2. 商丘美兰生物工程有限公司,柘城 476200;
3. 河南农业大学动物医学院,郑州 450046
2. Shangqiu Meilan Bioengineering Co., Ltd., Zhecheng 476200, China;
3. College of Veterinary Medicine of Henan Agricultural University, Zhengzhou 450046, China
猪丁型冠状病毒(porcine deltacoronavirus,PDCoV)属于冠状病毒科丁型冠状病毒属,PDCoV可以感染不同年龄的猪,其中,以哺乳仔猪最易感。近年来, PDCoV常与猪流行性腹泻病毒、传染性胃肠炎病毒混合感染,导致仔猪死亡率骤升[1-2]。2014年,Hu等[3]研究发现,该病毒可在LLC-PK1细胞上生长与传代,并产生明显的细胞病变。但是细胞的贴壁培养工艺无法适应疫苗的规模化生产要求,而无血清悬浮培养工艺正成为当前国内外疫苗生产的主要趋势[4]。
本研究对LLC-PK1细胞悬浮培养工艺及PDCoV在悬浮培养的LLC-PK1细胞上的增殖特性进行了初步探索,以期为利用悬浮培养工艺规模化生产PDCoV灭活疫苗提供理论依据。
1 材料与方法 1.1 材料PDCoV HNZK-04株P10代(GenBank No:MH708124;108.38TCID50·0.1 mL-1)、LLC-PK1细胞、抗PDCoV的猪多克隆抗体受赠于河南农业大学微生物学实验室;MEM培养基、TPCK胰酶、胎牛血清(fetal bovine serum, FBS)均购自Gibco;PK201培养基购自壹生科(深圳)有限公司。
1.2 方法1.2.1 PDCoV适应性LLC-PK1悬浮细胞株的筛选 采用不含FBS的PK201培养基逐渐代替含5% FBS的MEM培养基进行适应培养,每次改变培养基成分时依次用离心传代法和稀释传代法进行细胞传代,当细胞密度>1.5×106cells·mL-1,活率≥95%时即认为细胞已适应生长。采用有限稀释法筛选单克隆细胞株,将PDCoV HNZK-04株P10代接种于不同的单克隆细胞株,以收获病毒液的滴度筛选PDCoV高适应性悬浮细胞株命名为LLC-PK1Sa。
1.2.2 间接免疫荧光法(IFA)鉴定PDCoV对LLC-PK1细胞的感染性 分别将PDCoV HNZK-04株P10代及LLC-PK1Sa细胞增殖的PDCoV F10代(109.38TCID50·0.1 mL-1)病毒接种于LLC-PK1细胞,300 μL·孔-1。同时设不接毒对照组,按照已建立的IFA方法[5]进行检测,其中,一抗为抗PDCoV的猪多克隆抗体(1∶1 000),二抗为FITC标记的兔抗猪IgG抗体(1∶100),荧光显微镜下观察病毒对细胞的感染情况。
1.2.3 PDCoV在悬浮培养LLC-PK1细胞上增殖工艺的优化 分别对影响病毒增殖的主要参数进行优化。1)将细胞密度分别调整为5×105、1×106、2×106、3×106 cells·mL-1,TPCK胰酶浓度为5.0 μg·mL-1,按MOI为10-2接种PDCoV HNZK-04株P10代,待细胞病变达80%左右时收毒。2)在确定的最佳细胞密度条件下,TPCK胰酶浓度为5.0 μg·mL-1,分别按MOI为10-4、10-3、10-2接种PDCoV HNZK-04株P10代,于接种后24、36、48、60、72、84、96 h收毒。3)在最佳细胞密度及MOI条件下,TPCK胰酶浓度分别为1、2.5、5.0、7.5、10 μg·mL-1,每种浓度3个重复,待细胞病变达80%左右时收毒。分别测定所收获的PDCoV的TCID50。
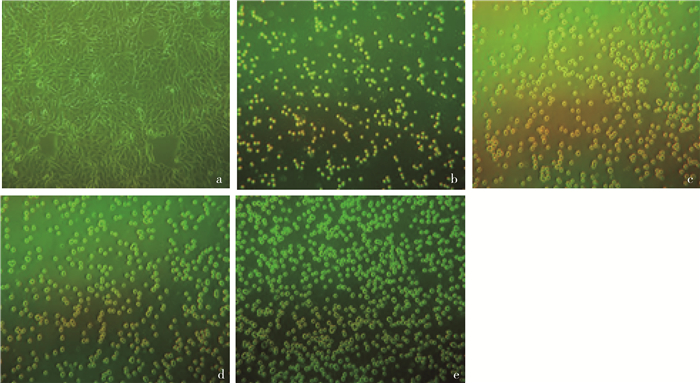
2 结果 2.1 PDCoV适应性LLC-PK1悬浮细胞株的筛选如图 1a所示,LLC-PK1细胞贴壁生长时呈规则纤维样,紧密排列;图 1b~d所示,细胞形态由纤维样变为圆形,密度逐渐增大,但是大小不一,有少量结团现象;图 1e所示LLC-PK1细胞形态和大小都趋于一致,无明显结团现象。通过对LLC-PK1细胞的单克隆及PDCoV HNZK-04株适应性培养,最终优选出可稳定增殖PDCoV的LLC-PK1Sa细胞株。
|
