卵母细胞的成熟是哺乳动物雌性生殖和胚胎发育的关键,需要经历复杂的减数分裂停滞和恢复,该过程受多种激素和生长因子的调控[1]。紧密围绕在卵母细胞周围的卵丘颗粒细胞参与了卵母细胞减数分裂的阻滞和恢复,促进卵母细胞成熟.。卵丘颗粒细胞与卵母细胞相互作用,进行双向的营养传递与信息交换,这种联系在卵母细胞成熟过程中必不可少[2-3]。体外培养裸卵会导致细胞核和细胞质发育异常,但培养卵丘卵母细胞复合体(cumulus-oocyte complexes,COCs)时,细胞则正常成熟[4-5]。在卵母细胞成熟过程中,卵丘颗粒细胞逐渐扩展,卵丘扩展需要卵丘扩展因子透明质酸合酶2(hyaluronan synthase 2, HAS2)、穿透素3(pentraxin 3, PTX3)和前列腺素内过氧化物合酶2(prostaglandin-endoperoxide synthase 2, PTGS2)的参与,其中HAS2是生成透明质酸的关键酶,透明质酸是卵丘扩展基质的基本成分,PTGS2和PTX3具有维持扩展基质的作用[6-7]。卵母细胞自身分泌的生长分化因子9(growth differentiation factor 9, GDF9)和骨形态发生蛋白15(bone morphogenetic protein 15, BMP15)通过诱导卵丘扩展因子(PTGS2、HAS2和PTX3)表达,引起细胞外基质分泌,促进卵丘细胞扩展[8-9]。王兆琛等[10]体外添加GDF9和BMP15培养绵羊卵母细胞发现,GDF9和BMP15有利于卵丘扩展,促进卵母细胞成熟。卵丘颗粒细胞产生的环磷酸腺苷(cyclic adenosine monophosphate, cAMP)被运送到卵母细胞内[11],细胞内不断累积的cAMP能够启动cAMP依赖性蛋白激酶(PKA)途径,阻止成熟促进因子(maturation promoting factor,MPF)活化,使卵母细胞停滞在M期[12]。MPF是由细胞周期蛋白依赖性激酶1(cyclin-dependent kinase 1,CDK1)和细胞周期相关蛋白B(cyclin B)组成的复合物,主要调控卵母细胞减数分裂的恢复。MPF的活性主要由细胞质中的调节亚基cyclin B在细胞周期中的不断活化和衰减来调节,随着卵母细胞的成熟,cyclin B含量逐渐上升,诱导CDK1蛋白发生去磷酸化,形成活化的MPF进而调控卵母细胞的成熟[13]。
Tsutsui等[14]于2000年在日本鹌鹑中发现了一种C端具有RF酰胺结构的神经肽,它以剂量依赖的方式抑制了体外培养的鹌鹑垂体中促性腺激素释放激素的释放,因此被命名为促性腺激素抑制激素(GnIH)。随后的研究表明,哺乳动物体内存在与鸟类GnIH具有相似生理作用的同源物RFRP-3[15]。在哺乳动物中,Li等[16]发现RFRP-3的同源物GnIH除定位在猪的下丘脑等中枢神经系统外,同样在其它器官中也有表达,尤其在生殖系统细胞中,推测RFRP-3可能在猪的生殖系统中也发挥调控作用。研究表明,使用不同剂量的RFRP-3处理猪下丘脑细胞可以显著抑制促性腺激素释放激素分泌及相关基因水平的表达,处理猪颗粒细胞可以显著降低雌二醇(estradiol, E2)的浓度[17]。汪瑶等[18]也证实RFRP-3可作用于母猪促性腺激素释放激素的神经元,抑制E2的产生与分泌。此外,RFRP-3处理垂体细胞还能够抑制发情周期中猪促黄体生成素(luteinizing hormone, LH)的合成和分泌[19]。RFRP-3不仅可以调控猪生殖激素的分泌,还能够通过降低CDK1和cyclin B1 mRNA的表达水平,抑制cyclinB-CDK1复合物的活化,使部分细胞阻滞在G2/M期,进而影响猪颗粒细胞的增殖[18]。综上,目前RFRP-3关于猪的研究主要集中在对生殖激素和颗粒细胞的调控,还没有关于RFRP-3对猪卵母细胞影响的研究,因此,本试验通过在猪卵母细胞培养基中添加RFRP-3,探究其对体外培养猪卵母细胞成熟的影响,为揭示RFRP-3对哺乳动物繁殖的调控作用提供理论依据。
1 材料与方法 1.1 试验试剂TCM199培养基购自Gibco公司,人源RFRP-3购自Phoenix Pharmaceuticals公司,青霉素-链霉素溶液购自碧云天生物技术有限公司,马绒毛膜促性腺激素(PMSG)和人绒毛膜促性腺激素(HCG)购自宁波第二激素厂,猪促成熟因子(MPF)和猪环磷酸腺苷(cAMP)检测试剂盒购自睿信生物科技有限公司,反转录试剂盒购自北京全式金生物技术有限公司,细胞生长因子(EGF)、丙酮酸钠、L-半胱氨酸、牛血清白蛋白(BSA)、D-葡萄糖、胰岛素转铁蛋白(ITS)和矿物油均购自Sigma公司。
