有较强的抗炎作用[47-48]。Yeh等[49]研究表明, 芦丁能显著抑制NF-κB表达,降低IL-1β、TNF-α、IL-6等多种促炎症因子的水平,具有较强的抗炎作用。本试验结果显示,日粮添加250、500和1 000 mg·kg-1芦丁均显著抑制了小鼠睾丸组织NF-κB mRNA的表达量,同时添加250和500 mg·kg-1芦丁可使TLR-4表达量显著降低,且当添加剂量为250 mg·kg-1时,HS组小鼠睾丸中MyD88与IL-1β mRNA表达量也显著下降,故推测热应激小鼠日粮中添加250 mg·kg-1芦丁可能通过抑制TLR-4/NF-κB信号通路下调相关炎症因子的表达发挥抗炎作用。
3.4 日粮添加芦丁对热应激小鼠睾丸增殖与凋亡基因表达的影响Bcl-2是一个重要细胞凋亡基因,能够抑制细胞凋亡、参与细胞增殖调控,在组织器官生长发育及正常功能发挥方面具有重要作用[50-51]。Bax是Bcl-2的同源基因,当Bax表达过量可自身结合成同源二聚体, 促进凋亡发生[52]。何萌萌等[22]研究发现,大鼠在43 ℃环境中水浴20 min可引起睾丸生精细胞凋亡,导致生殖障碍。口服75和150 mg·kg-1芦丁可抑制小鼠肾炎症和细胞凋亡[53]。本试验结果显示,连续热处理8 d(42 ℃,4 h·d-1)后使小鼠睾丸组织中Bax基因表达量显著增加,而日粮添加250 mg·kg-1芦丁显著抑制热应激小鼠睾丸Bax mRNA的表达;添加250和1 000 mg·kg-1芦丁显著增加Bcl-2 mRNA的表达量,这与添加250 mg·kg-1芦丁可降低热应激小鼠睾丸生精细胞脱落比率的结果相吻合,提示日粮添加适宜剂量芦丁对改善热应激小鼠睾丸组织损伤及抑制细胞凋亡具有重要作用。
4 结论本试验结果提示,日粮添加适宜剂量芦丁具有改善热应激小鼠睾丸组织形态及功能的效果,其机制可能与芦丁通过Nrf2信号通路缓解氧化应激,通过TLR-4/NF-κB信号通路抑制炎症反应及调控Bax/Bcl-2 mRNA表达密切相关。本试验条件下日粮添加250 mg·kg-1芦丁效果较好。
| [1] |
SEJIAN V, BHATTA R, GAUGHAN J B, et al. Review: Adaptation of animals to heat stress[J]. Animal, 2018, 12(S2): S431-S444. |
| [2] |
BARRETT N W, ROWLAND K, SCHMIDT C J, et al. Effects of acute and chronic heat stress on the performance, egg quality, body temperature, and blood gas parameters of laying hens[J]. Poult Sci, 2019, 98(12): 6684-6692. DOI:10.3382/ps/pez541 |
| [3] |
SLIMEN I B, NAJAR T, GHRAM A, et al. Heat stress effects on livestock: Molecular, cellular and metabolic aspects, a review[J]. J Anim Physiol Anim Nutr (Berl), 2016, 100(3): 401-412. DOI:10.1111/jpn.12379 |
| [4] |
SURAI P F, KOCHISH I I, FISININ V I, et al. Antioxidant defence systems and oxidative stress in poultry biology: An update[J]. Antioxidants (Basel), 2019, 8(7): 235. DOI:10.3390/antiox8070235 |
| [5] |
彭定伟, 张博, 张秀娟, 等. 脐带间充质干细胞移植对急性颅脑创伤大鼠超氧化物歧化酶及白细胞介素-12的影响[J]. 医学综述, 2020, 26(15): 3062-3066. PENG D W, ZHANG B, ZHANG X J, et al. Effects of umbilical cord mesenchymal stem cells transplantation on superoxide dismutase and interleukin-12 in rats with acute traumatic brain injury[J]. Medical Recapitulate, 2020, 26(15): 3062-3066. (in Chinese) |
| [6] |
李峰, 田冰锋, 魏小兵, 等. 重型颅脑创伤急性期和慢性期外周血炎症反应标志物和氧化应激临床研究[J]. 中国现代神经疾病杂志, 2018, 18(2): 128-133. LI F, TIAN B F, WEI X B, et al. Clinical study on the changes of peripheral inflammatory markers and oxidative stress during post-acute and chronic phase after severe traumatic brain injury[J]. Chinese Journal of Contemporary Neurology and Neurosurgery, 2018, 18(2): 128-133. DOI:10.3969/j.issn.1672-6731.2018.02.009 (in Chinese) |
| [7] |
雷方玉, 王玉芳, 叶天太, 等. 热应激对小鼠睾丸组织细胞的影响研究[J]. 浙江畜牧兽医, 2020, 45(3): 3-5. LEI F Y, WANG Y F, YE T T, et al. Study on the effect of heat stress on mouse testicular tissue cells[J]. Journal Animal Science and Veterinary Medicine, 2020, 45(3): 3-5. (in Chinese) |
| [8] |
