2. 甘肃省动物细胞技术创新中心, 兰州 730030;
3. 西北民族大学生物医学研究中心, 兰州 730030
2. Gansu Technology Innovation Center of Animal Cell, Lanzhou 730030, China;
3. Biomedical Research Center, Northwest Minzu University, Lanzhou 730030, China
高原地区具有强紫外线、牧草生长季短、低温、低氧等特殊的环境特点,牦牛(Bos grunniens)是青藏高原上特有的物种,其具有肺功能强大、觅食能力强和代谢能力高等一系列适应高原地区的特点。它不仅能够很好的生存,而且还能将其稳定的适应性遗传到下一代,是研究低氧适应性的最理想物种,其潜在的分子机制仍然很大程度上未知。在正常生理条件下,低氧会导致肺循环早期肺血管管径变化和后期肺血管重构,以及可能出现低氧性肺动脉高压等[1]。其中肺血管平滑肌细胞的异常增殖是肺血管重构的主要原因之一[2]。因此,本研究选用成年牦牛肺动脉平滑肌细胞为牦牛适应性调节蛋白奠定基础,以及为提高人或动物低氧损伤带来疾病的药物的研发提供一定基础数据。
同位素标记相对和绝对定量串联质谱标签(tandem mass tag, TMT)技术是一种新型多肽体外标记技术。现在TMT技术已经是探索动物生长发育、动物疾病调控机制以及了解细胞机体活动规律的应用技术手段。具有高通量、重复性高以及定量准确等特性[3-7]。研究表明,低氧条件下(1% O2)的蛋白质组学分析揭示了细胞代谢的变化,包括能量代谢、线粒体活性和脂质代谢相关的蛋白质[8]。更有研究者验证,在低氧培养条件下(1% O2浓度)培养细胞48 h后,观察到细胞的增殖抑制作用,但是随着培养时间的增加(72 h)会引起相反的效果,细胞增殖率增加[9]。因此,本研究旨在探索对低氧(1% O2)和常氧(20% O2)条件下培养的PASMCs采用TMT结合液相色谱-质谱(LC-MS/MS)技术检测差异表达蛋白(DEPs),从而进行DEPs的作用分析,以便对低氧与常氧下PASMCs的DEPs有广泛而全面的了解。
1 材料与方法 1.1 试验材料从青海省西宁某屠宰场采集成年牦牛肺动脉血管,置于25 ℃左右的生理盐水中,尽快带回实验室。用生理盐水和PBS分别冲洗3次,将PASMCs进行原代培养,之后将PASMCs分为低氧组(1% O2)和常氧组(20% O2),培养72 h。每组设3个重复。
1.2 SDT裂解法提取蛋白及SDS-PAGE电泳取低氧和常氧条件下培养的细胞样品并加入适量SDT裂解液, 采用超声处理, 沸水浴15 min, 14 000×g离心15 min,取上清后进行蛋白质定量。将保存样品各取蛋白质20 μg,分别加入6×上样缓冲液,沸水浴5 min,进行12% SDS-PAGE电泳(恒压250 V,40 min)。
1.3 肽段标记及高pH反向分离将样品进行FASP酶解,各酶解样品分别取100 μg肽段,按照TMT标记试剂盒(赛默飞世尔科技公司,90064CH)进行标记。将标记后的肽段混合后进行分级。色谱柱以100% A液(10 mmol·L-1 HCOONH4, 5% ACN, pH=10.0)平衡,分离梯度见表 1。洗脱过程吸光值为214 nm,每隔1 min收集一次,共收集洗脱组分约40份。将样品冻干后用0.1% FA复溶合并为10份。
|
|
表 1 高pH分离梯度 Table 1 High pH separation gradient |
每份样品采用纳升流速Easy nLC系统进行分离。缓冲液A液(0.1%甲酸水溶液)以100%平衡。样品由自动进样器进入分析柱进行分离。洗脱梯度见表 2。
|
|
表 2 液相色谱洗脱梯度 Table 2 Liquid chromatography elution gradients |
将得到的原始数据按照ScoreSequestHT>0且uniquepeptide≥1条件去除空白值,筛选可信蛋白。在筛选的可信蛋白基础上,根据FC(fold change)≥1.3或≤0.78,且P<0.05条件进行DEPs筛选。使用Blast2GO软件对鉴定的DEPs进行GO分析,分别从3个方面描述差异蛋白质的属性:包括参与的生物过程、分子功能和细胞组分。对DEPs进行KEGG通路富集分析其主要参与的信号通路,并应用Interpro数据库对DEPs进行功能结构域注释分析。
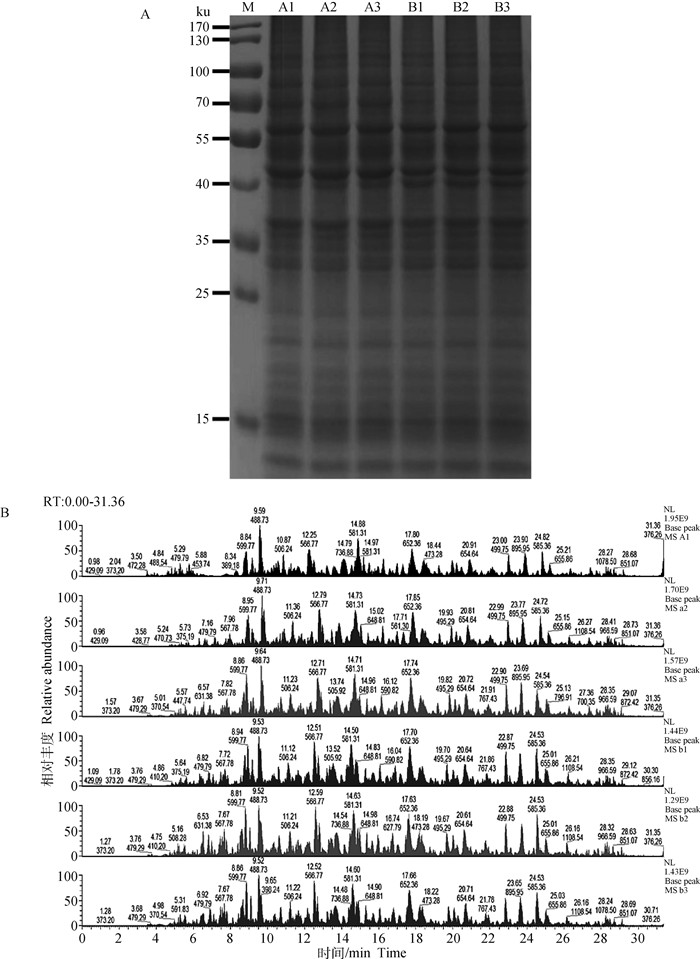
2 结果 2.1 蛋白样本质控经BCA法测定各组蛋白浓度,各样本蛋白浓度均大于5 g·L-1。取一定量蛋白样本进行SDS-PAGE,经考马斯亮蓝染色,结果显示各样本电泳条带均清晰丰富,分布均匀,且重复性好,可以满足下一步TMT标记蛋白组学试验要求(图 1A)。图 1B为质谱分析BasePeak图谱,主要反映样品的色谱分离度、肽段信号强度以及样品中蛋白质的构成复杂程度等。如果从色谱图上看到在不同时间洗脱的峰较多,而且相对丰度较高,则说明样品中的肽段种类较多,复杂程度较高。另一方面,可以通过比较不同样品信号峰的分布情况以及质谱信号强度的相似度,来判断样品酶解后肽段的平行性,从而为各样品间平行性的判断提供依据,如图 1B所示色谱质谱行为正常,组内和组间的所有样本平行性好。
