2. 晋城市农业农村局现代农业发展中心, 晋城 048000
2. The Center for Modern Agricultural Development, Bureau of Agriculture and Rural Affairs of Jincheng City, Jincheng 048000, China
动物体脂肪的合成与沉积对于胴体品质有重要影响,脂肪组织的合成需要经过前体脂肪细胞数目、体积的增加,细胞的融合分化等过程。其中脂肪细胞的分化受到多条信号通路调控,每条通路都包含多种转录因子,通过各种途径调控脂肪细胞的分化[1]。过氧化物酶体增殖物激活受体(peroxisome proliferators-activated receptors, PPARs)家族、脂肪酸结合蛋白(fatty acid-binding protein, FABP)、CCAAT/增强子结合蛋白(CCAAT /enhancer binding protein, C/EBP)、类固醇调节元件结合蛋白(sterol regulatory element binding protein, SREBP)等均为公认的与脂肪形成相关的转录因子,其中过氧化物酶体增殖物激活受体γ(peroxisome proliferator-activated receptor γ,PPARγ)是最熟知的转录调控因子[2]。碳水化合物反应元件结合蛋白(carbohydrate response element binding protein,ChREBP)也是与脂肪合成相关的转录因子,还能调控脂肪细胞的分化,对于脂质代谢和葡萄糖转运也有重要意义[3]。脂肪酸合成酶(fatty acid synthase,FAS)主要参与脂肪酸的合成代谢,通过调节甘油三酯的含量影响动物机体脂质的沉积[4]。
肝X受体(liver X receptors,LXRs)属于核受体超家族成员,受氧化型胆固醇调节[5-6],在脂质代谢中是一个不可缺少的调节因子[7-9]。核受体亚家族1H成员3(nuclear receptor subfamily 1 group H member 3,NR1H3)属于LXRs的一员,也称肝X受体α(liver X receptor α,LXRα),在人和多种动物组织中广泛表达,在肝、肾中的表达量较多,主要作为胆固醇的感受器,通过调控下游基因来影响胆固醇的合成与转化[10]。此外,NR1H3在碳水化合物、脂蛋白代谢等方面也发挥重要作用[9, 11-13]。敲除NR1H3的小鼠血脂含量偏低,脂肪酸和甘油三酯的表达受到影响,脂肪形成能力减弱,证明NR1H3参与到小鼠的成脂过程,对于脂质代谢过程的稳定运转具有重要意义[14-15]。NR1H3在鸭原代肝细胞中过表达后,高密度脂蛋白和三酰甘油表达量增加[16]。在脂肪肝细胞中敲低NR1H3后,三酰甘油含量降低,脂肪酸的合成过程受到影响[17]。这都说明NR1H3对于脂质合成、脂质代谢、脂肪沉积等过程具有重要意义。
目前,有关NR1H3基因在脂肪细胞脂质积累中作用的研究主要集中在小鼠和山羊等物种中,其对猪脂肪细胞中脂质积累的调控作用研究较少。本研究在探究NR1H3基因在猪皮下脂肪组织中的发育性表达规律的基础上,构建猪NR1H3过表达载体并筛选有效的猪NR1H3 siRNA,转染猪前体脂肪细胞并诱导其分化,采用油红O染色、qRT-PCR及Western blot技术检测脂滴形成情况及成脂分化关键基因的表达变化,探究NR1H3基因对猪前体脂肪细胞分化及脂肪生成的影响,为阐明猪NR1H3基因的功能及作用机制奠定理论基础。
1 材料与方法 1.1 试验动物来源于大同市种猪场的30、90和240日龄的马身猪各4头,同一阶段的个体按要求饲养,达到目标日龄时屠宰,采集背部脂肪组织,用于NR1H3基因发育性表达规律研究。5日龄杜长大仔猪(公)来源于山西省天禄丰种猪育种有限公司,屠宰后采集背部脂肪组织,分离培养猪前体脂肪细胞。本研究的试验设计经过山西农业大学动物伦理委员会批准后实施。
1.2 主要试剂与载体Prime Script RT reagent Kit with gDNA Eraser、RNAiso Plus regent和SYBR Premix Ex Taq II购于大连TaKaRa公司;Insulin和罗格列酮购自Sigma公司;I型胶原酶、DEX、IBMX、PBS粉末、青链霉素混合液、胰蛋白酶和油红O染液购于中国索莱宝公司;FBS和DMEM购自Gibco公司;Adiponectin抗体、NR1H3抗体、β-actin抗体购于博奥森公司;兔二抗购于LI-COR;转染试剂Lipofecter 3000、过表达载体pIRES2-EGFP-3×flag购自汉恒生物科技有限公司。
1.3 试验方法1.3.1 总RNA提取、反转录及qRT-PCR检测 参照TaKaRa RNAiso Plus和试剂盒说明书提取脂肪组织和细胞总RNA,按照PrimeScript RT reagent Kit with gDNA Eraser试剂盒说明书进行反转录,合成的cDNA于-20 ℃保存备用。
以18S rRNA为内参基因,采用qRT-PCR对NR1H3及相关标志基因的表达水平进行检测,引物信息见表 1。qRT-PCR反应体系:cDNA 2 μL,2×SYBR Premix Ex TaqⅡ10 μL,上、下游引物各0.5 μL,RNAase Free ddH2O补至20 μL。反应程序:95 ℃ 30 s;95 ℃ 5 s,58 ℃ 30 s,35个循环;熔解曲线程序为95 ℃ 15 s,60 ℃ 35 s,95 ℃。结果采用2-△△CT法进行分析,每个样本做3次技术重复。