EL-TARABANY M S, EL-TARABANY A A, ATTA M A. Physiological and lactation responses of Egyptian dairy Baladi goats to natural thermal stress under subtropical environmental conditions[J]. Int J Biometeorol, 2017, 61(1): 61-68. DOI:10.1007/s00484-016-1191-2 |
| [9] |
BONI R. Heat stress, a serious threat to reproductive function in animals and humans[J]. Mol Reprod Dev, 2019, 86(10): 1307-1323. DOI:10.1002/mrd.23123 |
| [10] |
祖晓宁, 王金宏, 于宛鑫. 槐米中芦丁的提取及抑菌活性研究[J]. 哈尔滨商业大学学报, 2018, 34(6): 648-652. ZU X N, WANG J H, YU W X. Study on extraction and antibacterial activity of rutin in locust[J]. Journal of Harbin University of Commerce (Natural Sciences Edition), 2018, 34(6): 648-652. DOI:10.3969/j.issn.1672-0946.2018.06.003 (in Chinese) |
| [11] |
臧志和, 曹丽萍, 钟铃. 芦丁药理作用及制剂的研究进展[J]. 医药导报, 2007, 26(7): 758-760. ZANG Z H, CAO L P, ZHONG L. Research progress on the pharmacological effects and preparations of Rutin[J]. Herald of Medicine, 2007, 26(7): 758-760. DOI:10.3870/j.issn.1004-0781.2007.07.021 (in Chinese) |
| [12] |
马溶, 庞广昌. 芦丁对现代文明病的作用[J]. 食品科学, 2013, 34(7): 307-311. MA R, PANG G C. Role of rutin in modern civilization diseases[J]. Food Science, 2013, 34(7): 307-311. (in Chinese) |
| [13] |
RUSSO A, ACQUAVIVA R, CAMPISI A, et al. Bioflavonoids as antiradicals, antioxidants and DNA cleavage protectors[J]. Cell Biol Toxicol, 2000, 16(2): 91-98. DOI:10.1023/A:1007685909018 |
| [14] |
王力, 闻赵燕, 阚梦颖, 等. 芦丁对肥胖诱发的雄性小鼠生殖损伤的保护作用[J]. 南方医科大学学报, 2017, 37(11): 1529-1534. WANG L, WEN Z Y, KAN M Y, et al. Protective effects of rutin against obesity-induced reproductive impairment in male mice[J]. Journal of Southern Medical University, 2017, 37(11): 1529-1534. DOI:10.3969/j.issn.1673-4254.2017.11.17 (in Chinese) |
| [15] |
MEHFOOZ A, WEI Q W, ZHENG K Z, et al. Protective roles of rutin against restraint stress on spermatogenesis in testes of adult mice[J]. Tissue Cell, 2018, 50: 133-143. DOI:10.1016/j.tice.2018.01.003 |
| [16] |
CAGLAYAN C, KANDEMIR F M, DARENDELIOG ˇ LU E, et al. Rutin ameliorates mercuric chloride-induced hepatotoxicity in rats via interfering with oxidative stress, inflammation and apoptosis[J]. J Trace Elem Med Biol, 2019, 56: 60-68. DOI:10.1016/j.jtemb.2019.07.011 |
| [17] |
LI Y S, HUANG Y, PIAO Y, et al. Protective effects of nuclear factor erythroid 2-related factor 2 on whole body heat stress-induced oxidative damage in the mouse testis[J]. Reprod Biol Endocrinol, 2013, 11(1): 23. DOI:10.1186/1477-7827-11-23 |
| [18] |
LI Y S, LI Z J, CAO Y, et al. Chronic excessive Zn intake increases the testicular sensitivity to high ambient temperature in Bama miniature pigs[J]. Environ Pollut, 2020, 257: 113629. DOI:10.1016/j.envpol.2019.113629 |
| [19] |
SHEN J K, PERVEEN A, KAKA N, et al. Maternal exposure to T-2 toxin induces changes in antioxidant system and testosterone synthesis in the testes of mice offspring[J]. Animals (Basel), 2019, 10(1): 74. |
| [20] |