|
A. 样品SDS-PAGE电泳图:每个样品使用5 μg蛋白质进行检测, 并用考马斯亮蓝进行染色; M. 蛋白质相对分子质量标准; A1、A2、A3表示低氧组(1% O2)3个重复; B1、B2、B3表示常氧组(20% O2)3个重复。B.样品质谱峰图:横坐标为肽段在色谱中的保留时间,纵坐标为质谱信号强度;主要峰上的数字标记分别为信号峰强度最高的肽段的保留时间和质荷比 A. SDS-PAGE of the sample: 5 μg protein of each sample was used for isolation, and the proteins were stained with Coomassie brilliant blue; M. Molecular weight of protein marker; A1, A2 and A3 represent 3 replicates in the hypoxia group (1% O2); B1, B2 and B3 represent 3 replicates in normoxia group (20% O2). B. Sample quality spectrum peak: The abscissa is the retention time of the peptide in the chromatography, the ordinate is the mass spectrum signal intensity; The digital markers on the main peaks are the retention time and mass charge ratio of the peptide with the highest signal peak intensity 图 1 定量蛋白质组学分析中蛋白质样品的鉴定 Fig. 1 Identification of protein samples in quantitative proteomic analysis |
通过TMT蛋白定量技术和串联质谱分析,将得到的原始数据按照ScoreSequestHT>0且uniquepeptide≥1条件去除空白值,筛选可信蛋白。得到谱图总数为488 006个,肽段谱图数(peptide spectrum match, PSM)为116 752个,肽段总数为70 444个,唯一肽段总数为59 583个,蛋白质总数为7 600种(表 3)。
|
|
表 3 串联质谱法测定蛋白质样品的定量结果 Table 3 Quantitative results of protein samples determined by tandem mass spectrometry |
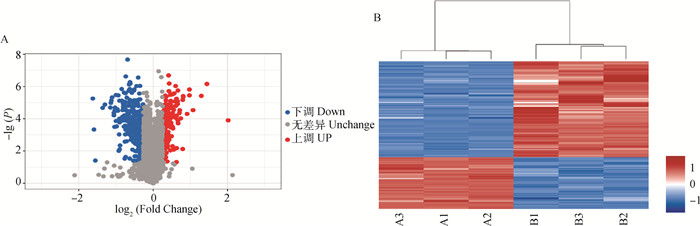
在两组样品中筛选的可信蛋白基础上,根据FC(fold change)≥1.3或≤0.78,且P<0.05条件进行DEPs筛选。共鉴定了6 859种蛋白质,其中差异比较组所有差异表达蛋白质有531种,上调差异表达蛋白质有186种,下调差异表达蛋白质有345种(图 2A)。差异表达蛋白聚类图(图 2B)显示,两种条件的样品生物学重复性较好。
|
A. DEPs火山图:横坐标表示Fold Change差异表达倍数值,纵坐标表示P值以10为底取负对数值;红色散点表示上调蛋白(186个),蓝色散点表示下调蛋白(345个),灰色散点表示没有变化蛋白。B. DEPs聚类热图:每列代表一组样品,其中红色代表显著性上调蛋白,蓝色代表显著性下调蛋白,灰色部分代表无蛋白信息;横坐标为样品信息,纵坐标为显著性差异表达蛋白;A1、A2、A3表示低氧组(1% O2)3个重复组, B1、B2、B3表示常氧组(20% O2)3个重复组 A. DEPs volcano figure: The abscissa represents the differential expression multiple value of Fold Change, and the ordinate represents the negative log value of P value to the base 10; Red scatter dots show upregulated proteins (186), blue scatter dots represent down-regulated proteins (345), grey scatter dots indicate no changing protein. B. DEPs clustering heat map: Each column represents a group of samples, in which red represents significantly up-regulated protein, blue represents significantly down-regulated protein, and gray represents no protein information; the abscissa is the sample information, and the ordinate is the significantly differentially expressed protein; A1, A2 and A3 represent 3 replicates in the hypoxia group (1% O2), while B1, B2 and B3 represent 3 replicates in the normoxia group (20% O2) 图 2 低氧与常氧条件下牦牛PASMCs比较蛋白质组学分析 Fig. 2 Proteomic analysis in PASMCs of yaks under hypoxia and normoxia conditions |