1.2 猪卵母细胞的采集与体外培养猪卵巢采集于长沙市红星盛业屠宰场,将卵巢置于含有2%双抗的37 ℃的生理盐水中,在2 h内运回实验室。使用10 mL的注射器抽取卵巢上直径约为3~6 mm的卵泡,静置15 min后弃去上清,在显微镜下用自制口吸管挑取胞质均匀、且包被3层及以上颗粒细胞的卵丘-卵母细胞复合体(COCs),将COCs培养于含激素的TCM199成熟培养基(Ⅰ液:卵泡液、丙酮酸钠、L-半胱氨酸、BSA、D-葡萄糖、孕马血清促性腺激素、人绒毛膜促性腺激素、表皮细胞生长因子和胰岛素转铁蛋白)中培养,培养条件为饱和湿度,38.5 ℃,5% CO2,培养22~24 h后换液,换成不含激素的TCM199成熟培养基(Ⅱ液:卵泡液、丙酮酸钠、L-半胱氨酸、BSA、D-葡萄糖)继续培养20~22 h。在培养基中添加10-6和10-8mol·L-1 RFRP-3为RFRP-3处理组,不添加RFRP-3为空白对照组。
1.3 卵丘细胞的扩展与卵母细胞成熟的判定体外培养猪卵母细胞44 h后,通过体式显微镜观察卵丘扩展情况并计算卵丘扩展指数(cumulus expansion index, CEI),卵丘扩展指数=组内COCs卵丘扩展情况分值总和/组内所有COCs数。卵丘扩展分级:0级表示卵丘无扩展,计0分; 1级表示仅最外围1~2层卵丘细胞发生扩展,计2分; 3级表示除放射冠之外所有卵丘细胞均发生扩展,计3分; 4级表示所有卵丘细胞均发生扩展,计4分。
体外培养猪卵母细胞44 h后,用0.3%的透明质酸酶脱去卵丘颗粒层,以第一极体的排出作为COCs成熟的判定标准,并统计各组的第一极体排出率。
1.4 实时荧光定量PCR从各组随机挑取150个卵母细胞,使用微量RNA提取试剂盒(QIAGEN,德国)提取卵母细胞RNA,根据反转录试剂盒说明书将RNA反转录为cDNA。实时荧光定量反应体系为:5 μL TB Green Premix Ex Taq II, 0.2 μL ROX Reference Dye, 正、反向引物各0.2 μL, cDNA 2 μL,DEPC水2.4 μL,每孔3个重复。反应条件:95 ℃ 30 s、95 ℃ 5 s、57 ℃ 30 s、95 ℃ 15 s、61 ℃ 60 s、95 ℃ 15 s、40个循环。各基因mRNA的相对表达量通过2-ΔΔCT法计算[20]。引物序列见表 1。
|
|
表 1 实时荧光定量PCR引物序列 Table 1 Primer sequences used in real-time PCR |
收集各组培养44 h的COCs,用0.3%透明质酸酶脱去颗粒层,使用蛋白酶溶解透明带,经过PBS洗净后,按照ELISA试剂盒说明书进行操作,检测卵母细胞cAMP和MPF的含量。
1.6 放射免疫检测雌二醇和孕酮的浓度分别收集COCs培养22和44 h的培养基,送北京北方生物技术研究所有限公司,通过放射免疫检测雌二醇和孕酮的浓度。
1.7 数据分析本试验数据均采用SPSS19.0软件进行分析,试验结果用“平均数±标准误(Mean±SEM)”表示,P < 0.05表示差异具有统计学意义,P < 0.01表示差异极显著。
2 结果 2.1 RFRP-3对猪COCs体外成熟率的影响体外添加不同浓度(0、10-6和10-8 mol·L-1) RFRP-3培养猪卵丘卵母细胞复合体44 h,然后脱去卵丘颗粒层,统计第一极体排出率。结果显示,与对照组((71.8±3.39)%)相比,10-8 mol·L-1 RFRP-3添加组的成熟率((53±1.80)%)极显著降低(P < 0.01),但添加10-6 mol·L-1 RFRP-3对猪COCs的成熟率((69.27±0.35)%)无显著影响(P>0.05)(图 1)。表明10-8 mol·L-1 RFRP-3可抑制猪COCs的体外成熟。

|
“*”表示与空白对照组相比差异显著(P < 0.05);“**”表示与空白对照组相比差异极显著(P < 0.01),下同 "*" indicates a significant difference compared with the control group (P < 0.05); "**" indicates a very significant difference compared to the control group (P < 0.01).The same as below 图 1 RFRP-3对猪COCs体外成熟率的影响 Fig. 1 Effect of RFRP-3 on in vitro maturation rate of porcine COCs |
根据上述试验结果,本研究后续试验将选择10-8 mol·L-1 RFRP-3作为RFRP-3处理组。添加10-8 mol·L-1 RFRP-3培养猪COCs 44 h后,通过显微镜观察各组的卵丘扩展情况并计算卵丘扩展指数。结果显示,RFRP-3添加组的卵丘扩展指数(3.46±0.1)与对照组(3.24±0.08)相比无明显变化(P>0.05)(图 2A-E)。采用qRT-PCR检测RFRP-3添加组中卵丘扩展相关因子PTGS2、HAS2和PTX3基因的表达水平。结果如图 2F所示,10-8 mol·L-1 RFRP-3处理COCs可极显著下调PTGS2、HAS2和PTX3基因的表达水平(P < 0.01)。表明添加RFRP-3对猪COCs卵丘扩展无影响,但可降低相关卵丘扩展因子的表达水平。