ZHANG L G, ZHANG J Q, YAN E F, et al. Dietary supplemented curcumin improves meat quality and antioxidant status of intrauterine growth retardation growing pigs via Nrf2 signal pathway[J]. Animals (Basel), 2020, 10(3): 539. |
| [21] |
LIVAK K J, SCHMITTGEN T D. Analysis of relative gene expression data using real-time quantitative PCR and the Method[J]. Methods, 2001, 25(4): 402-408. DOI:10.1006/meth.2001.1262 |
| [22] |
何萌萌, 张婷婷, 马杰, 等. 热应激对大鼠睾丸影响的研究[J]. 中日友好医院学报, 2015, 29(2): 92-95. HE M M, ZHANG T T, MA J, et al. Effects of heat stress on male rat testis[J]. Journal of China-Japan Friendship Hospital, 2015, 29(2): 92-95. DOI:10.3969/j.issn.1001-0025.2015.02.007 (in Chinese) |
| [23] |
KANTER M, AKTAS C, ERBOGA M. Heat stress decreases testicular germ cell proliferation and increases apoptosis in short term: an immunohistochemical and ultrastructural study[J]. Toxicol Ind Health, 2013, 29(2): 99-113. DOI:10.1177/0748233711425082 |
| [24] |
OTSUKA S, NAMIKI Y, ICHⅡ O, et al. Analysis of factors decreasing testis weight in MRL mice[J]. Mamm Genome, 2010, 21(3-4): 153-161. DOI:10.1007/s00335-010-9251-0 |
| [25] |
饶猛, 朱长虹. 热应激诱导睾丸生殖细胞凋亡的机制[C]//中华医学会第十届全国计划生育学学术会议论文集. 深圳: 中华医学会, 2014: 353-355. RAO M, ZHU C H. The mechanism of heat stress inducing the apoptosis of testicular germ cells[C]//National Family Planning Conference of Chinese Medical Association. Shenzhen: Chinese Medical Association, 2014: 353-355. (in Chinese) |
| [26] |
吴璟, 郑婕, 金学琴, 等. 葫芦巴碱对热应激致小鼠睾丸损伤的保护作用[J]. 中国比较医学杂志, 2019, 29(7): 47-52. WU J, ZHENG J, JIN X Q, et al. Protective effect of trigonelline on testicular tissue injury induced by heat stress in mice[J]. Chinese Journal of Comparative Medicine, 2019, 29(7): 47-52. DOI:10.3969/j.issn.1671-7856.2019.07.008 (in Chinese) |
| [27] |
MAURYA P K, DUA K. Role of oxidative stress in pathophysiology of diseases[M]. Singapore: Springer Singapore, 2020.
|
| [28] |
张艺, 叶升. 还原型谷胱甘肽生理功能及其临床应用[J]. 生命的化学, 2020, 40(12): 2226-2235. ZHANG Y, YE S. Physiological functions and clinical applications of glutathione[J]. Chemistry of Life, 2020, 40(12): 2226-2235. (in Chinese) |
| [29] |
WEI S M, YAN Z Z, ZHOU J. Protective effect of rutin on testicular ischemia-reperfusion injury[J]. J Pediatr Surg, 2011, 46(7): 1419-1424. DOI:10.1016/j.jpedsurg.2010.09.044 |
| [30] |
OZDEMIR D, UYSAL N, TUGYAN K, et al. The effect of melatonin on endotoxemia-induced intestinal apoptosis and oxidative stress in infant rats[J]. Intensive Care Med, 2007, 33(3): 511-516. DOI:10.1007/s00134-006-0492-z |
| [31] |
韩雨薇, 王晨辰, 李晓明. 芦丁通过抗氧化抗凋亡对蛛网膜下腔出血大鼠早期脑损伤的保护作用[J]. 现代药物与临床, 2020, 35(3): 421-425. HAN Y W, WANG C C, LI X M. Protective effect of rutin on early brain injury in mice with subarachnoid hemorrhage by anti-oxidation and anti-apoptosis[J]. Drugs & Clinic, 2020, 35(3): 421-425. (in Chinese) |
| [32] |
SHIN S M, YANG J H, KI S H. Role of the Nrf2-ARE pathway in liver diseases[J]. Oxid Med Cell Longev, 2013, 2013: 763257. |
