鸡作为一种重要的农业养殖鸟类,与其他畜禽相比消化道较短,肠道黏膜屏障较脆弱,更容易受到各种疾病的侵害[1]。在规模化的肉鸡养殖中,为降低死淘率并追求更快的生长速度,通常会使用饲料添加剂。但某些饲料添加剂如抗生素和激素等使用不当会导致肉鸡体内药物残留、内分泌紊乱和腹部脂肪沉积过多等问题[2]。过多的腹部脂肪不仅影响肉鸡的口感,还会影响饲料转化率、胴体产量、生殖性能以及对疾病的免疫力,而肌内脂肪(intramuscular fat,IMF)对肉质嫩度、色泽和风味等均有积极影响,消费者通常偏爱IMF含量较高的鸡,因此,促生长的同时调节脂肪沉积已成为现在家禽养殖业急需解决的问题[3-5]。
益生菌能够通过积极调节肠道菌群来减轻肠道黏膜损伤、增强免疫应答,并促进肠道消化吸收、调节糖脂代谢,进而改善机体的生长性能和健康状况[6-8]。饲料添加益生菌不会产生药物残留,且对肉鸡的脂质代谢有积极影响。研究表明,嗜酸乳杆菌可降低白羽肉鸡血清和肝的胆固醇含量,并调控胆固醇转化、合成以及酯化相关基因的表达[9]。Shokryazdan等[10]和Wang等[11]研究发现,益生菌可促进肉鸡生长,降低血清总胆固醇、甘油三酯(triglyceride,TG)以及腹部脂肪沉积。其中,干酪乳杆菌(Lactobacillus casei)、嗜酸乳杆菌(Lactobacillus acidophilus)和双歧杆菌(Bifidobacterium) 共称为“健康三益菌”,在肠道中均有定植,较其他菌种安全性能更高,被广泛用于食品和饲料添加[12]。本课题组前期研究发现,健康三益菌可有效促进肉鸡生长,当干酪乳杆菌、嗜酸乳杆菌和双歧杆菌的比例为1∶1∶2时效果较佳。益生菌作用的最直接部位是肠道,肉鸡中25%的脂肪酸也来自小肠对饮食的消化吸收,与十二指肠和空肠相比,回肠是小肠中最长的部分且菌群数量更高,有更强的发酵能力[13-14]。
本研究通过对黄麻肉鸡的肉品质和回肠转录组测序结果进行分析,探索健康三益菌复合制剂(干酪乳杆菌∶嗜酸乳杆菌∶双歧杆菌=1∶1∶2)对肉鸡脂质代谢的调控,为进一步研究健康三益菌对肉鸡肠道脂质代谢的调节及分子机制提供理论基础。
1 材料与方法 1.1 饲养管理及试验设计试验黄麻肉鸡购自山西省太谷县田建胜养殖场,按要求接种疫苗,饲养于山西农业大学动物房,定期消毒打扫,每日08:00和20:00饲喂,肉鸡自由采食饮水,动物房内光照23 h,黑暗1 h,育雏第一周温度为33 ℃,此后每日降低1 ℃,直至23 ℃保持恒温。试验期为42 d,随机将200只1日龄雄性黄麻肉雏鸡分为2组,每组5个重复,每个重复20只。对照组正常饮水,益生菌组将健康三益菌(干酪乳杆菌、嗜酸乳杆菌、双歧杆菌) 按1∶1∶2的比例混合,在饮水中添加1%复合制剂,均按标准饲喂基础日粮(表 1)。健康三益菌菌种均购自上海丹尼斯克添加剂有限公司,有效活菌数大于3.4×109 CFU·g-1。
|
|
表 1 基础日粮组成及营养成分 Table 1 Composition and nutrients of basic diets |
42 d时禁食12 h、自由饮水,每组抽取接近平均体重肉鸡20只进行屠宰试验,称重测量胸肌率和腹脂率; 取回肠用生理盐水将其冲洗干净,液氮速冻,并于-80 ℃保存,用于转录组测序; 利用索氏抽提法测胸肌IMF含量; 肉品嫩度仪(MAQC-12)测定剪切力。
1.3 转录组测序及分析在对照组和益生菌组中各自随机抽取4个回肠样本送至上海美吉生物医药科技有限公司,进行转录组测序。Illumina HiSeq Xten/NovaSeq600上机测序。使用SeqPrep软件对原始测序数据(raw reads)进行过滤,从而得到高质量的测序数据(clean reads)。将质控后的clean reads利用HiSat2软件比对到鸡(Gallus gallus)的参考基因组,获得mapped reads,同时进行质量评估,剔除1个离群样本。
1.4 差异表达基因筛选使用DESeq2进行差异表达分析:P < 0.05和log2FC ≥1,当一个基因同时满足这两个条件时,则视为显著差异表达基因(significantly differentially expressed genes, SDEGs)。
1.5 GO富集和KEGG富集将筛选后的SDEGs利用R脚本进行GO富集和KEGG Pathway富集,使用Fisher精确检验,利用Bonferroni多重检验方法来控制计算假阳性率,P < 0.05则认为显著富集。
1.6 实时荧光定量PCR(qRT-PCR)验证随机选择5个SDEGs进行qRT-PCR,验证转录组测序的结果。取测序相同样品,TRIzol法提取回肠总RNA,使用反转录试剂盒(TaKaRa, RR036A)得到cDNA。使用SYBR Premix Ex TaqTM Ⅱ Kit试剂盒(TaKaRa)进行反应。qRT-PCR扩增程序为:95 ℃预变性5 min; 95 ℃变性5 s,60 ℃退火35 s,72 ℃延伸20 s,循环40次; 每5 s增加0.5 ℃至95 ℃,5 min制作熔解曲线。由NCBI获得基因序列,Primer 3.0设计引物,β-actin为内参,按照公式P=2-ΔΔCT计算基因相对表达量。
1.7 统计分析使用SPSS 24.0进行ANOVA分析,Duncan’s法进行组间多重比较,GraphPad Prism 8进行绘图。P>0.05表示差异不显著,P < 0.05表示差异显著。