|
|
表 1 qRT-PCR引物信息 Table 1 Primer information of qRT-PCR |
1.3.2 猪前体脂肪细胞的分离、鉴定与诱导分化 5日龄杜长大仔猪放血处死后用75%酒精消毒,取背部皮下脂肪组织,用含2%青链霉素的PBS清洗3次后移入超净工作台;去除筋膜及结缔组织,置于小烧杯中剪成肉糜状;加入等体积Ⅰ型胶原酶消化液(2 mg·mL-1),37 ℃消化30 min;加入等体积终止培养基(3% FBS+DMEM)终止消化;依次用70、200目的筛子过滤,1 100 r·min-1离心5 min,获得细胞沉淀;完全培养基重悬沉淀,加入10 cm培养皿中,于CO2恒温培养箱中培养。
采用细胞免疫荧光法检测脂肪细胞因子Adiponectin含量以鉴定细胞的纯度。细胞密度达70%~80%时,弃掉培养基,PBS洗3次;4%多聚甲醛固定40 min;0.1%的Triton-100通透10 min,PBS洗3次;5% BSA封闭60 min,每孔加入100 μL Adiponectin鼠一抗(1∶100),室温孵育6 h,PBS洗3次;加入适量FITC标记的荧光二抗(1∶100),覆盖孔板底部,37 ℃孵育2 h,PBS洗3次;加入100 μL DAPI染液,避光染色10~15 min,PBS清洗数次;荧光显微镜下观察并拍照。
前体脂肪细胞成脂分化的诱导采用“激素鸡尾酒”法[18]。待细胞长满后,更换诱导分化培养基(DMEM+10% FBS+5 μg·mL-1 Insulin+0.5 mmoL·L-1 IBMX+1 μmoL·L-1 DEX+1 gmoL·L-1罗格列酮+1%青链霉素),培养2 d更换维持培养基(DMEM +10% FBS+1%青链霉素+5 μg·mL-1胰岛素),培养8 d后收集细胞,期间2 d换1次液。
1.3.3 NR1H3基因过表达载体的构建、转染及相关标志基因的检测 以实验室前期获得的猪NR1H3基因菌液为模板,利用同源重组引物(F: CTGGTTTAGTGAACCGTCAGATCCGCCACC-ATGTCCTTGTGGGTGGAGG;R: CACCGTCA-TGGTCTTTGTAGTCCATCTCGTGCACATCC-CAGATCTCAG)扩增含有过表达载体同源臂的目的片段,将目的基因片段通过同源重组连接到过表达载体pIRES2-EGFP-3×Flag上,构建NR1H3基因过表达载体。
试验设置过表达组和对照组,过表达组添加NR1H3-OE,对照组添加NR1H3-NC。当猪前体脂肪细胞生长至70%~80%时进行转染。分别将转染试剂和载体加入到无血清的DMEM中,室温静置5 min后将两者混合,静置20 min,进行转染。转染48 h后,诱导分化,8 d后收集细胞,检测NR1H3过表达效率及下游靶基因SREBP-1c和ChREBP及成脂关键基因FAS、PPARγ、FABP4和C/EBPβ的表达量变化。
1.3.4 猪NR1H3 siRNA的合成、转染及相关标志基因的检测 根据NCBI上猪NR1H3的基因序列(登录号:NM_001101814.1),选择不同位点设计3对NR1H3 siRNA,命名为siRNA-1、siRNA-2和siRNA-3,对照组为siRNA-NC,由汉恒生物公司合成(表 2)。
|
|
表 2 NR1H3 siRNA序列信息 Table 2 Sequence information of NR1H3 siRNA |
采用脂质体法分别将siRNA-1、siRNA-2、siRNA-3和siRNA-NC转染入猪前体脂肪细胞,48 h后收集细胞,检测NR1H3基因的表达量,筛选干扰效果最佳的siRNA进行后续试验。在猪前体脂肪细胞中分别转染siRNA-NC和siRNA-2,诱导分化10 d后收集细胞,检测NR1H3下游靶基因SREBP-1c和ChREBP及成脂关键基因FAS、PPARγ、FABP4和C/EBPβ的表达情况。
1.3.5 Western blot检测成脂关键基因的表达 细胞成脂分化10 d后,收集蛋白质样品,100 ℃变性10 min;制备分离胶和浓缩胶进行SDS-PAGE,电泳条件为90 V 15 min,120 V 60 min,保持恒压进行;5%奶粉封闭1 h;加入一抗(1∶1 000)4 ℃过夜;洗涤3次,加入二抗(1∶10 000),避光孵育1 h;洗涤后观察蛋白条带,分析灰度值。
1.3.6 细胞油红O染色 细胞成脂分化第10天,PBS漂洗3次,4% 的多聚甲醛37 ℃固定30 min;PBS漂洗3次,加入油红O染液37 ℃染色60 min;PBS漂洗5次,在光学显微镜下观察并拍照。
1.3.7 统计分析 应用SPSS version 22.0进行统计分析。采用单因素方差分析探讨马身猪不同日龄背部皮下脂肪组织中NR1H3基因表达量的差异,采用Duncan’s法进行多重比较;试验组与对照组相关基因及蛋白质表达量之间的差异采用独立样本t检验进行比较;P < 0.05为差异显著,P < 0.01为差异极显著。
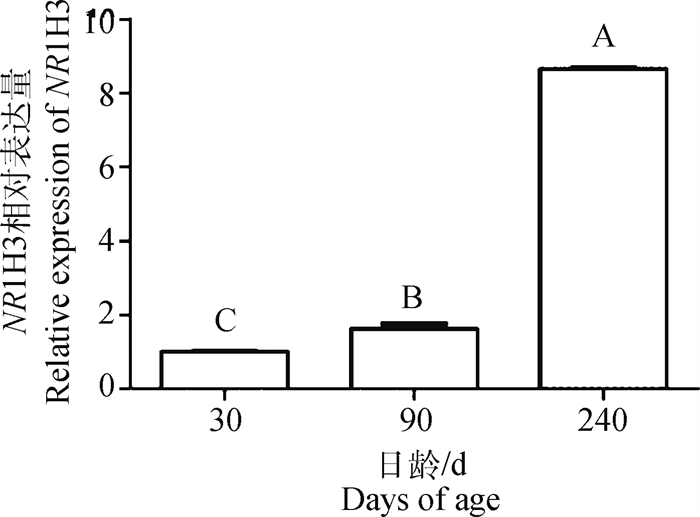