|
A.Control组卵丘细胞扩展情况(40×); B. Control组卵丘细胞扩展情况(100×); C.RFRP-3组卵丘细胞扩展情况(40×); D. RFRP-3组卵丘细胞扩展情况(100×); E. RFRP-3对卵丘扩展的影响; F. RFRP-3对卵丘扩展因子表达的影响 A. Cumulus cell expansion in the control group (40×); B. Cumulus cell expansion in the control group (100×); C. Cumulus cell expansion in the RFRP-3 group (40×); D. Cumulus cell expansion in the RFRP-3 group (100×); E. Effect of RFRP-3 on cumulus expansion; F. Effect of RFRP-3 on expression of cumulus expansion factors 图 2 RFRP-3对猪COCs卵丘细胞扩展的影响 Fig. 2 Effect of RFRP-3 on the expansion of porcine COCs cumulus cells |
为进一步探索RFRP-3抑制猪COCs体外成熟的机制,添加RFRP-3培养COCs成熟后,脱去卵丘颗粒层,收集卵母细胞裂解物,采用ELISA检测卵母细胞胞质中MPF和cAMP的含量。结果表明,RFRP-3添加组与空白对照组相比可极显著降低细胞质中MPF的含量(P < 0.01),但对cAMP的含量无明显影响(P >0.05)(图 3A, 3B)。采用qRT-PCR检测添加RFRP-3培养COCs后对GDF9和BMP15表达的影响。结果如图 3C所示,与对照组相比,添加RFRP-3可极显著提高卵母细胞GDF9基因的表达(P < 0.01),显著提高BMP15的表达(P < 0.05)。
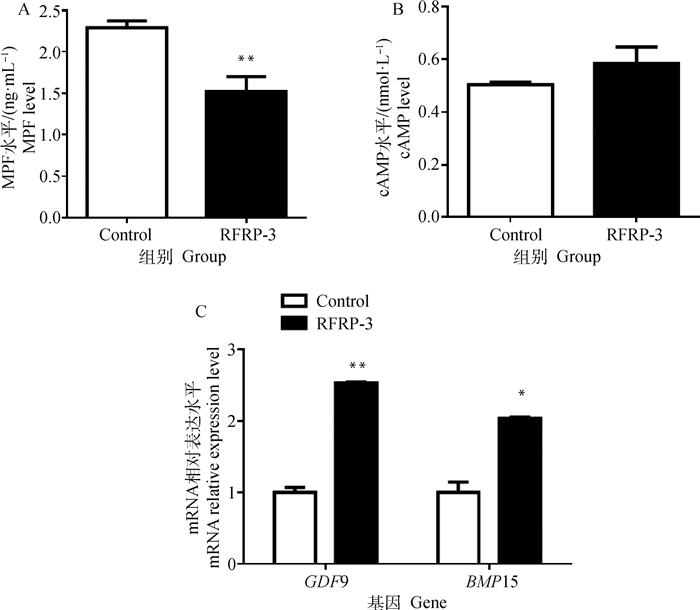
|
A.MPF水平; B. 环磷酸腺苷水平; C. 生长分化因子9和骨形态发生蛋白15基因的表达 A. The concentration of MPF; B. The concentration of cyclic adenosine monophosphate; C. Expression of growth differentiation factor 9 and bone morphogenetic protein 15 genes 图 3 RFRP-3对猪COCs成熟相关因子的影响 Fig. 3 Effects of RFRP-3 on maturation related factors of porcine COCs |
添加RFRP-3培养COCs成熟后,脱去卵丘颗粒层,收集卵母细胞裂解物,利用qRT-PCR检测猪卵母细胞中细胞周期相关基因CCNB1和CDK1的表达水平。结果表明,与对照组相比,添加RFRP-3培养猪COCs可以显著降低卵母细胞中CCNB1和CDK1基因的表达水平(P < 0.05,图 4)。

|
图 4 RFRP-3对猪卵母细胞周期相关基因表达的影响 Fig. 4 Effects of RFRP-3 on the expression of cell cycle related genes in porcine COCs |
添加RFRP-3培养COCs后,分别收集22(Ⅰ液)和44 h(Ⅱ液)的培养基,检测培养基中雌二醇和孕酮的浓度。结果表明,RFRP-3可极显著降低培养基中雌二醇(E2)和孕酮(P4)的浓度(P < 0.01)(图 5)。

|
A.雌二醇浓度; B.孕酮浓度 A. Concentration of estradiol; B. Concentration of progesterone 图 5 RFRP-3对猪COCs激素分泌的影响 Fig. 5 Effects of RFRP-3 on COCS hormone secretion in porcine |
在哺乳动物卵巢中,RFRP-3主要表达在颗粒细胞和黄体细胞中,在卵母细胞中的表达研究较少。Wang等[21]研究表明,添加10-6和10-8 mol·L-1RFRP-3可显著降低猪颗粒细胞cyclin B1和CDK1的表达水平。cyclin B和CDK1可以组成异二聚复合体(即成熟促进因子,MPF),MPF对于卵母细胞的减数分裂恢复是必不可少的,因此本试验选择添加0、10-6和10-8 mol·L-1RFRP-3体外培养猪COCs,结果显示,添加10-8 mol·L-1RFRP-3可极显著降低卵母细胞的成熟率,而添加10-6 mol·L-1 RFRP-3对猪COCs的体外成熟率无显著影响。Singh等[22]用RFRP-3处理小鼠,也表明RFRP-3可以明显抑制卵泡发育,使得卵母细胞无法成熟。