2 结果 2.1 健康三益菌对肉鸡肉品质的影响由表 2可知,与对照组相比,益生菌组的肉鸡腹脂率和剪切力降低,而胸肌率和IMF均提高(P>0.05)。
|
|
表 2 荧光定量PCR引物 Table 2 Primers for Real-time quantitative PCR |
测序结果如表 3,样本过滤后的clean reads占raw reads的98.26%~98.91%,测序碱基平均错误率均在0.026%以下,GC含量所占比例为49.09%~51.12%。由图 1可知,皮尔逊相关系数(R2)均大于0.925,表明测序结果适合后续分析; Venn分析表明,对照组和益生菌组的回肠组织中分别检测到13 317和12 971个基因,在两组均有表达的基因有12 754个。在检测到的全部13 534个基因中,益生菌组与对照组比较得到641个SDEGs,其中上调274个,下调367个(图 2)。图 3为SDEGs的聚类分析结果,同一处理下的重复组基因表达模式更加接近,说明本试验的数据可靠,组内一致性和组间差异较好,且健康三益菌可调控黄麻肉鸡中基因的表达。
|
|
表 2 健康三益菌对肉鸡肉品质的影响 Table 2 Effects of healthy tribeneficial bacteria on meat quality of broilers |
|
|
表 3 序列质量和比对信息汇总统计 Table 3 Summary statistics for sequence quality and alignment information |

|
图 1 转录组Pearson相关系数(a)和转录组Venn分析(b) Fig. 1 Pearson correlation coefficient(a)and Venn analysis(b)of transcriptome |

|
图 2 差异表达基因火山图 Fig. 2 Volcano map of differentially expressed genes |

|
图 3 差异表达基因聚类图 Fig. 3 Heat cluster of differentially expressed genes |
如表 4所示,基于641个SDEGs,筛选出主要在脂质代谢中起调控作用的差异表达基因12个,与对照组相比,益生菌组中上调基因10个,下调基因2个(P < 0.05)。
|
|
表 4 脂质代谢显著差异表达基因 Table 4 Significantly different ially expressed genes involved in lipid metabolism |
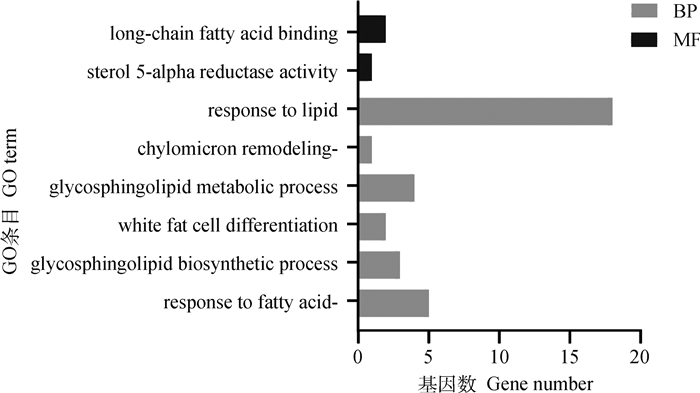
图 4为利用GO富集分析SDEGs参与的脂质代谢相关的生物过程,包括对脂肪酸的反应、鞘糖脂代谢过程、乳糜微粒重构和长链脂肪酸结合等。KEGG数据库分析发现,SDEGs参与的与脂质代谢有关代谢通路包括17条,包括甾类激素生物合成、PPAR信号通路和花生四烯酸的代谢等(图 5)。
|
BP、MF分别代表生物学过程和分子功能 BP and MF represents biological process and molecular function, respectively 图 4 GO富集脂质代谢条目 Fig. 4 GO enrichment lipid metabolism terms |

|
图 5 KEGG富集脂质代谢通路 Fig. 5 KEGG enrichment lipid metabolism pathway |
随机选择5个基因LPL、FABP4、GOS2、THRSP和CYP7B1进行qRT-PCR验证。如图 6所示,与对照组相比,益生菌组LPL、FABP4、GOS2和THRSP表达极显著上调(P < 0.001),CYP7B1表达量极显著下调(P < 0.001),这与转录组测序结果的趋势一致,说明测序结果可靠。

|
***表示差异极显著(P < 0.001) *** indicate extremely significant differences (P < 0.001) 图 6 qPCR验证差异基因表达 Fig. 6 Differential gene expression was verified by quantitative real-time PCR |
肉质品质可直观地反映出肉鸡脂质代谢情况。研究表明,益生菌对肉鸡的肉品质有积极影响[15],可通过提高氨基酸和蛋白质的含量增加胸肌重量,并通过减少腹部脂肪沉积来改善家禽胴体质量[16-17]。本研究发现,益生菌组肉鸡的胸肌重和IMF均有所增加,腹脂减少。陈婷等[18]在乌骨鸡饲料中添加复合益生菌产生了相似的结果。Resnyk等[19]发现,生长性能相同的肥系肉鸡会将更多的能量和营养物质分配到腹脂中,而瘦系肉鸡会将更多的蛋白质沉积到胸肌中。表明本试验中的健康三益菌可调节营养物质的分配,改善肉鸡肉品质。鸡IMF含量是决定鸡肉品质和健康的最重要因素[20],IMF的适度增加可以减少单位面积肌纤维的数量,而肌纤维和肌肉结缔组织、肌浆蛋白是构成肌肉嫩度的主要物质基础,因此,肌内脂肪的增加可使肌肉嫩度提升[21-22]。剪切力是评估肉类嫩度的直观指标[23]。陈婷等[18]发现,复合益生菌可使鸡胸肌剪切力降低,与本试验结果相同。表明健康三益菌可通过提高IMF沉积,增加肌肉嫩度,降低剪切力,改善鸡肉脂质代谢。