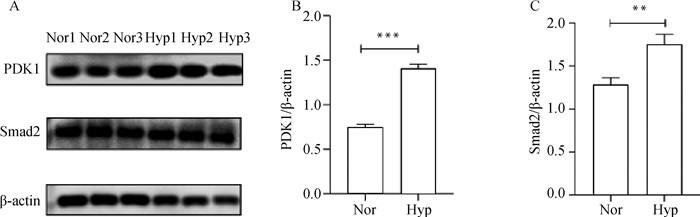
将筛选出的DEPs随机挑选出进行免疫蛋白印迹验证(图 3),结果与TMT检测结果一致。说明该技术具有可靠性。
|
A.PDK1和Smad2蛋白检测结果; B. PDK1蛋白相对表达量; C. Smad2蛋白相对表达量。内参蛋白为β-actin; Nor表示常氧组,Hyp表示低氧组;**.P<0.01 A. PDK1 and Smad2 protein detection results; B. Relative expression of PDK1 protein; C. Relative expression of Smad2 protein. β-actin is internal reference protein; Nor stands for normoxia group, Hyp stands for hypoxia group; **. P<0.01 图 3 DEPs的Western blot验证 Fig. 3 Western blot analysis of DEPs |
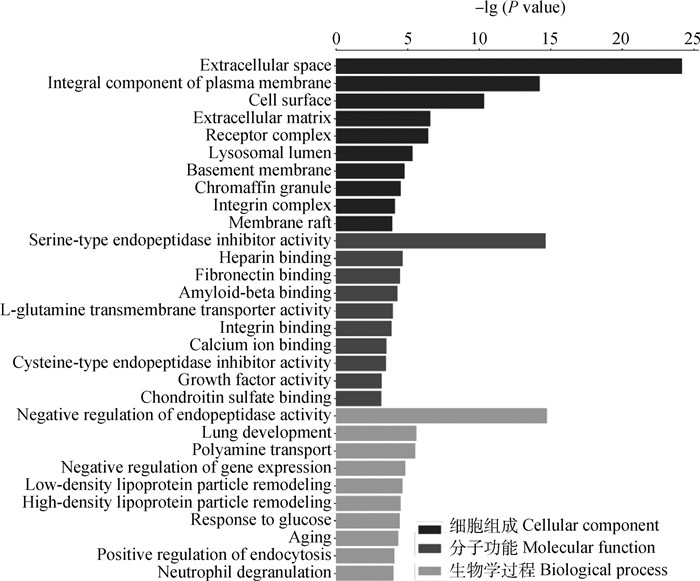
为了进一步了解低氧与常氧下DEPs的生物学意义,通过GO功能注释和KEGG通路富集对DEPs进行富集分析。GO条目被分为细胞组成(cellular component, CC)、分子功能(molecular function, MF)和生物学过程(biological process, BP)3个部分(图 4),其中差异表达蛋白富集数量最多的几个类别为细胞外空隙(extracellular space),质膜的组成部分(integral component of plasma membrane),细胞表面(cell surface),丝氨酸型内肽酶抑制剂活性(serine-type endopeptidase inhibitor activity),肝素结合(heparin binding),钙离子结合(calcium ion binding),内肽酶活性的负调控(negative regulation of endopeptidase activity),衰老(aging),嗜中性粒细胞脱颗粒(neutrophil degranulation)。
|
横坐标表示P值以10为底取负对数值,纵坐标表示GO功能注释名称 The abscissa represents the negative log of P to the base 10, and the vertical axis represents the GO functional annotation names 图 4 GO富集分析图 Fig. 4 GO enrichment analysis diagram |
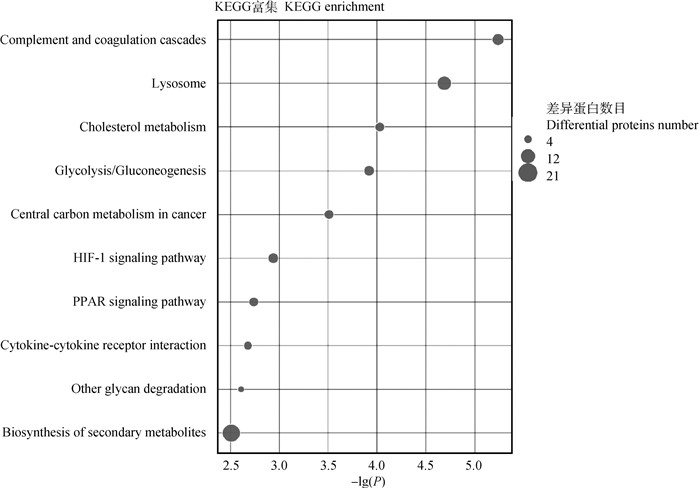
进一步KEGG通路富集数据分析,图中显示了富集前10的通路(图 5),结果表明,显著DEPs富集在次生代谢产物的生物合成(biosynthesis of secondary metabolites;21种蛋白),溶酶体(lysosome;14种蛋白),补体系统(complement and coagulation cascades;10种蛋白),HIF-1信号通路(HIF-1 signaling pathway;8种蛋白),糖酵解与糖代谢合成(glycolysis/gluconeogenesis;8种蛋白),胆固醇代谢(cholesterol metabolism;7种蛋白),中枢碳代谢(central carbon metabolism in cancer;7种蛋白),PPAR信号通路(PPAR signaling pathway;7种蛋白),细胞因子受体相互作用(cytokine-cytokine receptor interaction;6种蛋白),其他多糖降解(other glycan degradation;4种蛋白)。
|
横坐标表示P值以10为底取负对数值,纵坐标表示KEGG通路名称 The abscissa represents the negative log of P value to the base 10, and the ordinate indicates the KEGG pathway names 图 5 KEGG富集分析图 Fig. 5 KEGG enrichment analysis diagram |