卵母细胞成熟的实质就是减数分裂的阻滞与恢复,该过程主要由cAMP和MPF调控。研究表明,高水平的cAMP可以抑制cyclin B的表达,磷酸化CDK1的苏氨酸Thr-14和酪氨酸Tyr-15残基,导致CDK/cyclin B蛋白复合物失活,MPF的活性被抑制[23],但随着卵母细胞的发育成熟,cAMP被cAMP磷酸二酯酶降解,cAMP浓度降低,诱导MPF活化,引起卵母细胞减数分裂恢复[24]。郭振伟等[25]体外添加褪黑素培养水牛卵母细胞发现,褪黑素能够抑制cAMP合成, 进而促进卵母细胞成熟。本研究利用ELISA法检测了猪卵母细胞cAMP和MPF的浓度,结果发现,RFRP-3可以使卵母细胞中MPF的浓度极显著降低,但cAMP的浓度无明显变化,推测正是这一变化导致卵母细胞减数分裂停滞,抑制卵母细胞的成熟。李赞[26]的研究也表明,添加减数分裂的抑制剂AMH,可降低MPF的浓度,维持高水平的cAMP,阻碍卵母细胞成熟。本试验通过qRT-PCR检测发现,卵母细胞CCNB1和CDK1的基因表达水平显著下降,推测RFRP-3可能是通过降低CCNB1和CDK1基因的表达,从而下调了MPF。与本研究结果类似,Wang等[21]研究表明,添加RFRP-3可以降低猪颗粒细胞cyclin B和CDK1的表达水平,抑制cyclin B-CDK1复合物(MPF)的激活从而诱导细胞G2/M期停滞,阻碍猪颗粒细胞增殖。推测RFRP-3可能通过调节MPF和cAMP的水平,进而调控卵母细胞的成熟。
GDF9和BMP15作为卵母细胞分泌因子,可以与卵丘颗粒细胞相互作用,促进卵丘扩展,调控卵母细胞成熟[27]。魏莉娜等[28]研究发现,卵母细胞中GDF9和BMP15参与调控卵母细胞的成熟。在本试验中,RFRP-3显著抑制了猪卵母细胞的成熟,但RFRP-3处理组中卵母细胞GDF9和BMP15的基因表达水平却显著升高。有研究表明,体外添加高浓度(300 ng·mL-1)的GDF9可以显著降低卵母细胞的成熟率[29],过表达BMP15可以阻碍卵母细胞的成熟[30],据此推测,体外添加RFRP-3可能通过增加卵母细胞内的GDF9和BMP15表达,进而抑制猪卵母细胞的成熟。
卵母细胞的成熟与排卵受多种细胞和信号因子的调控,其中卵丘细胞通过复杂的缝隙连接为卵母细胞提供部分营养物质,维持卵母细胞减数分裂阻滞和恢复,促进卵母细胞成熟[31]。赵子墨等[32]研究证明,培养COCs的存活率与成熟率显著高于裸卵组,表明卵丘细胞对于卵母细胞的成熟是必不可少的。其中卵丘细胞扩展在卵母细胞成熟过程中发挥着重要作用,卵丘扩展后卵丘细胞才能与卵母细胞进行营养传递与信息交换。本试验结果表明,在猪COCs体外培养体系中添加RFRP-3对卵丘细胞的扩展无明显影响。Nikoloff等[33]研究也证明,添加不饱和脂肪酸EPA,体外培养COCs虽然可以显著降低卵母细胞的成熟率,但对卵丘细胞扩展无明显影响,这一点与本研究结果一致。卵丘扩展需要卵丘基质来维持,卵丘基质的合成依赖于卵丘扩展因子[34]。Pan等[35]研究发现,在卵母细胞培养基中添加E2可以增强卵丘扩展因子(PTGS2、HAS2和PTX3)基因的表达,从而促进卵母细胞成熟。Nagyova等[36]研究证明,添加10 μmol·L-1表皮生长因子受体酪氨酸激酶的抑制剂(lapatinib),可以使卵丘扩展因子TNFAIP6和PTGS2的表达显著降低,阻碍猪卵母细胞成熟。本试验结果显示,RFRP-3可通过抑制卵丘扩展因子(PTGS2、HAS2和PTX3)的表达来降低卵母细胞的成熟。研究表明,卵丘颗粒细胞不仅通过卵丘扩展因子影响卵母细胞的成熟,颗粒细胞分泌的E2和P4也可以维持卵母细胞减数分裂的停滞与恢复,促进卵母细胞的成熟[37-38]。本研究结果显示,添加RFRP-3体外培养卵母细胞,可极显著降低培养基中E2和P4的浓度,表明RFRP-3可通过抑制卵丘颗粒细胞分泌E2和P4,进而抑制卵母细胞的成熟。
4 结论RFRP-3可以通过降低猪COCs卵丘扩展因子(PTGS2、HAS2和PTX3)、周期相关因子(CCNB1和CDK1)的基因表达,增加卵母细胞分泌因子(GDF9和BMP15)的基因表达,抑制卵母细胞MPF的生成,抑制卵丘颗粒细胞雌二醇和孕酮的分泌,进而抑制猪卵母细胞的成熟。本研究可以为揭示RFRP-3对哺乳动物卵母细胞的调控作用及机制提供理论依据。
| [1] |
张羽芳, 阳美霞, 汤亚茹, 等. 哺乳动物卵母细胞成熟相关因子研究[J]. 医学信息, 2020, 33(14): 17-20. ZHANG Y F, YANG M X, TANG Y R, et al. Study on factors related to maturation of mammalian oocytes[J]. Medical Information, 2020, 33(14): 17-20. DOI:10.3760/cma.j.issn.1000-8039.2020.14.127 (in Chinese) |