脂质代谢是由多个基因和调控因子通过不同的信号途径所调节的复杂的过程,本试验通过肉鸡回肠的转录组测序结果分析发现,健康三益菌调控的主要脂质代谢SDEGs有12个,包括LPL、SCD、FABP4、FABP3、PLIN1、GOS2、GGT5、RBP7、CYP7B1、CYP2C18、THRSP和THEM4,先前的报道显示,这些基因参与脂肪生成、活化、转运、分解和沉积等过程的调控[24-25]。其中,LPL在641个SDEGs中差异最显著,编码称为脂蛋白脂肪酶的糖蛋白酶。这种糖蛋白酶可水解乳糜微粒和TG,生成的游离脂肪酸、甘油单酯和乳糜微粒残留物可供机体使用或贮存[26]。Lee等[27]研究表明,植物乳杆菌可降低小鼠血清中TG,提高肝中LPL mRNA水平。LPL的高表达被认为与脂肪沉积有关[28]。Zhao等[29]认为,添加丁酸梭菌的肉鸡肌肉组织中LPL的高表达可能是IMF增加的原因。本试验益生菌组LPL的过表达可能也与IMF增加有关。SCD、FABP3、FABP4和THRSP也与IMF沉积有关。SCD能够将棕榈酸和硬脂酸转化为棕榈油酸和油酸,棕榈酸和油酸是最丰富的长链脂肪酸,主要用于合成TG[30]。Choi等[31]发现,植物乳杆菌通过下调肝中SCD的表达来减轻小鼠肥胖。本研究中,肉鸡回肠的SCD在益生菌组中上调,但腹脂减少,并未导致肉鸡的过度肥胖,产生这种结果的原因可能是在不同物种SCD的作用不同,肉鸡肠道中高SCD表达导致IMF的增加而非腹部脂肪的增加。FABP3和FABP4属于FABP家族,其编码的蛋白质负责脂肪酸的转运和代谢,且在脂肪生成、分解以及稳态维持等方面起作用[32]。FABP3可将脂肪酸从细胞膜转运至细胞内特定位点,FABP4可调控PPARγ在脂肪酸转运和代谢中起重要作用[25]。据报道,FABP可促进脂肪酸的转运[33]。本试验益生菌组中FABP3和FABP4的回肠表达水平增加,说明健康三益菌可促进肉鸡肠道脂肪酸向特定位点的转运。研究表明,THRSP与牛肉中大理石花纹的含量高度相关,其在脂肪生成的调节方面起重要作用[34]。Yin等[32]表明,THRSP在遗传性肥胖鸟类中高度表达。Schering等[35]认为,THRSP表达增加是IMF含量高的结果而非原因。这就可以解释为什么本试验健康三益菌使肉鸡回肠THRSP表达增加却未导致肥胖。CYP7B1在大脑、肝、生殖道等多个部位表达,在不同组织中生理功能不同[36]。CYP7B1作为与脂肪分解有关的基因,在瘦系鸡腹部脂肪中表达高于肥系鸡[19]。本试验中,CYP7B1表达量在益生菌组的下调,说明健康三益菌可能通过降低其表达来抑制肉鸡脂肪分解。脂质代谢相关SDEGs中,PLIN1和GOS2也在脂肪的分解中起调控作用。机体处于稳态时,PLIN1包被在脂滴表面; 当所需能量增加时,其表达量下降,促进脂肪分解[37]。PLIN1的高表达被认为促进了鸡体内的脂肪积累[30],PLIN1的下调导致鸟类空肠脂质水平降低[32]。Martinez-Botas等[38]表明PLIN1活性的丧失降低了脂肪细胞内的脂质水平,导致小鼠消瘦。GOS2参与TG的分解代谢[39],某些药物可通过下调大鼠中GOS2的表达来促进脂肪分解达到减肥的目的[40]。本试验PLIN1和GOS2的过表达进一步说明了健康三益菌抑制了肉鸡的脂肪分解。RBP7、CYP2C18、THEM4和GGT5也参与脂质代谢,但其在肉鸡体内的调控作用尚且有待研究。
基于已鉴定的SDEGs,通过GO富集和KEGG通路富集探索健康三益菌对肉鸡脂质代谢的调控网络。GO富集表示,LPL、SCD参与到对脂肪酸的反应、乳糜微粒重构以及对脂质反应的生物过程中; FABP3和FABP4参与白脂肪细胞分化以及长链脂肪酸结合等过程。KEGG富集发现,PPAR信号通路是脂质代谢的主要信号通路,脂肪沉积和转运基因(FABP3、FABP4、PLIN1、LPL、SCD)在此通路中富集,脂质代谢相关SDEGs还富集于甾类激素生物合成、亚油酸新陈代谢、不饱和脂肪酸的生物合成、脂肪细胞中的脂肪分解等脂肪沉积、分解相关通路。结果表明,健康三益菌通过调控以上通路促进肠道对脂肪的吸收转运并使肉鸡脂肪生成和沉积增加。但SDEGs在以上通路中的具体作用机制尚不明确,且目前尚缺乏研究表明益生菌对肉鸡脂质代谢通路的影响,因此其具体调控机制需要进一步的探索研究。
4 结论健康三益菌可通过调节LPL、PLIN1、GOS2、SCD、FABP3、FABP4、RBP7、THRSP、CYP7B1、CYP2C18、THEM4和GGT5等基因的表达以及PPAR信号通路、对脂质的反应等通路,可能促进肉鸡肠道对脂肪的吸收转运以及IMF沉积,抑制脂肪分解,降低腹脂含量,进而影响肉鸡脂质代谢但不会引起过度肥胖。
| [1] |
樊红平, 侯水生, 黄苇, 等. 鸡、鸭消化系统解剖组织学的比较研究[C]//中国畜牧兽医学会家禽学分会第七次代表大会暨第十二次全国家禽学术讨论会论文集. 成都: 中国畜牧兽医学会, 2005: 596-600. FAN H P, HOU S S, HUANG W, et al. Comparative study on anatomy and histology of digestive system in chicken and duck[C]//Proceedings of the 7th Congress of Poultry Science Branch of Chinese Society of Animal Husbandry and Veterinary Medicine and the 12th National Poultry Symposium. Chengdu: Chinese Society of Animal Husbandry and Veterinary Medicine, 2005: 596-600. (in Chinese) |