通过对DEPs功能分析,在次生代谢产物的生物合成、细胞因子受体相互作用、糖酵解与糖代谢合成、HIF-1信号通路中筛选出7个与低氧适应性相关的DEPs,见表 4。
|
|
表 4 重要DEPs筛选 Table 4 Screening of important DEPs |
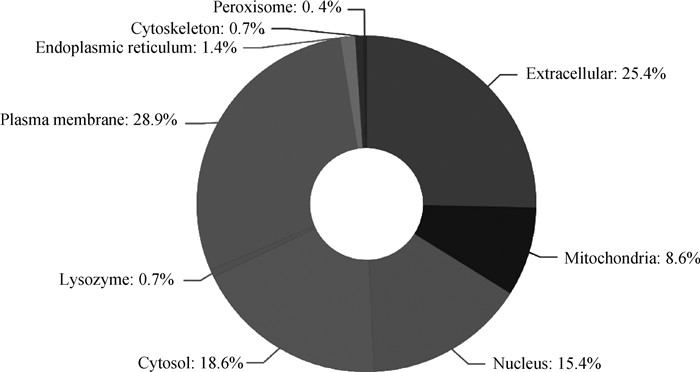
亚细胞定位是指某种蛋白或表达产物在细胞内的具体存在部位,蛋白质的亚细胞定位是进行蛋白质功能研究的重要信息,蛋白质合成后只有被转运到正确的细胞器中才能参与各项生命活动,并有效地发挥功能。因此,本研究使用软件WoLF PSORT对差DEPs进行亚细胞定位分析。结果显示(图 6),质膜(plasma membrane)占28.9%,细胞外基质(extracellular)占25.4%,细胞溶质(cytosol)占18.6%,细胞核(nucleus)占15.4%。
|
图 6 差异蛋白质亚细胞定位分析图 Fig. 6 Subcellular localization analysis of differential proteins |
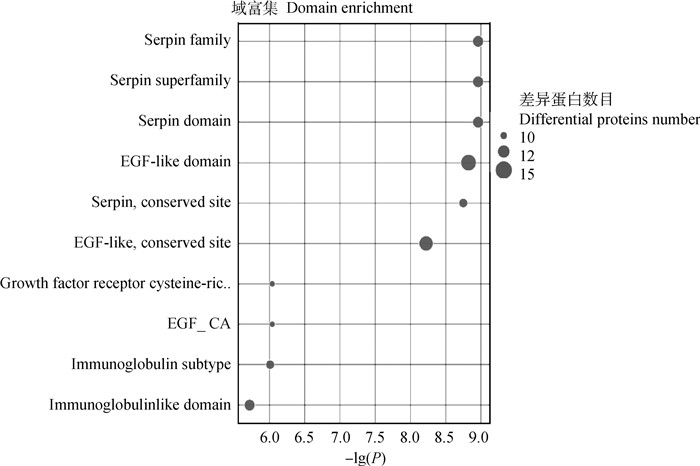
结构域是蛋白质结构、功能和进化的单位。研究蛋白质的结构域对于理解蛋白质的生物学功能及其进化具有重要的意义。因此,本研究采用Interpro资源数据库分析差异蛋白质的功能结构域的注释及富集情况。DEPs结构域结果显示(图 7),富集在表皮生长因子样结构域(EGF-like domain)的DEPs比较多。
|
横坐标表示P值以10为底取负对数值,纵坐标表示结构域名称 The abscissa represents the negative log of P value to the base 10, and the ordinate indicates the domain names 图 7 差异蛋白质结构域分析图 Fig. 7 Domains analysis of differential proteins |
TMT技术用于定量蛋白质组学,具有高通量和高重现性。在这项研究中,基于TMT技术以牦牛PASMCs作为研究对象,对在低氧和常氧不同培养条件下的DEPs进行筛选,并进行生物学信息分析。结果表明,经TMT分析,在比较低氧和常氧下的PASMCs的2组蛋白样品中共获得6 859个蛋白,以FC≥1.3或≤0.78,且P<0.05条件进行筛选,获得DEPs共531个,其中上调DEPs有186个,下调DEPs有345个。分别富集在糖代谢、酶活性等低氧适应相关类别上。这些DEPs主要富集在中枢碳代谢、糖酵解与糖代谢合成、HIF-1信号通路、次生代谢产物的生物合成等低氧适应相关通路中。推测这些DEPs给机体的提供氧和能量在低氧下起重要作用。低氧状态下通过诱导激活HIF-1α对局部组织和细胞的缺氧、缺血,并改变糖代谢和氧代谢的平衡[10]来维持机体的生理稳态,并且通过氧化应激和自噬调节来维持组织细胞生存。
本研究通过GO与KEGG对DEPs进行功能富集和分析,其中在次生代谢产物的生物合成、细胞因子受体相互作用、糖酵解与糖代谢合成、HIF-1信号通路中筛选出7个重要的DEPs(PGK、HK、LDH、PGAM、pfkA、IL6ST、PDK1),在缺氧状态下以糖酵解作为主要的供能方式。磷酸甘油激酶(phosphoglycerate kinase, PGK)是一种胞内蛋白,是产生能量的糖酵解酶,可催化1, 3-二磷酸甘油酸与ADP的可逆转移,产生3-磷酸甘油酸和ATP[11]。PGK不仅在协调糖酵解和三羧酸循环中发挥重要作用,而且还作为二硫化物还原酶参与血管生成过程[12]。PGK可被O-GlcNAc糖基化激活并诱导至线粒体。在线粒体内PGK作为一种激酶激活丙酮酸脱氢酶激酶1(PDHK1)磷酸化并抑制丙酮酸脱氢酶(PDH)复合物,降低线粒体丙酮酸利用率以减少氧化磷酸化,同时增加乳酸的产生[13]。在低氧条件下,HIF-1α上调PGK的表达, 利用糖酵解途径为机体提供能量, 并参与血管生成过程,满足机体活动需要,进而适应外界环境[14-15]。己糖激酶(hexokinases, HK)将葡萄糖转化为葡萄糖-6-磷酸,是调节糖酵解的关键酶[16]。缺氧条件下HK的上调增强了糖酵解,增加了细胞的自噬和上皮-间质转化[17]。己糖激酶的上调可通过与线粒体外膜结合促进糖酵解[18],为细胞代谢提供ATP[19]。乳酸脱氢酶(lactate dehydrogenase, LDH)有LDHA和LDHB两种亚型,在低氧条件下,LDHA通过HIF信号调节TME, HIF-1α或HIF-2α均与LDH启动子中的HRE-D相互作用,LDH的上调表达提高了糖酵解水平和细胞代谢能力[20-21]。在缺氧微环境中,HIF-1α可以通过稳定上调HK、LDH、PGK等糖酵解酶的表达促进葡萄糖吸收/糖酵解和线粒体代谢,为细胞提供能量[22]。磷酸甘油酸变位酶(phosphoglycerate Mutase, PGAM)是一种参与糖酵解过程的关键酶,可以通过将3-磷酸甘油酸转变为2-磷酸甘油酸促进糖酵解[23],在低氧下,HIF可诱导PGAM表达并与其启动子区低氧反应原件结合发挥转录调控作用,从而导致PGAM活性升高[24],进而促进糖酵解。