| [2] |
WIGGLESWORTH K, LEE K B, O'BRIEN M J, et al. Bidirectional communication between oocytes and ovarian follicular somatic cells is required for meiotic arrest of mammalian oocytes[J]. Proc Natl Acad Sci U S A, 2013, 110(39): E3723-E3729. |
| [3] |
RUSSELL D L, GILCHRIST R B, BROWN H M, et al. Bidirectional communication between cumulus cells and the oocyte: old hands and new players?[J]. Theriogenology, 2016, 86(1): 62-68. DOI:10.1016/j.theriogenology.2016.04.019 |
| [4] |
GE L, SUI H S, LAN G C, et al. Coculture with cumulus cells improves maturation of mouse oocytes denuded of the cumulus oophorus: observations of nuclear and cytoplasmic events[J]. Fertil Steril, 2008, 90(6): 2376-2388. DOI:10.1016/j.fertnstert.2007.10.054 |
| [5] |
田长永, 杨景晁, 马泽芳, 等. 卵丘细胞对辽宁绒山羊卵母细胞体外成熟与孤雌发育的影响[J]. 畜牧兽医学报, 2010, 41(5): 543-548. TIAN C Y, YANG J C, MA Z F, et al. Effects of cumulus cells on in vitro maturation and parthenogenetic development of Liaoning cashmere goats oocytes[J]. Acta Veterinaria et Zootechnica Sinica, 2010, 41(5): 543-548. (in Chinese) |
| [6] |
MAREI W F, ABAYASEKARA D R E, WATHES D C, et al. Role of PTGS2-generated PGE2 during gonadotrophin-induced bovine oocyte maturation and cumulus cell expansion[J]. Reprod Biomed Online, 2014, 28(3): 388-400. DOI:10.1016/j.rbmo.2013.11.005 |
| [7] |
CHAUBEY G K, KUMAR S, KUMAR M, et al. Induced cumulus expansion of poor quality buffalo cumulus oocyte complexes by Interleukin-1beta improves their developmental ability[J]. J Cell Biochem, 2018, 119(7): 5750-5760. DOI:10.1002/jcb.26688 |
| [8] |
LI Q L, RAJANAHALLY S, EDSON M A, et al. Stable expression and characterization of N-terminal tagged recombinant human bone morphogenetic protein 15[J]. Mol Hum Reprod, 2009, 15(12): 779-788. DOI:10.1093/molehr/gap062 |
| [9] |
PANGAS S A, MATZUK M M. The art and artifact of GDF9 activity: cumulus expansion and the cumulus expansion-enabling factor[J]. Biol Reprod, 2005, 73(4): 582-585. DOI:10.1095/biolreprod.105.042127 |
| [10] |