| [2] |
CHEN G H, CHEN J H, WU J W, et al. Integrative analyses of mRNA expression profile reveal SOCS2 and CISH play important roles in GHR mutation-induced excessive abdominal fat deposition in the sex-linked dwarf chicken[J]. Front Genet, 2021, 11: 610605. DOI:10.3389/fgene.2020.610605 |
| [3] |
ZHANG T, ZHANG X Q, HAN K P, et al. Genome-wide analysis of lncRNA and mRNA expression during differentiation of abdominal preadipocytes in the chicken[J]. G3 (Bethesda), 2017, 7(3): 953-966. DOI:10.1534/g3.116.037069 |
| [4] |
ZHANG X Y, WU M Q, WANG S Z, et al. Genetic selection on abdominal fat content alters the reproductive performance of broilers[J]. Animal, 2018, 12(6): 1232-1241. DOI:10.1017/S1751731117002658 |
| [5] |
LIU L, LIU X J, CUI H X, et al. Transcriptional insights into key genes and pathways controlling muscle lipid metabolism in broiler chickens[J]. BMC Genomics, 2019, 20(1): 863. DOI:10.1186/s12864-019-6221-0 |
| [6] |
SHAH M, ZANEB H, MASOOD S, et al. Effect of zinc and probiotics supplementation on performance and immune organs morphology in heat stressed broilers[J]. South African J Anim Sci, 2018, 48(6): 1017-1025. |
| [7] |
TABASHSUM Z, PENG M F, ALVARADO-MARTINEZ Z, et al. Competitive reduction of poultry-borne enteric bacterial pathogens in chicken gut with bioactive Lactobacillus casei[J]. Sci Rep, 2020, 10(1): 16259. DOI:10.1038/s41598-020-73316-5 |
| [8] |
BILAL M, SI W, BARBE F, et al. Effects of novel probiotic strains of Bacillus pumilus and Bacillus subtilis on production, gut health, and immunity of broiler chickens raised under suboptimal conditions[J]. Poult Sci, 2021, 100(3): 100871. DOI:10.1016/j.psj.2020.11.048 |
| [9] |
叶思霖. 嗜酸乳杆菌对肉鸡肝脏胆固醇代谢的影响及机制[D]. 杨凌: 西北农林科技大学, 2020. YE S L. Effect and mechanism of lactobacillus acidophilus on liver cholesterol metabolism in broilers[D]. Yangling: Northwest A&F University, 2020. (in Chinese) |
| [10] |
SHOKRYAZDAN P, JAHROMI M F, LIANG J B, et al. Effects of a Lactobacillus salivarius mixture on performance, intestinal health and serum lipids of broiler chickens[J]. PLoS One, 2017, 12(5): e0175959. DOI:10.1371/journal.pone.0175959 |
| [11] |