磷酸果糖激酶(phosphofructose kinase, pfkA)是细胞糖酵解代谢途径中的限速酶,可以催化果糖-6-磷酸发生磷酸化变成果糖-1, 6-二磷酸。近几年的研究表明,在低氧微环境中pfkA表达上调[25-26],促进细胞代谢,给机体提供能量。白介素6信号传感器(IL6ST)在低氧下显著下调,近几年研究发现IL6ST为抑癌蛋白[27-28],可用于肿瘤诊断和治疗。已成为多种疾病治疗的潜在新靶点,抑制IL6ST表达在肿瘤的治疗中具有重要作用。目前已经开发出抑制IL6ST的方法并进行了临床试验[29-30],但通过缺氧抑制该蛋白表达尚未见报道。丙酮酸脱氢酶激酶(pyruvate dehydrogenase kinase, PDK) 是糖酵解过程中重要的调节酶,在调节体内葡萄糖和脂肪酸代谢过程中发挥着至关重要的作用[31]。研究发现,HIF-1α可上调PDK1的表达,促使肿瘤细胞的糖酵解[32-33]。近年来发现,PDK1特异性地在肿瘤细胞中高表达[34],有可能成为肿瘤治疗的新靶点[35-38]。本试验也采用WB验证了PDK1在低氧和常氧下的蛋白表达量,结果显示,PDK1在低氧和常氧下的表达量差异极显著(图 3)。
随着组学技术的发展,目前对于物种的适应性研究分析也越来越多,在本研究中筛选出的PGK、HK、LDH和PGAM与前期研究低氧胁迫杂交鱼筛选的蛋白(PGK、HK、LDH和PGAM)一致,在低氧和常氧条件下的表达差异性较大[39],证实了本试验的准确性。本试验筛选的pfkA与前期低氧对大鼠肝脂代谢的试验结果一致,低氧和常氧条件下pfkA表达的差异性比较显著[40]。对于本试验筛选的IL6ST和PDK1,目前在癌症的研究中较多,也有研究证实它们与低氧相关[41-42],但是具体在低氧下的机制尚未明确。
4 结论本研究采用TMT技术对缺氧和常氧条件下牦牛PASMCs的DEPs进行筛选和功能分析。结果表明,DEPs分别富集在代谢、酶活性、低氧适应性等相关类别与通路中。在中枢碳代谢、糖酵解与糖代谢合成、HIF-1信号通路、次生代谢产物的生物合成等低氧适应相关通路中发现7个重要的DEPs(PGK、HK、LDH、PGAM、pfkA、IL6ST、PDK1),可能与低氧环境的适应有关。本研究是探索牦牛PASMCs在低氧下适应性差异蛋白的初步试验,这些结果为进一步研究牦牛低氧适应性提供了基础数据。
| [1] |
黄佳, 魏新川. 慢性低氧对肺循环作用的研究进展[J]. 实用医院临床杂志, 2021, 18(3): 191-193. HUANG J, WEI X C. Advances in pulmonary circulation effects of chronic hypoxia[J]. Practical Journal of Clinical Medicine, 2021, 18(3): 191-193. DOI:10.3969/j.issn.1672-6170.2021.03.057 (in Chinese) |
| [2] |
阳一栋. 内质网-线粒体相关膜在低氧诱导的平滑肌细胞增殖和内皮细胞损伤中的作用及机制研究[D]. 重庆: 中国人民解放军陆军军医大学, 2019. YANG Y D. The role of MAMs in hypoxia-induced smooth muscle cell proliferation and endothelial cell injury[D]. Chongqing: Army Medical University, 2019. (in Chinese) |
| [3] |
彭梦玲, 胡文业, 李乃馨, 等. 组学技术分析肉鸡胚胎发育过程中肝脏蛋白表达的变化[J]. 畜牧兽医学报, 2020, 51(2): 252-259. PENG M L, HU W Y, LI N X, et al. The changes of hepatic proteins during chicken embryonic development based on proteomics analysis[J]. Acta Veterinaria et Zootechnica Sinica, 2020, 51(2): 252-259. (in Chinese) |
| [4] |
SA R N, XING H, LUAN S J, et al. Atmospheric ammonia alters lipid metabolism-related genes in the livers of broilers (Gallus gallus)[J]. J Anim Physiol Anim Nutr (Berl), 2018, 102(2): e941-e947. DOI:10.1111/jpn.12859 |
| [5] |
徐梦飞, 卢平萍, 马勋, 等. 细粒棘球绦虫蛋白质组学研究进展[J]. 畜牧兽医学报, 2018, 49(3): 466-476. XU M F, LU P P, MA X, et al. Advances in Echinococcus granulosus proteomics[J]. Acta Veterinaria et Zootechnica Sinica, 2018, 49(3): 466-476. (in Chinese) |