王兆琛, 赵勇超, 杜炜, 等. AREG对绵羊小腔卵泡卵母细胞体外成熟的影响[J]. 畜牧兽医学报, 2020, 51(6): 1238-1247. WANG Z C, ZHAO Y C, DU W, et al. Effect of AREG on in vitro maturation of small antral follicular oocytes of sheep[J]. Acta Veterinaria et Zootechnica Sinica, 2020, 51(6): 1238-1247. (in Chinese) |
| [11] |
EDRY I, SELA-ABRAMOVICH S, DEKEL N. Meiotic arrest of oocytes depends on cell-to-cell communication in the ovarian follicle[J]. Mol Cell Endocrinol, 2006, 252(1-2): 102-106. DOI:10.1016/j.mce.2006.03.009 |
| [12] |
LEAL G R, MONTEIRO C A S, SOUZA-FABJAN J M G, et al. Role of cAMP modulator supplementations during oocyte in vitro maturation in domestic animals[J]. Anim Reprod Sci, 2018, 199: 1-14. DOI:10.1016/j.anireprosci.2018.11.002 |
| [13] |
VALENCIA C, PÉREZ F A, MATUS C, et al. Activation of bovine oocytes by protein synthesis inhibitors: new findings on the role of MPF/MAPKs[J]. Biol Reprod, 2021, 104(5): 1126-1138. DOI:10.1093/biolre/ioab019 |
| [14] |
TSUTSUI K, SAIGOH E, UKENA K, et al. A novel avian hypothalamic peptide inhibiting gonadotropin release[J]. Biochem Biophys Res Commun, 2000, 275(2): 661-667. DOI:10.1006/bbrc.2000.3350 |
| [15] |
UBUKA T, INOUE K, FUKUDA Y, et al. Identification, expression, and physiological functions of Siberian hamster gonadotropin- inhibitory hormone[J]. Endocrinology, 2012, 153(1): 373-385. DOI:10.1210/en.2011-1110 |
| [16] |
LI X, SU J, LEI Z H, et al. Gonadotropin-inhibitory hormone (GnIH) and its receptor in the female pig: cDNA cloning, expression in tissues and expression pattern in the reproductive axis during the estrous cycle[J]. Peptides, 2012, 36(2): 176-185. DOI:10.1016/j.peptides.2012.05.008 |
| [17] |
LI X, SU J, FANG R, et al. The effects of RFRP-3, the mammalian ortholog of GnIH, on the female pig reproductive axis in vitro[J]. Mol Cell Endocrinol, 2013, 372(1-2): 65-72. DOI:10.1016/j.mce.2013.03.015 |
| [18] |
汪瑶, 李珣, 李敏婕, 等. GnIH对母猪生殖调控的研究[J]. 中国农业科学, 2014, 47(18): 3716-3724. WANG Y, LI X, LI M J, et al. Regulation of GnIH on reproduction of female pig[J]. Scientia Agricultura Sinica, 2014, 47(18): 3716-3724. DOI:10.3864/j.issn.0578-1752.2014.18.020 (in Chinese) |
| [19] |