a. 贴壁培养的细胞;b. 3%FBS悬浮培养的细胞;c. 2%FBS悬浮培养的细胞;d. 1%FBS悬浮培养的细胞;e. 无血清悬浮培养的细胞 a. Monolayer culture's cells; b. 3%FBS suspension culture's cells; c. 2%FBS suspension culture's cells; d. 1%FBS suspension culture's cells; e. Serum-free suspension culture's cells 图 1 LLC-PK1细胞在不同血清含量的培养基中生长状态(100×) Fig. 1 The growth status of LLC-PK1 cells in medium with different serum contents (100×) |
如图 2所示,对照组细胞质无绿色荧光,PDCoV接种LLC-PK1细胞后,绝大部分细胞的细胞质均呈现明亮的绿色荧光,表明经贴壁细胞和悬浮细胞增殖的PDCoV均可特异性的感染LLC-PK1细胞。

|
A. 悬浮细胞增殖的PDCoV感染的细胞;B. 贴壁细胞增殖的PDCoV感染的细胞;C. 对照细胞;其中,感染细胞为绿色荧光,细胞核为蓝色荧光 A. Infected cells by PDCoV with suspension cell proliferation; B. Infected cells by PDCoV with monolayer cell proliferation; C.Control cells; PDCoV-infected cells stained as green and nuclei stained as blue 图 2 PDCoV感染LLC-PK1细胞荧光图(100×) Fig. 2 Immunofluorescence staining of LLC-PK1 cells infected with PDCoV(100×) |
从表 1结果可知,当PDCoV接种于细胞密度为2×106 cells·mL-1时,病毒滴度可达109.76TCID50·0.1 mL-1;当MOI为10-3时,在48 h时收获的病毒液滴度可达109.88TCID50·mL-1;当TPCK胰酶浓度达7.5 μg·mL-1时,收获病毒液的滴度最高,可达108.88TCID50~109.59TCID50。
|
|
表 1 PDCoV在悬浮培养LLC-PK1细胞上增殖的工艺优化结果 Table 1 Optimization of the process for proliferation of PDCoV by LLC-PK1 cells in suspension |
由于血清中存在不明或有害成分,不仅可能降低疫苗的安全性,而且可以中和胰酶,进而使病毒不能有效感染细胞而增殖[6],因此实现PDCoV在无血清悬浮培养LLC-PK1细胞中增殖具有切实意义。为了实现PDCoV的高效增殖,不仅需要理想的宿主细胞,还需要对影响病毒增殖的关键参数进行优化[7]。在病毒接种细胞时存在细胞密度效应,在利用LLC-PK1Sa细胞增殖PDCoV时也证实这一现象的存在,过高或者过低的MOI都不利于PDCoV的高效增殖。另外,本研究发现PDCoV对TPCK胰酶的依懒性较强,但随着TCPK胰酶浓度的增加,病毒的滴度出现先增加后减低的趋势。这种现象的产生可能是由于较低浓度的TPCK胰酶可以促进病毒利用宿主的氨基肽酶N(APN)作为进入受体,通过介导细胞间融合而促进病毒的复制以及细胞病变的产生;随着TPCK胰酶浓度的增加,致使细胞不能耐受而提前脱落,导致病毒滴度随之下降[8-11]。
4 结论以LLC-PK1细胞为母本,筛选出PDCoV高适应性悬浮细胞株LLC-PK1Sa,且PDCoV按MOI为10-3接种密度为2×106cells·mL-1的LLC-PK1Sa细胞,当TPCK胰酶终浓度达到7.5 μg·mL-1时,接毒后48 h收获的病毒液滴度最高。本研究结果可为生物反应器规模化增殖PDCoV提供候选细胞株。
| [1] |
DONG N, FANG L R, ZENG S L, et al. Porcine deltacoronavirus in mainland China[J]. Emerg Infect Dis, 2015, 21(12): 2254-2255. DOI:10.3201/eid2112.150283 |
| [2] |
ZHANG J Q. Porcine deltacoronavirus: overview of infection dynamics, diagnostic methods, prevalence and genetic evolution[J]. Virus Res, 2016, 226: 71-84. DOI:10.1016/j.virusres.2016.05.028 |
| [3] |