| [6] |
熊火, 蔡欣, 刘静波, 等. 高脂饲粮对生长育肥猪肉品质和骨骼肌蛋白质组的影响[J]. 畜牧兽医学报, 2016, 47(10): 2052-2059. XIONG H, CAI X, LIU J B, et al. Effects of high-fat diet on meat quality traits and skeletal muscle proteome of growing-finishing pigs[J]. Acta Veterinaria et Zootechnica Sinica, 2016, 47(10): 2052-2059. DOI:10.11843/j.issn.0366-6964.2016.10.014 (in Chinese) |
| [7] |
赵畅, 张江, 白云龙, 等. 基于iTRAQ技术的卵巢静止奶牛血清差异蛋白分析[J]. 畜牧兽医学报, 2019, 50(5): 972-982. ZHAO C, ZHANG J, BAI Y L, et al. Analysis of serum differential proteins in cows with inactive ovaries based on iTRAQ technology[J]. Acta Veterinaria et Zootechnica Sinica, 2019, 50(5): 972-982. (in Chinese) |
| [8] |
GUGLIANDOLO A, DIOMEDE F, SCIONTI D, et al. The role of hypoxia on the neuronal differentiation of gingival mesenchymal stem cells: a transcriptional study[J]. Cell Transplant, 2019, 28(5): 538-552. DOI:10.1177/0963689718814470 |
| [9] |
BURAVKOVA L B, ANDREEVA E R, GOGVADZE V, et al. Mesenchymal stem cells and hypoxia: where are we?[J]. Mitochondrion, 2014, 19: 105-112. DOI:10.1016/j.mito.2014.07.005 |
| [10] |
HIRAI K, FURUSHO H, HIROTA K, et al. Activation of hypoxia-inducible factor 1 attenuates periapical inflammation and bone loss[J]. Int J Oral Sci, 2018, 10(2): 12. DOI:10.1038/s41368-018-0015-0 |
| [11] |
LI F X, ZHANG Y S, YAO C L. Characterization and role of PGK from Litopenaeus vannamei in WSSV infection[J]. Fish Shellfish Immunol, 2019, 93: 144-152. DOI:10.1016/j.fsi.2019.07.048 |
| [12] |
LAY A J, JIANG X M, KISKER O, et al. Phosphoglycerate kinase acts in tumour angiogenesis as a disulphide reductase[J]. Nature, 2000, 408(6814): 869-873. DOI:10.1038/35048596 |
| [13] |
NIE H, JU H X, FAN J Y, et al. O-GlcNAcylation of PGK1 coordinates glycolysis and TCA cycle to promote tumor growth[J]. Nat Commun, 2020, 11(1): 36. DOI:10.1038/s41467-019-13601-8 |
| [14] |
WILLSON J A, ARIENTI S, SADIKU P, et al. Neutrophil HIF-1α stabilization is augmented by mitochondrial ROS produced via the glycerol 3-phosphate shuttle[J]. Blood, 2022, 139(2): 281-286. DOI:10.1182/blood.2021011010 |
| [15] |
DUNCAN L, SHAY C, TENG Y. PGK1: an essential player in modulating tumor metabolism[M]//GUEST P C. Physical Exercise and Natural and Synthetic Products in Health and Disease. New York: Humana, 2022: 57-70.