WANG H S, NI X Q, QING X D, et al. Live probiotic Lactobacillus johnsonii BS15 promotes growth performance and lowers fat deposition by improving lipid metabolism, intestinal development, and gut microflora in broilers[J]. Front Microbiol, 2017, 8: 1073. DOI:10.3389/fmicb.2017.01073 |
| [12] |
MARKOWIAK P, ŚLIŻEWSKA K. The role of probiotics, prebiotics and synbiotics in animal nutrition[J]. Gut Pathog, 2018, 10(1): 21. DOI:10.1186/s13099-018-0250-0 |
| [13] |
ALVARENGA R R, ZANGERONIMO M G, PEREIRA L J, et al. Lipoprotein metabolism in poultry[J]. World Poultry Sci J, 2011, 67(3): 431-440. DOI:10.1017/S0043933911000481 |
| [14] |
ZHANG C J, LIU Y M, CHEN S S, et al. Effects of intranasal pseudorabies virus AH02LA infection on microbial community and immune status in the ileum and colon of piglets[J]. Viruses, 2019, 11(6): 518. DOI:10.3390/v11060518 |
| [15] |
KRYSIAK K, KONKOL D, KORCZYŃSKI M. Overview of the use of probiotics in poultry production[J]. Animals (Basel), 2021, 11(6): 1620. |
| [16] |
AZIZ N H, KHIDHIR Z K, HAMA Z O, et al. Influence of probiotic (miaclost) supplementation on carcass yield, chemical composition and meat quality of broiler chick[J]. J Anim Poult Prod, 2020, 11(1): 9-12. |
| [17] |
HIDAYAT M N, MALAKA R, AGUSTINA L, et al. Abdominal fat percentage and carcass quality of broiler given probiotics Bacillus spp.[J]. Sci Res J, 2016, 4(10): 33-37. |
| [18] |
陈婷, 廉俊红, 吴佳韩, 等. 复合益生菌发酵饲料对雪峰乌骨鸡日粮表观消化率及肉品质的影响[J]. 中国畜牧兽医, 2019, 46(10): 2964-2972. CHEN T, LIAN J H, WU J H, et al. Effects of compound probiotics fermentation feed on nutritional apparent digestibility and meat quality of Xuefeng Black-boned chicken[J]. China Animal Husbandry & Veterinary Medicine, 2019, 46(10): 2964-2972. (in Chinese) |
| [19] |
RESNYK C W, CARRÉ W, WANG X F, et al. Transcriptional analysis of abdominal fat in genetically fat and lean chickens reveals adipokines, lipogenic genes and a link between hemostasis and leanness[J]. BMC Genomics, 2013, 14(1): 557. DOI:10.1186/1471-2164-14-557 |
| [20] |
PAMPOUILLE E, BERRI C, BOITARD S, et al. Mapping QTL for white striping in relation to breast muscle yield and meat quality traits in broiler chickens[J]. BMC Genomics, 2018, 19(1): 202. DOI:10.1186/s12864-018-4598-9 |
| [21] |
王贝贝, 李琦华, 李玉珑, 等. 肉鸡肌内脂肪沉积的研究进展[J]. 黑龙江畜牧兽医, 2014(11): 65-67. WANG B B, LI Q H, LI Y L, et al. Research progress of intramuscular fat deposition in broilers[J]. Heilongjiang Animal Science and Veterinary Medicine, 2014(11): 65-67. (in Chinese) |