ZMIJEWSKA A, CZELEJEWSKA W, DZIEKONSKI M, et al. Effect of kisspeptin and RFamide-related peptide-3 on the synthesis and secretion of LH by pituitary cells of pigs during the estrous cycle[J]. Anim Reprod Sci, 2020, 214: 106275. DOI:10.1016/j.anireprosci.2020.106275 |
| [20] |
PFAFFL M W. A new mathematical model for relative quantification in real-time RT-PCR[J]. Nucleic Acids Res, 2001, 29(9): e45. DOI:10.1093/nar/29.9.e45 |
| [21] |
WANG X Y, LI X, HU C H. RFRP-3, the mammalian ortholog of GnIH, induces cell cycle arrest at G2/M in porcine ovarian granulosa cells[J]. Peptides, 2018, 101: 106-111. DOI:10.1016/j.peptides.2018.01.006 |
| [22] |
SINGH P, KRISHNA A, TSUTSUI K. Effects of gonadotropin-inhibitory hormone on folliculogenesis and steroidogenesis of cyclic mice[J]. Fertil Steril, 2011, 95(4): 1397-1404. DOI:10.1016/j.fertnstert.2010.03.052 |
| [23] |
JOSEFSBERG L B Y, GALIANI D, LAZAR S, et al. Maturation-promoting factor governs mitogen-activated protein kinase activation and interphase suppression during meiosis of rat oocytes[J]. Biol Reprod, 2003, 68(4): 1282-1290. DOI:10.1095/biolreprod.102.006882 |
| [24] |
PAN B, LI J L. The art of oocyte meiotic arrest regulation[J]. Reprod Biol Endocrinol, 2019, 17(1): 8. DOI:10.1186/s12958-018-0445-8 |
| [25] |
郭振伟, 黄永军, 范威宏, 等. 褪黑素对水牛卵母细胞体外成熟的影响及其受体介导机制的探究[J]. 畜牧兽医学报, 2019, 50(2): 314-322. GUO Z W, HUANG Y J, FAN W H, et al. The effect of melatonin on the maturation of oocyte in vitro and its receptor-mediated mechanism in buffalo[J]. Acta Veterinaria et Zootechnica Sinica, 2019, 50(2): 314-322. (in Chinese) |
| [26] |
李赞. AMH对小鼠卵母细胞体外成熟及卵丘扩展的调控作用研究[D]. 武汉: 华中农业大学, 2018. LI Z. Regulation effect of AMH on in-vitro maturation of oocytes and cumulus expansion in mouse[D]. Wuhan: Huazhong Agricultural University, 2018. (in Chinese) |
| [27] |
GILCHRIST R B, LANE M, THOMPSON J G. Oocyte-secreted factors: regulators of cumulus cell function and oocyte quality[J]. Hum Reprod Update, 2008, 14(2): 159-177. DOI:10.1093/humupd/dmm040 |
| [28] |
魏莉娜, 方丛, 黄睿, 等. GDF9和BMP15在PCOS患者刺激周期卵母细胞不同成熟阶段的表达变化及意义[J]. 中华妇产科杂志, 2012, 47(11): 818-822. WEI L N, FANG C, HUANG R, et al. Change and significance of growth differentiation factor 9 and bone morphogenetic protein expression during oocyte maturation in polycystic ovary syndrome patients with ovarian stimulation[J]. Chinese Journal of Obstetrics and Gynecology, 2012, 47(11): 818-822. DOI:10.3760/cma.j.issn.0529-567x.2012.11.005 (in Chinese) |