2 结果 2.1 NR1H3基因在猪皮下脂肪组织中的发育性表达特性马身猪皮下脂肪组织NR1H3基因表达量随日龄增加呈逐渐升高的趋势,240日龄表达量最高,30日龄表达量最低,差异极显著(P < 0.01,图 1),表明其对脂肪沉积可能发挥促进作用。
|
不同大写字母代表差异极显著(P < 0.01) Different capital letters represent the extremely significant difference (P < 0.01) 图 1 不同日龄马身猪皮下脂肪组织中NR1H3的相对表达量 Fig. 1 Relative expression of NR1H3 in subcutaneous adipose tissues of Mashen pigs at different ages |
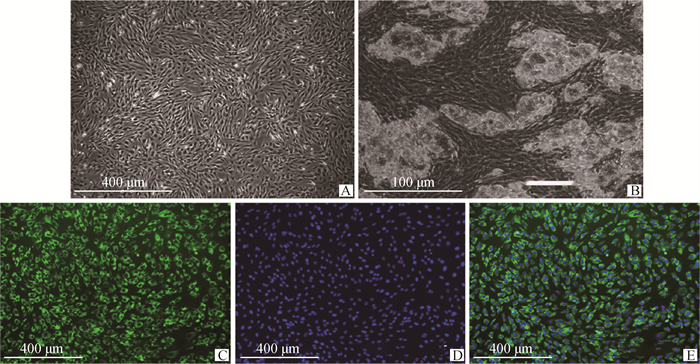
分离的猪前体脂肪细胞形态为圆形、椭圆形、梭形等不规则形状,6 d左右汇合度达到70%~80%(图 2A)。诱导分化后,前体脂肪细胞膨大变形,出现脂滴。随着细胞密度不断增加,脂滴逐渐增多,相邻的小脂滴发生融合(图 2B)。
|
A. 猪前体脂肪细胞;B. 成脂诱导的猪前体脂肪细胞;C~E. Adiponectin标记的猪前体脂肪细胞纯度鉴定 A. Porcine preadipocytes; B. Adipogenic induction of porcine preadipocytes; C-E. Purity identification of porcine preadipocytes marked by Adiponectin 图 2 猪前体脂肪细胞的分离与纯度鉴定 Fig. 2 Isolation and purity identification of porcine preadipocytes |
免疫荧光染色检测Adiponectin的表达情况,发现绿色荧光均匀分布于胞浆(图 2C),细胞核蓝染(图 2D),99%以上的细胞带有绿色荧光,为阳性结果(图 2E)。因此,可鉴定所培养的细胞为猪前体脂肪细胞,纯度在99%以上。
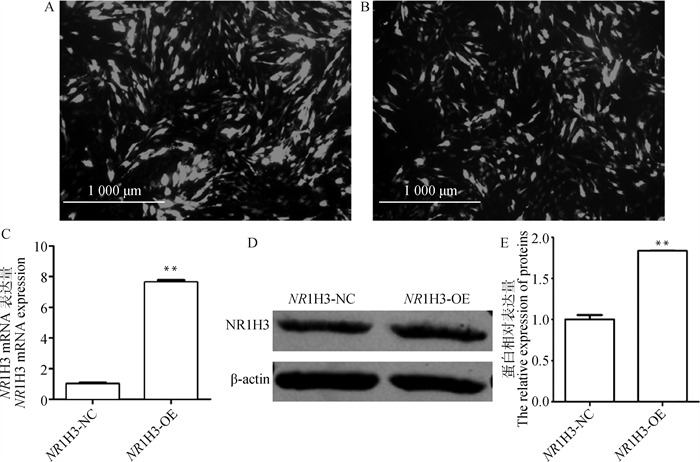
2.3 过表达NR1H3基因促进猪前体脂肪细胞的成脂分化细胞培养到70%~80%融合时,将NR1H3-NC和NR1H3-OE转染入猪前体脂肪细胞,48 h后,显微镜观察GFP的表达情况,NR1H3-NC组和NR1H3-OE组的绿色荧光达到80%,转染效率相似(图 3A、3B)。qRT-PCR和Western blot检测结果显示,与NR1H3-NC组相比,NR1H3-OE组NR1H3的mRNA表达水平上升7.63倍(P < 0.01,图 3C),蛋白表达水平上升1.83倍(P < 0.01,图 3D、3E),表明过表达成功。
|
A、B. 转染NR1H3-NC和NR1H3-OE的猪前体脂肪细胞;C. NR1H3基因mRNA表达量;D、E. NR1H3蛋白表达量。**.P < 0.01,下同 A, B. Porcine preadipocytes transfected with NR1H3-NC and NR1H3-OE; C. The mRNA expression of NR1H3 gene; D, E. NR1H3 protein expression level.**. P < 0.01, the same as below 图 3 NR1H3基因在猪前体脂肪细胞中的过表达效率 Fig. 3 The over-expression efficiency of NR1H3 gene in porcine preadipocytes |
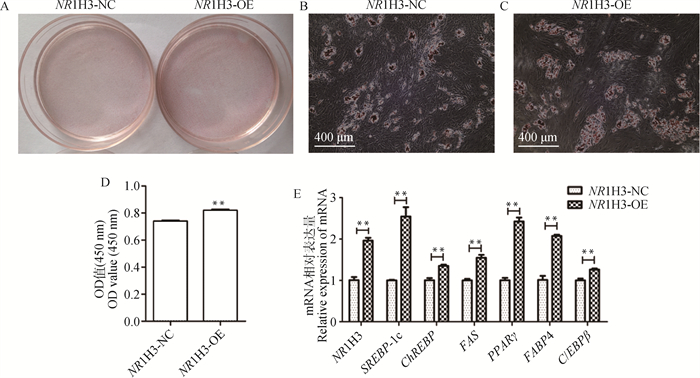
将转染NR1H3-NC和NR1H3-OE的猪前体脂肪细胞诱导分化后进行油红O染色,结果显示,与NR1H3-NC组相比,NR1H3-OE组的油红O染色着色深(图 4A、4B、4C);用酶标仪测定细胞的OD450 nm值,结果同样存在极显著差异(P < 0.01,图 4D);NR1H3-OE组成脂分化关键基因FAS、PPARγ、C/EBPβ和FABP4及NR1H3基因下游靶标SREBP-1c和ChREBP的mRNA水平均极显著升高(P < 0.01,图 4E)。综上结果表明,过表达猪NR1H3基因促进猪前体脂肪细胞的分化。
|
A. 油红O染色结果;B、C. 细胞油红O染色;D. NR1H3-NC和NR1H3-OE组OD450 nm值;E. NR1H3-NC和NR1H3-OE组NR1H3及成脂关键基因mRNA表达量 A. Oil red O staining results; B, C. Oil red O staining of cells; D. The OD value at 450 nm in NR1H3-NC and NR1H3-OE groups; E. The mRNA expression of NR1H3 and the key adipogenic genes in NR1H3-NC and NR1H3-OE groups 图 4 过表达NR1H3对猪前体脂肪细胞分化的影响 Fig. 4 The effect of NR1H3 over-expression on differentiation of porcine preadipocytes |
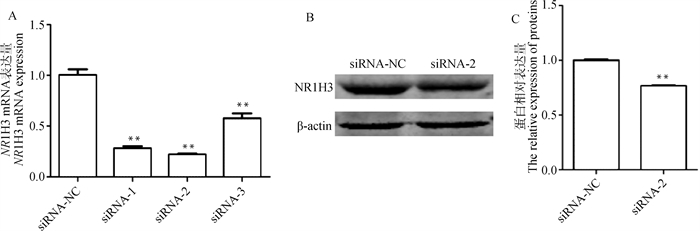