| [22] |
YU L H, PENG Z, DONG L, et al. Enterococcus faecium NCIMB 10415 supplementation improves the meat quality and antioxidant capacity of muscle of broilers[J]. J Anim Physiol Anim Nutr (Berl), 2019, 103(4): 1099-1106. |
| [23] |
PURCHAS R W, BURNHAM D L, MORRIS S T. Effects of growth potential and growth path on tenderness of beef longissimus muscle from bulls and steers[J]. J Anim Sci, 2002, 80(12): 3211-3221. DOI:10.2527/2002.80123211x |
| [24] |
ALBERDI G, RODRÍGUEZ V M, MIRANDA J, et al. Changes in white adipose tissue metabolism induced by resveratrol in rats[J]. Nutr Metab (Lond), 2011, 8(1): 29. DOI:10.1186/1743-7075-8-29 |
| [25] |
NEMATBAKHSH S, PEI C P, SELAMAT J, et al. Molecular regulation of lipogenesis, adipogenesis and fat deposition in chicken[J]. Genes(Basel), 2021, 12(3): 414. |
| [26] |
PAPAH M B, ABASHT B. Dysregulation of lipid metabolism and appearance of slow myofiber-specific isoforms accompany the development of wooden breast myopathy in modern broiler chickens[J]. Sci Rep, 2019, 9(1): 17170. DOI:10.1038/s41598-019-53728-8 |
| [27] |
LEE E, JUNG S R, LEE S Y, et al. Lactobacillus plantarum strain Ln4 attenuates diet-induced obesity, insulin resistance, and changes in hepatic mRNA levels associated with glucose and lipid metabolism[J]. Nutrients, 2018, 10(5): 643. DOI:10.3390/nu10050643 |
| [28] |
VOSHOL P J, JONG M C, DAHLMANS V E H, et al. In muscle-specific lipoprotein lipase-overexpressing mice, muscle triglyceride content is increased without inhibition of insulin-stimulated whole-body and muscle-specific glucose uptake[J]. Diabetes, 2001, 50(11): 2585-2590. DOI:10.2337/diabetes.50.11.2585 |
| [29] |
ZHAO X, GUO Y M, GUO S S, et al. Effects of Clostridium butyricum and Enterococcus faecium on growth performance, lipid metabolism, and cecal microbiota of broiler chickens[J]. Appl Microbiol Biotechnol, 2013, 97(14): 6477-6488. DOI:10.1007/s00253-013-4970-2 |
| [30] |
LIU L, LIU X J, CUI H X, et al. Transcriptional insights into key genes and pathways controlling muscle lipid metabolism in broiler chickens[J]. BMC Genomics, 2019, 20(1): 863. DOI:10.1186/s12864-019-6221-0 |
| [31] |
CHOI W J, DONG H J, JEONG H U, et al. Lactobacillus plantarum LMT1-48 exerts anti-obesity effect in high-fat diet-induced obese mice by regulating expression of lipogenic genes[J]. Sci Rep, 2020, 10(1): 869. DOI:10.1038/s41598-020-57615-5 |