|
| [16] |
张凯, 郭明洲, 张志谦. 有氧糖酵解在胰腺癌中的研究[J]. 胃肠病学和肝病学杂志, 2021, 30(7): 735-739, 747. ZHANG K, GUO M Z, ZHANG Z Q. Research of aerobic glycolysis in pancreatic cancer[J]. Chinese Journal of Gastroenterology and Hepatology, 2021, 30(7): 735-739, 747. DOI:10.3969/j.issn.1006-5709.2021.07.004 (in Chinese) |
| [17] |
CHEN G H, ZHANG Y D, LIANG J F, et al. Deregulation of hexokinase II is associated with glycolysis, autophagy, and the epithelial-mesenchymal transition in tongue squamous cell carcinoma under hypoxia[J]. Biomed Res Int, 2018, 2018: 8480762. |
| [18] |
樊知桐. 己糖激酶II与Warburg效应[J]. 临床与病理杂志, 2016, 36(12): 2053-2059. FAN Z T. Hexokinase-II and Warburg effect[J]. Journal of Clinical and Pathological Research, 2016, 36(12): 2053-2059. DOI:10.3978/j.issn.2095-6959.2016.12.030 (in Chinese) |
| [19] |
HONG C F, ZHUANG H E, CAI B R, et al. β-Elemene attenuates fibrosis after esophageal endoscopic submucosal dissection via modulating the HIF-1α/HK2/p38-MAPK signaling axis[J]. ACS Biomater Sci Eng, 2021, 7(7): 3399-3408. DOI:10.1021/acsbiomaterials.1c00047 |
| [20] |
KHAN J, NORDBACK I, SAND J. Serum lipid levels are associated with the severity of acute pancreatitis[J]. Digestion, 2013, 87(4): 223-228. DOI:10.1159/000348438 |
| [21] |
HOU T L, MA H P, WANG H X, et al. Sevoflurane preconditioning attenuates hypoxia/reoxygenation injury of H9c2 cardiomyocytes by activation of the HIF-1/PDK-1 pathway[J]. PeerJ, 2020, 8: e10603. DOI:10.7717/peerj.10603 |
| [22] |
AHMAD S S, GLATZLE J, BAJAEIFER K, et al. Phosphoglycerate kinase 1 as a promoter of metastasis in colon cancer[J]. Int J Oncol, 2013, 43(2): 586-590. DOI:10.3892/ijo.2013.1971 |
| [23] |
MIKAWA T, SHIBATA E, SHIMADA M, et al. Phosphoglycerate mutase cooperates with Chk1 kinase to regulate glycolysis[J]. Iscience, 2020, 23(7): 101306. DOI:10.1016/j.isci.2020.101306 |
| [24] |
崔国岐. PGAM1在肺动脉高压中的作用与机制研究[D]. 杨凌: 西北农林科技大学, 2021. CUI G Q. The role and mechanism of PGAM1 in pulmonary hypertension[D]. Yangling: Northwest A & F University, 2021. (in Chinese) |
| [25] |
刘鹏. 6-磷酸果糖-2-激酶3在胃癌中的表达及对胃癌细胞生长和凋亡的影响研究[D]. 南昌: 南昌大学, 2020. LIU P. Study on the expression of PFKFB3 in gastric cancer and its effect on the growth and apoptosis of gastric cancer cells[D]. Nanchang: Nanchang University, 2020. (in Chinese) |
| [26] |
高瑞芳, 荀敬, 李宁昕, 等. 6-磷酸果糖-2-激酶/果糖-2, 6-二磷酸酶4对癌细胞的增殖及迁移的影响及机制研究[J]. 中国中西医结合外科杂志, 2021, 27(3): 503-508. GAO R F, XUN J, LI N X, et al. PFKFB4 promotes the proliferation and migration of ovarian cancer cells through regulating glycolysis[J]. Chinese Journal of Surgery of Integrated Traditional and Western Medicine, 2021, 27(3): 503-508. DOI:10.3969/j.issn.1007-6948.2021.03.025 (in Chinese) |
| [27] |
JIA R, WENG Y J, LI Z X, et al. Bioinformatics analysis identifies IL6ST as a potential tumor suppressor gene for triple-negative breast cancer[J]. Reprod Sci, 2021, 28(8): 2331-2341. DOI:10.1007/s43032-021-00509-2 |
| [28] |
MARTÍNEZ-PÉREZ C, LEUNG J, KAY C, et al. The signal transducer IL6ST (gp130) as a predictive and prognostic biomarker in breast cancer[J]. J Pers Med, 2021, 11(7): 618. DOI:10.3390/jpm11070618 |
| [29] |
WANG X, DING Y Y, CHEN Y, et al. MiR-223-3p alleviates vascular endothelial injury by targeting IL6ST in Kawasaki disease[J]. Front Pediatr, 2019, 7: 288. DOI:10.3389/fped.2019.00288 |