与siRNA-NC组相比,siRNA-1、siRNA-2、siRNA-3组中NR1H3基因mRNA表达水平分别降低了71.9%、78.0%和42.3%(P < 0.01,图 5A),siRNA-2干扰效果最佳,用于后续试验。Western blot结果显示,与siRNA-NC组相比,siRNA-2组NR1H3表达水平下调了23.5%(P < 0.01,图 5B、5C),表明干扰成功。
|
A. 不同NR1H3 siRNA组别NR1H3基因mRNA表达量;B~C. siRNA-NC和siRNA-2组NR1H3蛋白表达量 A. The NR1H3 mRNA expressions in different NR1H3 siRNA groups; B-C. The NR1H3 protein expressions in NR1H3 siRNA-NC and siRNA-2 groups 图 5 NR1H3干扰效率的检测 Fig. 5 NR1H3 interference efficiency detection |
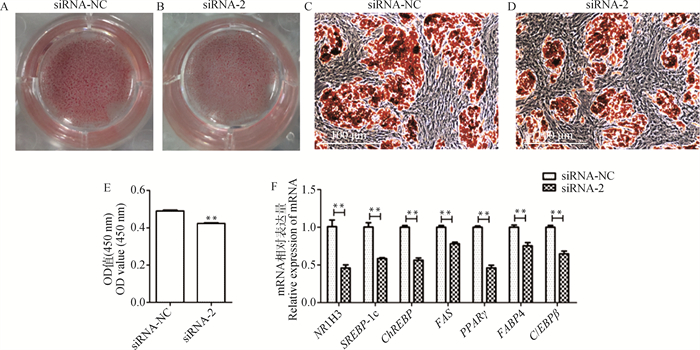
将转染入干扰载体的猪前体脂肪细胞诱导分化后进行油红O染色,通过肉眼和倒置显微镜同样明显观察到油红O着色差异(图 6A、6B、6C、6D);用酶标仪测定细胞的OD450 nm值,也存在极显著差异(P < 0.01,图 6E);与siRNA-NC组相比,siRNA-2组的NR1H3基因下游靶标SREBP-1c和ChREBP及成脂分化关键基因FAS、PPARγ、C/EBPβ和FABP4的mRNA水平均极显著降低(P < 0.01,图 6F)。综上结果表明,干扰猪NR1H3基因抑制猪前体脂肪细胞的分化。
3 讨论脂肪的含量与分布会影响胴体品质和肉的风味。脂肪的形成主要依赖脂肪细胞的增殖、分化,之后,三酰甘油不断沉积,而成脂相关转录因子在脂质沉积过程中发挥着关键调控作用[19-21]。NR1H3是近年来在脂肪生成过程中发挥关键作用的转录因子[22],与多种脂肪酸合成酶的表达相关。研究发现,NR1H3-/-小鼠下调了SREBP-1c和重要的脂肪形成酶FAS、SCD-1和ACC表达水平[23],导致小鼠的脂肪生成减弱。敲低NR1H3抑制了山羊肌内前体脂肪细胞分化[9]。经过LXR激动剂处理可能增加脂肪细胞中甘油三酯的含量及脂质蓄积[24-26]。此外,NR1H3在乳腺脂质合成过程中也发挥作用[27-28]。研究发现,NR1H3可以通过直接与FASN启动子相互作用,影响SREBP-1的表达,从而促进乳脂合成[29];也可作用于SREBP-1c,然后影响FAS的表达,调控脂肪酸的生成[30]。
|
A、B. 油红O染色结果;C、D. 细胞油红O染色; E. siRNA-NC组和siRNA-2组OD 450 nm值;F. siRNA-NC组和siRNA-2组成脂关键基因mRNA表达量 A, B. Oil red O staining results; C, D. Oil red O staining of cells; E. The OD values at 450 nm in siRNA-NC and siRNA-2 groups; F. The mRNA expression of key adipogenic genes in siRNA-NC and siRNA-2 groups 图 6 干扰NR1H3基因对猪前体脂肪细胞分化的影响 Fig. 6 The effect of NR1H3 siRNA on differentiation of porcine preadipocytes |
本研究中,过表达NR1H3后,SREBP-1c和ChREBP的表达量显著上调(P < 0.01),脂肪生成标记基因PPARγ、C/EBPβ和FABP4的表达量同样极显著上升(P < 0.01),脂滴形成增加;干扰NR1H3则呈现相反趋势,表明NR1H3在猪前体脂肪细胞的分化过程中具有促进作用,进而促进成脂过程。研究表明,SREBP-1c是NR1H3的关键靶标[31-33],参与调控脂肪酸生成相关酶的表达,进而调控脂肪细胞分化[34-35]。ChREBP显著影响成脂相关基因FAS和ACC1的表达量,是脂肪组织必需的调控因子[36-37],NR1H3可激活ChREBPα的转录,从而激活脂肪形成基因,促进脂肪细胞分化。可见,NR1H3通过调控其下游靶标SREBP-1c和ChREBP的表达水平影响成脂关键基因PPARγ、C/EBPβ和FABP4的表达,进而影响猪前体脂肪细胞的分化,是猪前体脂肪细胞成脂分化的正调节剂。
尽管NR1H3在多个物种中都有研究,但其对于脂肪细胞分化的作用并不统一,可能是脂肪细胞分化正反两方面的调节器。Ross等[38]报道,LXR活性抑制原代前体脂肪细胞的分化和脂质蓄积。NR1H3通过Wnt /β-catenin信号传导对鼠间充质干细胞(MSCs)的成脂分化具有抑制作用[39]。这些不一致的结果可能反映了物种、细胞类型和培养条件等的差异。
NR1H3基因近年来受到广泛的关注,已在小鼠和山羊等物种中证实与脂肪细胞脂质积累相关,但在猪脂肪沉积中的作用鲜有报道。Zhang等[40]研究发现,NR1H3-exon-5-A201C的C等位基因与背膘厚度有关,可能促进猪的脂质沉积,并增加皮下脂肪和肌内脂肪含量,推断与高脂质沉积有关。本研究中,细胞试验结果表明NR1H3是猪前体脂肪细胞成脂分化的正调节剂,而且NR1H3基因在马身猪皮下脂肪组织的表达量随着日龄的增长极显著上升,与猪脂肪沉积的趋势一致,表明该基因在调控猪脂肪代谢过程中可能发挥重要作用。