| [32] |
YIN F G, YU H, LEPP D, et al. Transcriptome analysis reveals regulation of gene expression for lipid catabolism in young broilers by butyrate glycerides[J]. PLoS One, 2016, 11(8): e0160751. DOI:10.1371/journal.pone.0160751 |
| [33] |
CLARKE D C, MISKOVIC D, HAN X X, et al. Overexpression of membrane-associated fatty acid binding protein (FABPpm) in vivo increases fatty acid sarcolemmal transport and metabolism[J]. Physiol Genomics, 2004, 17(1): 31-37. DOI:10.1152/physiolgenomics.00190.2003 |
| [34] |
MOISÁ S J, SHIKE D W, FAULKNER D B, et al. Central role of the PPARγ gene network in coordinating beef cattle intramuscular adipogenesis in response to weaning age and nutrition[J]. Gene Regul Syst Biol, 2014, 8. DOI:10.4137/GRSB.S11782 |
| [35] |
SCHERING L, ALBRECHT E, KOMOLKA K, et al. Increased expression of thyroid hormone responsive protein (THRSP) is the result but not the cause of higher intramuscular fat content in cattle[J]. Int J Biol Sci, 2017, 13(5): 532-544. DOI:10.7150/ijbs.18775 |
| [36] |
STILES A R, MCDONALD J G, BAUMAN D R, et al. CYP7B1:one cytochrome P450, two human genetic diseases, and multiple physiological functions[J]. J Biol Chem, 2009, 284(42): 28485-28489. DOI:10.1074/jbc.R109.042168 |
| [37] |
雷召雄, 魏大为, 汪书哲, 等. 牛PLIN1基因CDS区扩增及时序表达[J]. 西北农业学报, 2020, 29(10): 1472-1478. LEI Z X, WEI D W, WANG S Z, et al. Coding region sequence and temporal expression of bovine PLIN1 gene[J]. Acta Agriculturae Boreali-occidentalis Sinica, 2020, 29(10): 1472-1478. (in Chinese) |
| [38] |
MARTINEZ-BOTAS J, ANDERSON J B, TESSIER D, et al. Absence of perilipin results in leanness and reverses obesity in Leprdb/db mice[J]. Nat Genet, 2000, 26(4): 474-479. DOI:10.1038/82630 |
| [39] |
岑王敏. 猪G0S2分子作用机理及H-FABP和ACSL4基因SNPs位点对脂肪性状遗传效应的研究[D]. 雅安: 四川农业大学, 2013. CEN W M. Molecular mechanism of the porcine GOS2 and the genetic effect of H-FABP and ACSL4 SNPs locus on fat traits[D]. Ya'an: Sichuan Agricultural University, 2013. (in Chinese) |
| [40] |
洪士聪. 复方决明对SD营养性肥胖大鼠脂肪组织G0S2和ATGL基因表达的影响[D]. 武汉: 华中科技大学, 2013. HONG S C. Effects of jueming prescription on the expressions of G0S2 and ATGL in adipose tissue of SD diet-induced obese rats[D]. Wuhan: Huazhong University of Science and Technology, 2013. (in Chinese) |
(编辑 郭云雁)