HU H, JUNG K, VLASOVA A N, et al. Isolation and characterization of porcine deltacoronavirus from pigs with diarrhea in the United States[J]. J Clin Microbiol, 2015, 53(5): 1537-1548. DOI:10.1128/JCM.00031-15 |
| [4] |
GAO X, ZHAO D H, ZHOU P, et al. Characterization, pathogenicity and protective efficacy of a cell culture-derived porcine deltacoronavirus[J]. Virus Res, 2020, 282: 197955. DOI:10.1016/j.virusres.2020.197955 |
| [5] |
JUNG K, HU H, SAIF L J, et al. Porcine deltacoronavirus induces apoptosis in swine testicular and LLC porcine kidney cell lines in vitro but not in infected intestinal enterocytes in vivo[J]. Vet Microbiol, 2016, 182: 57-63. DOI:10.1016/j.vetmic.2015.10.022 |
| [6] |
ALIMPERTI S, WEN Y, LEI P, et al. Serum-free spheroid suspension culture maintains high proliferation and differentiation potentials of mesenchymal stem cells[J]. Biotechnol Prog, 2014, 30(4): 974-983. DOI:10.1002/btpr.1904 |
| [7] |
SHEN C F, GUILBAULT C, LI X L, et al. Development of suspension adapted Vero cell culture process technology for production of viral vaccines[J]. Vaccine, 2019, 37(47): 6996-7002. DOI:10.1016/j.vaccine.2019.07.003 |
| [8] |
HU H, JUNG K, KENNEY S P, et al. Isolation and tissue culture adaptation of porcine deltacoronavirus: a case study[J]. Methods Mol Biol, 2020, 2203: 77-88. |
| [9] |
ZHANG J L, CHEN J F, LIU Y, et al. Pathogenicity of porcine deltacoronavirus (PDCoV) strain NH and immunization of pregnant sows with an inactivated PDCoV vaccine protects 5-day-old neonatal piglets from virulent challenge[J]. Transbound Emerg Dis, 2020, 67(2): 572-583. DOI:10.1111/tbed.13369 |
| [10] |
ZHU X Y, LIU S D, WANG X L, et al. Contribution of porcine aminopeptidase N to porcine deltacoronavirus infection[J]. Emerg Microbes Infect, 2018, 7(1): 65. |
| [11] |
YANG Y L, MENG F D, QIN P, et al. Trypsin promotes porcine deltacoronavirus mediating cell-to-cell fusion in a cell type-dependent manner[J]. Emerg Microbes Infect, 2020, 9(1): 457-468. DOI:10.1080/22221751.2020.1730245 |
(编辑 白永平)