4 结论在马身猪脂肪组织生长发育过程中,NR1H3基因的表达量随年龄的增长呈逐渐升高趋势,与猪脂肪沉积规律相符合。NR1H3是猪前体脂肪细胞成脂分化的正调节剂,通过调控其下游靶标SREBP-1c和ChREBP的表达水平影响成脂关键基因PPARγ、C/EBPβ和FABP4的表达,进而影响猪前体脂肪细胞的分化。
| [1] |
TANG Q Q, LANE M D. Adipogenesis: from stem cell to adipocyte[J]. Annu Rev Biochem, 2012, 81: 715-736. DOI:10.1146/annurev-biochem-052110-115718 |
| [2] |
FARMER S R. Transcriptional control of adipocyte formation[J]. Cell Metab, 2006, 4(4): 263-273. DOI:10.1016/j.cmet.2006.07.001 |
| [3] |
HERMAN M A, PERONI O D, VILLORIA J, et al. A novel ChREBP isoform in adipose tissue regulates systemic glucose metabolism[J]. Nature, 2012, 484(7394): 333-338. DOI:10.1038/nature10986 |
| [4] |
HOFBAUER H F, SCHOPF F H, SCHLEIFER H, et al. Regulation of gene expression through a transcriptional repressor that senses acyl-chain length in membrane phospholipids[J]. Dev Cell, 2014, 29(6): 729-739. DOI:10.1016/j.devcel.2014.04.025 |
| [5] |
WANG B, TONTONOZ P. Liver X receptors in lipid signalling and membrane homeostasis[J]. Nat Rev Endocrinol, 2018, 14(8): 452-463. DOI:10.1038/s41574-018-0037-x |
| [6] |
DALTON G D, OH S H, TANG L D, et al. Hepatocyte activity of the cholesterol sensor smoothened regulates cholesterol and bile acid homeostasis in mice[J]. iScience, 2021, 24(9): 103089. DOI:10.1016/j.isci.2021.103089 |
| [7] |
SCHULMAN I G. Liver X receptors link lipid metabolism and inflammation[J]. FEBS Lett, 2017, 591(19): 2978-2991. DOI:10.1002/1873-3468.12702 |
| [8] |
BECARES N, GAGE M C, VOISIN M, et al. Impaired LXRα phosphorylation attenuates progression of fatty liver disease[J]. Cell Rep, 2019, 26(4): 984-995. DOI:10.1016/j.celrep.2018.12.094 |
| [9] |
JALIL A, BOURGEOIS T, MÉNÉGAUT L, et al. Revisiting the role of LXRs in PUFA metabolism and phospholipid homeostasis[J]. Int J Mol Sci, 2019, 20(15): 3787. DOI:10.3390/ijms20153787 |
| [10] |
XIONG Y, XU Q, LIN S, et al. Knockdown of LXRα inhibits goat intramuscular preadipocyte differentiation[J]. Int J Mol Sci, 2018, 19(10): 3037. DOI:10.3390/ijms19103037 |
| [11] |
FAN Q, NØRGAARD R C, BINDESBØLL C, et al. LXRα regulates hepatic ChREBPα activity and lipogenesis upon glucose, but not fructose feeding in mice[J]. Nutrients, 2017, 9(7): 678. DOI:10.3390/nu9070678 |
| [12] |