| [30] |
WANG M, ZHANG H L, YANG F, et al. miR-188-5p suppresses cellular proliferation and migration via IL6ST: a potential noninvasive diagnostic biomarker for breast cancer[J]. J Cell Physiol, 2020, 235(5): 4890-4901. DOI:10.1002/jcp.29367 |
| [31] |
DI R M, YANG Z Z, XU P, et al. Silencing PDK1 limits hypoxia-induced pulmonary arterial hypertension in mice via the Akt/p70S6K signaling pathway[J]. Exp Ther Med, 2019, 18(1): 699-704. |
| [32] |
SLOMINSKI A, KIM T K, BROZ·YNA A A, et al. The role of melanogenesis in regulation of melanoma behavior: melanogenesis leads to stimulation of HIF-1α expression and HIF-dependent attendant pathways[J]. Arch Biochem Biophys, 2014, 563: 79-93. DOI:10.1016/j.abb.2014.06.030 |
| [33] |
OGNIBENE M, CANGELOSI D, MORINI M, et al. Immunohistochemical analysis of PDK1, PHD3 and HIF-1α expression defines the hypoxic status of neuroblastoma tumors[J]. PLoS One, 2017, 12(11): e0187206. DOI:10.1371/journal.pone.0187206 |
| [34] |
PAI S, YADAV V K, KUO K T, et al. PDK1 inhibitor BX795 improves cisplatin and radio-efficacy in oral squamous cell carcinoma by downregulating the PDK1/CD47/Akt-mediated glycolysis signaling pathway[J]. Int J Mol Sci, 2021, 22(21): 11492. DOI:10.3390/ijms222111492 |
| [35] |
JIANG Q W, ZHENG N N, BU L, et al. SPOP-mediated ubiquitination and degradation of PDK1 suppresses AKT kinase activity and oncogenic functions[J]. Mol Cancer, 2021, 20(1): 100. DOI:10.1186/s12943-021-01397-5 |
| [36] |
YAO S S, SHANG W W, HUANG L, et al. The oncogenic and prognostic role of PDK1 in the progression and metastasis of ovarian cancer[J]. J Cancer, 2021, 12(3): 630-643. DOI:10.7150/jca.47278 |
| [37] |
KITAMURA S, YAMAGUCHI K, MURAKAMI R, et al. PDK2 leads to cisplatin resistance through suppression of mitochondrial function in ovarian clear cell carcinoma[J]. Cancer Sci, 2021, 112(11): 4627-4640. DOI:10.1111/cas.15125 |
| [38] |
许晓巍, 赵初娴, 李肃, 等. HIF-1α-PDK1信号系统参与急性单核细胞白血病糖代谢和耐药的机制研究[J]. 上海交通大学学报: 医学版, 2018, 38(11): 1294-1299. XU X W, ZHAO C X, LI S, et al. Mechanism of HIF-1α-PDK1 signaling system involved in glucose metabolism and drug resistance in acute monocytic leukemia[J]. Journal of Shanghai Jiaotong University: Medical Science, 2018, 38(11): 1294-1299. DOI:10.3969/j.issn.1674-8115.2018.11.004 (in Chinese) |
| [39] |
裴雪莹. 杂交黄颡鱼"黄优1号"应对低氧胁迫的生理响应及基因表达研究[D]. 南京: 南京师范大学, 2020. PEI X Y. Physiological response and gene expression of hybrid Yellow Catfish "Huangyou 1" to hypoxia stress[D]. Nanjing: Nanjing Normal University, 2020. (in Chinese) |
| [40] |
孟昶. 低氧训练对肥胖大鼠肝脏脂代谢关联基因及血清外泌体的影响[D]. 曲阜: 曲阜师范大学, 2020. MENG C. Effects of hypoxia training on genes associated with liver lipid metabolism and serum exosomes in obese rats[D]. Qufu: Qufu Normal University, 2020. (in Chinese) |
| [41] |
PENG F, WANG J H, FAN W J, et al. Glycolysis gatekeeper PDK1 reprograms breast cancer stem cells under hypoxia[J]. Oncogene, 2018, 37(8): 1062-1074. DOI:10.1038/onc.2017.368 |
| [42] |
BOSCO M C, PUPPO M, SANTANGELO C, et al. Hypoxia modifies the transcriptome of primary human monocytes: modulation of novel immune-related genes and identification of CC-chemokine ligand 20 as a new hypoxia-inducible gene[J]. J Immunol, 2006, 177(3): 1941-1955. DOI:10.4049/jimmunol.177.3.1941 |
(编辑 郭云雁)