| [33] |
郑蕾, 李国芳, 杨盼盼, 等. 基于Nrf2/ARE通路研究银杏叶滴剂对口腔溃疡大鼠的作用机制[J]. 中医学报, 2021, 36(5): 1029-1034. ZHENG L, LI G F, YANG P P, et al. Study on the mechanism of Ginkgo Biloba drops on oral ulcer rats based on Nrf2/ARE pathway[J]. Acta Chinese Medicine, 2021, 36(5): 1029-1034. (in Chinese) |
| [34] |
王甜甜, 陈淳媛, 杨雷, 等. Nrf2/HO-1信号轴在氧化应激性疾病中的机制[J]. 中南大学学报: 医学版, 2019, 44(1): 74-80. WANG T T, CHEN C Y, YANG L, et al. Role of Nrf2/HO-1 signal axis in the mechanisms for oxidative stress-relevant diseases[J]. Journal of Central South University: Medical Science, 2019, 44(1): 74-80. (in Chinese) |
| [35] |
王斌, 赵明, 陈志勇, 等. 芦丁对梗阻性肾病大鼠肾脏氧化应激损伤及Nrf2/HO-1通路的影响[J]. 重庆医学, 2019, 48(2): 212-216. WANG B, ZHAO M, CHEN Z Y, et al. Effects of rutin on kidney oxidative stress and Nrf2/HO-1 signaling pathway in rats with obstructive nephropathy[J]. Chongqing Medicine, 2019, 48(2): 212-216. DOI:10.3969/j.issn.1671-8348.2019.02.008 (in Chinese) |
| [36] |
BENT S, KO R. Commonly used herbal medicines in the United States: a review[J]. Am J Med, 2004, 116(7): 478-485. DOI:10.1016/j.amjmed.2003.10.036 |
| [37] |
FÉRES C A O, MADALOSSO R C, ROCHA O A, et al. Acute and chronic toxicological studies of Dimorphandra mollis in experimental animals[J]. J Ethnopharmacol, 2006, 108(3): 450-456. DOI:10.1016/j.jep.2006.06.002 |
| [38] |
李颖平, 庞全海, 秦红, 等. 芦丁对AA肉鸡生长性能肉鸡生长性能、血清生化指标和肉鸡腹水综合征的影响[J]. 中国家禽, 2019, 41(17): 30-33. LI Y P, PANG Q H, QIN H, et al. Effects of rutin on growth performance, serum biochemical indexes and ascites syndrome of AA broilers[J]. China Poultry, 2019, 41(17): 30-33. (in Chinese) |
| [39] |
LIU Z F, SUN X G, TANG J, et al. Intestinal inflammation and tissue injury in response to heat stress and cooling treatment in mice[J]. Mol Med Rep, 2011, 4(3): 437-443. |
| [40] |
王业杨, 李贵涛, 王明森, 等. 槲皮素抑制TLR4/NF-κB通路介导的炎症反应减轻脊髓损伤[J]. 中国矫形外科杂志, 2020, 28(14): 1311-1316. WANG Y Y, LI G T, WANG M S, et al. Quercetin attenuates spinal cord injury by inhibiting TLR4 /NF-κB-mediated inflammatory response[J]. Orthopedic Journal of China, 2020, 28(14): 1311-1316. (in Chinese) |
| [41] |
何俊慧, 韦洁, 李冬梅, 等. 基于TLR4/NF-κB通路探讨蛤蚧提取物对利血平诱导抑郁大鼠神经炎症的影响[J]. 中国实验方剂学杂志, 2021, 27(9): 56-62. HE J H, WEI J, LI D M, et al. Effect of gecko extract on neuroinflammation in rats with reserpine-induced depression via TLR4/NF-κB pathway[J]. Chinese Journal of Experimental Traditional Medical Formulae, 2021, 27(9): 56-62. (in Chinese) |
| [42] |
邓婷婷. Toll样受体信号与Gas6/ProS-TAM系统在调节小鼠巨噬细胞炎症反应中的功能[D]. 北京: 北京协和医学院, 2012. DENG T T. The interplay of Toll-like receptor signaling and Gas6/ProS-TAM system in regulating inflammatory response of mouse macrophages[D]. Beijing: Peking Union Medical College, 2012. (in Chinese) |
| [43] |