PAN Y X, ZHUO M Q, LI D D, et al. SREBP-1 and LXRα pathways mediated Cu-induced hepatic lipid metabolism in zebrafish Danio rerio[J]. Chemosphere, 2019, 215: 370-379. DOI:10.1016/j.chemosphere.2018.10.058 |
| [13] |
曾德飞, 李薇, 汤琼健, 等. 肝X受体调节胆固醇代谢研究新进展[J]. 南通大学学报: 医学版, 2019, 39(2): 109-113. ZENG D F, LI W, TANG Q J, et al. Research progress of liver X receptor regulating cholesterol metabolism[J]. Journal of Nantong University: Medical Science, 2019, 39(2): 109-113. (in Chinese) |
| [14] |
REY M, KRUSE M S, MAGRINI-HUAMÁN R N, et al. High-fat diets and LXRs expression in rat liver and hypothalamus[J]. Cell Mol Neurobiol, 2019, 39(7): 963-974. DOI:10.1007/s10571-019-00692-6 |
| [15] |
ARCHER A, LAURENCIKIENE J, AHMED O, et al. Skeletal muscle as a target of LXR agonist after long-term treatment: focus on lipid homeostasis[J]. Am J Physiol Endocrinol Metab, 2014, 306(5): E494-E502. DOI:10.1152/ajpendo.00410.2013 |
| [16] |
罗华伦, 李万贵, 张依裕, 等. 鸭LXRα基因过表达对原代肝细胞及PC3细胞脂质代谢的效应研究[J]. 畜牧兽医学报, 2019, 50(1): 52-60. LUO H L, LI W G, ZHANG Y Y, et al. Effect of duck LXRα gene overexpression on lipid metabolism in primary hepatocytes and PC3 cells[J]. Acta Veterinaria et Zootechnica Sinica, 2019, 50(1): 52-60. (in Chinese) |
| [17] |
FILALI-MOUNCEF Y, HUNTER C, ROCCIO F, et al. The ménage à trois of autophagy, lipid droplets and liver disease[J]. Autophagy, 2022, 18(1): 50-72. DOI:10.1080/15548627.2021.1895658 |
| [18] |
RUIZ-OJEDA F J, RUPÉREZ A I, GOMEZ-LLORENTE C, et al. Cell models and their application for studying adipogenic differentiation in relation to obesity: a review[J]. Int J Mol Sci, 2016, 17(7): 1040. DOI:10.3390/ijms17071040 |
| [19] |
张宁芳, 成志敏, 乐宝玉, 等. 猪肌源性前体脂肪细胞的分离培养和成脂诱导分化研究[J]. 畜牧兽医学报, 2018, 49(12): 2612-2621. ZHANG N F, CHENG Z M, LE B Y, et al. Isolation, culture and adipogenic differention of pig myogenic preadipocytescell[J]. Acta Veterinaria et Zootechnica Sinica, 2018, 49(12): 2612-2621. DOI:10.11843/j.issn.0366-6964.2018.12.010 (in Chinese) |
| [20] |
BOUGHANEM H, CABRERA-MULERO A, MILLÁN-GÓMEZ M, et al. Transcriptional analysis of FOXO1, C/EBP-α and PPAR-γ2 genes and their association with obesity-related insulin resistance[J]. Genes (Basel), 2019, 10(9): 706. DOI:10.3390/genes10090706 |
| [21] |
MANUEL A M, WALLA M D, DORN M T, et al. Fumarate and oxidative stress synergize to promote stability of C/EBP homologous protein in the adipocyte[J]. Free Radic Biol Med, 2020, 148: 70-82. DOI:10.1016/j.freeradbiomed.2019.12.037 |
| [22] |
RUSSO-SAVAGE L, SCHULMAN I G. Liver X receptors and liver physiology[J]. Biochim Biophys Acta-Mol Basis Dis, 2021, 1867(6): 166121. DOI:10.1016/j.bbadis.2021.166121 |
| [23] |
PEET D J, TURLEY S D, MA W Z, et al. Cholesterol and bile acid metabolism are impaired in mice lacking the nuclear oxysterol receptor LXRα[J]. Cell, 1998, 93(5): 693-704. DOI:10.1016/S0092-8674(00)81432-4 |
| [24] |
XU H F, LUO J, ZHANG X Y, et al. Activation of liver X receptor promotes fatty acid synthesis in goat mammary epithelial cells via modulation of SREBP1 expression[J]. J Dairy Sci, 2019, 102(4): 3544-3555. DOI:10.3168/jds.2018-15538 |
| [25] |
LASCH A, ALARCAN J, LAMPEN A, et al. Combinations of LXR and RXR agonists induce triglyceride accumulation in human HepaRG cells in a synergistic manner[J]. Arch Toxicol, 2020, 94(4): 1303-1320. DOI:10.1007/s00204-020-02685-7 |
| [26] |
CHEN Y Q, POTTANAT T G, ZHEN E Y, et al. ApoA5 lowers triglyceride levels via suppression of ANGPTL3/8-mediated LPL inhibition[J]. J Lipid Res, 2021, 62: 100068. DOI:10.1016/j.jlr.2021.100068 |
| [27] |
GRINMAN D Y, CAREAGA V P, WELLBERG E A, et al. Liver X receptor-α activation enhances cholesterol secretion in lactating mammary epithelium[J]. Am J Physiol Endocrinol Metab, 2019, 316(6): E1136-E1145. DOI:10.1152/ajpendo.00548.2018 |
| [28] |
HU X Y, ZHANG N S, FU Y H. Role of liver X receptor in mastitis therapy and regulation of milk fat synthesis[J]. J Mammary Gland Biol Neoplasia, 2019, 24(1): 73-83. DOI:10.1007/s10911-018-9403-5 |
| [29] |
ZHANG Y Y, FAN X Y, QIU L H, et al. Liver X receptor α promotes milk fat synthesis in buffalo mammary epithelial cells by regulating the expression of FASN[J]. J Dairy Sci, 2021, 104(12): 12980-12993. DOI:10.3168/jds.2021-20596 |
| [30] |
JOSEPH S B, LAFFITTE B A, PATEL P H, et al. Direct and indirect mechanisms for regulation of fatty acid synthase gene expression by liver X receptors[J]. J Biol Chem, 2002, 277(13): 11019-11025. DOI:10.1074/jbc.M111041200 |
| [31] |
RONG S X, CORTÉS V A, RASHID S, et al. Expression of SREBP-1c requires SREBP-2-mediated generation of a sterol ligand for LXR in livers of mice[J]. eLife, 2017, 6: e25015. DOI:10.7554/eLife.25015 |