李琳娜, 李坚, 贺熙乔, 等. TLR4/MyD88/AMPK通路参与调节高脂饮食诱导的肥胖小鼠脂肪组织炎症和细胞凋亡[J]. 中国免疫学杂志, 2021: 1-14. (2021-03-05)[2022-02-18]. http://kns.cnki.net/kcms/detail/22.1126.R.20210304.1720.002.html. LI L N, LI J, HE X Q, et al. TLR4/MyD88/AMPK pathways involves in regulating adipose tissue inflammation and apoptosis in high-fat diet-induced obese mice[J]. Chinese Journal of Immunology, 2021: 1-14. (2021-03-05)[2022-02-18]. http://kns.cnki.net/kcms/detail/22.1126.R.20210304.1720.002.html. (in Chinese) |
| [44] |
巩栋梁. TLR4/NF-κB信号通路调控猪热应激性炎性肠病的分子机制[D]. 湛江: 广东海洋大学, 2018. GONG D L. The molecular mechanism of TLR4/NF-κB signaling pathway contributing to heat stress-induced porcine inflammatory bowel disease[D]. Zhanjiang: Guangdong Ocean University, 2018. (in Chinese) |
| [45] |
GANESHPURKAR A, SALUJA A K. Protective effect of rutin on humoral and cell mediated immunity in rat model[J]. Chem Biol Interact, 2017, 273: 154-159. DOI:10.1016/j.cbi.2017.06.006 |
| [46] |
PAL P B, PAL S, DAS J, et al. Modulation of mercury-induced mitochondria-dependent apoptosis by glycine in hepatocytes[J]. Amino Acids, 2012, 42(5): 1669-1683. DOI:10.1007/s00726-011-0869-3 |
| [47] |
KANDEMIR F M, OZKARACA M, YILDIRIM B A, et al. Rutin attenuates gentamicin-induced renal damage by reducing oxidative stress, inflammation, apoptosis, and autophagy in rats[J]. Ren Fail, 2015, 37(3): 518-525. DOI:10.3109/0886022X.2015.1006100 |
| [48] |
ARJUMAND W, SETH A, SULTANA S. Rutin attenuates cisplatin induced renal inflammation and apoptosis by reducing NFκB, TNF-α and caspase-3 expression in wistar rats[J]. Food Chem Toxicol, 2011, 49(9): 2013-2021. DOI:10.1016/j.fct.2011.05.012 |
| [49] |
YEH C H, YANG J J, YANG M L, et al. Rutin decreases lipopolysaccharide-induced acute lung injury via inhibition of oxidative stress and the MAPK-NF-κB pathway[J]. Free Radic Biol Med, 2014, 69: 249-257. DOI:10.1016/j.freeradbiomed.2014.01.028 |
| [50] |
付倩梅. Bcl-2基因的研究进展[J]. 西南军医, 2014, 16(1): 76-79. FU Q M. Research progress of Bcl-2 gene[J]. Journal of Military Surgeon in Southwest China, 2014, 16(1): 76-79. DOI:10.3969/j.issn.1672-7193.2014.01.030 (in Chinese) |
| [51] |
GIORDANO M V, LUCAS H D S, FIORELLI R K A, et al. Expression levels of BCL2 and MKI67 in endometrial polyps in postmenopausal women and their correlation with obesity[J]. Mol Clin Oncol, 2020, 13(6): 69. |
| [52] |
李亚丽, 冉战玲, 楚伟, 等. 中药六味地黄汤对衰老大鼠卵巢组织Bax/Bcl-2及Caspese-3蛋白表达的影响[J]. 中国优生与遗传杂志, 2012, 20(10): 36-37, 28. LI Y L, RAN Z L, CHU W, et al. Effects of Liuweidihuangtang on the expressions of apoptosis- associated genes Bax/Bcl-2 and Caspese-3 protein[J]. Chinese Journal of Birth Health & Heredity, 2012, 20(10): 36-37, 28. (in Chinese) |
| [53] |
MA J Q, LIU C M, YANG W. Protective effect of rutin against carbon tetrachloride-induced oxidative stress, inflammation and apoptosis in mouse kidney associated with the ceramide, MAPKs, p53 and calpain activities[J]. Chem Biol Interact, 2018, 286: 26-33. DOI:10.1016/j.cbi.2018.03.003 |
(编辑 范子娟)