| [32] |
BENÍTEZ-SANTANA T, HUGO S E, SCHLEGEL A. Role of intestinal LXRα in regulating post-prandial lipid excursion and diet-Induced hypercholesterolemia and hepatic lipid accumulation[J]. Front Physiol, 2017, 8: 280. DOI:10.3389/fphys.2017.00280 |
| [33] |
ZHANG Y X, GU Y Y, CHEN Y H, et al. Dingxin Recipe IV attenuates atherosclerosis by regulating lipid metabolism through LXR-α/SREBP1 pathway and modulating the gut microbiota in ApoE-/- mice fed with HFD[J]. J Ethnopharmacol, 2021, 266: 113436. DOI:10.1016/j.jep.2020.113436 |
| [34] |
DE QUEIROZ J C F, ALONSO-VALE M I C, CURI R, et al. Controle da adipogênese por ácidos graxos[J]. Arq Bras Endocrinol Metabol, 2009, 53(5): 582-594. DOI:10.1590/S0004-27302009000500011 |
| [35] |
NGUYEN T T P, KIM D Y, LEE Y G, et al. SREBP-1c impairs ULK1 sulfhydration-mediated autophagic flux to promote hepatic steatosis in high-fat-diet-fed mice[J]. Mol Cell, 2021, 81(18): 3820-3832. DOI:10.1016/j.molcel.2021.06.003 |
| [36] |
卢建雄, 张国华, 李昌辉, 等. ChREBP基因siRNA表达质粒构建及对原代培养猪脂肪细胞生脂的影响[J]. 中国农业科学, 2013, 46(24): 5196-5204. LU J X, ZHANG G H, LI C H, et al. Construction of siRNA expression plasmids targeting ChREBP gene and its effect on lipogenesis in primary cultured porcine adipocytes[J]. Scientia Agricultura Sinica, 2013, 46(24): 5196-5204. DOI:10.3864/j.issn.0578-1752.2013.24.014 (in Chinese) |
| [37] |
IIZUKA K, TAKAO K, YABE D. ChREBP-Mediated regulation of lipid metabolism: involvement of the gut microbiota, liver, and adipose tissue[J]. Front Endocrinol (Lausanne), 2020, 11: 587189. DOI:10.3389/fendo.2020.587189 |
| [38] |
ROSS S E, ERICKSON R L, GERIN I, et al. Microarray analyses during adipogenesis: understanding the effects of Wnt signaling on adipogenesis and the roles of liver X receptor alpha in adipocyte metabolism[J]. Mol Cell Biol, 2002, 22(16): 5989-5999. DOI:10.1128/MCB.22.16.5989-5999.2002 |
| [39] |
MATSUSHITA K, MORELLO F, ZHANG Z P, et al. Nuclear hormone receptor LXRα inhibits adipocyte differentiation of mesenchymal stem cells with Wnt/beta-catenin signaling[J]. Lab Invest, 2016, 96(2): 230-238. DOI:10.1038/labinvest.2015.141 |
| [40] |
ZHANG B, SHANG P, QIANGBA Y Z, et al. The association of NR1H3 gene with lipid deposition in the pig[J]. Lipids Health Dis, 2016, 15: 99. DOI:10.1186/s12944-016-0269-5 |
(编辑 郭云雁)