| [29] |
彭中友, 孙俊铭, 李燕, 等. GDF9和FST调控猪卵母细胞成熟和胚胎早期发育[J]. 江苏农业学报, 2015, 31(3): 583-589. PENG Z Y, SUN J M, LI Y, et al. Growth differentiation factor 9 and follistatin regulating porcine oocyte maturation and early embryo development[J]. Jiangsu Journal of Agricultural Sciences, 2015, 31(3): 583-589. DOI:10.3969/j.issn.1000-4440.2015.03.019 (in Chinese) |
| [30] |
CLELLAND E S, TAN Q, BALOFSKY A, et al. Inhibition of premature oocyte maturation: a role for bone morphogenetic protein 15 in zebrafish ovarian follicles[J]. Endocrinology, 2007, 148(11): 5451-5458. DOI:10.1210/en.2007-0674 |
| [31] |
HSIEH M, ZAMAH A M, CONTI M. Epidermal growth factor-like growth factors in the follicular fluid: role in oocyte development and maturation[J]. Semin Reprod Med, 2009, 27(1): 52-61. DOI:10.1055/s-0028-1108010 |
| [32] |
赵子墨, 王庆凯, 郑梓, 等. 卵丘细胞对猪卵母细胞体外成熟发育及GPX3、GPX4基因表达的影响[J]. 黑龙江畜牧兽医, 2020(12): 51-55, 59, 160. ZHAO Z M, WANG Q K, ZHENG Z, et al. Effects of cumulus cells on porcine oocyte maturation-development and the expression GPX3 and GPX4 genes in vitro[J]. Heilongjiang Animal Science and Veterinary Medicine, 2020(12): 51-55, 59, 160. (in Chinese) |
| [33] |
NIKOLOFF N, CAMPAGNA A, LUCHETTI C, et al. Effects of EPA on bovine oocytes matured in vitro with antioxidants: impact on the lipid content of oocytes and early embryo development[J]. Theriogenology, 2020, 146: 152-161. DOI:10.1016/j.theriogenology.2019.11.028 |
| [34] |
CAMAIONI A, HASCALL V C, YANAGISHITA M, et al. Effects of exogenous hyaluronic acid and serum on matrix organization and stability in the mouse cumulus cell-oocyte complex[J]. J Biol Chem, 1993, 268(27): 20473-20481. DOI:10.1016/S0021-9258(20)80750-9 |
| [35] |
PAN Y Y, WANG M, WANG L B, et al. Estrogen improves the development of yak (Bos grunniens) oocytes by targeting cumulus expansion and levels of oocyte-secreted factors during in vitro maturation[J]. PLoS One, 2020, 15(9): e0239151. DOI:10.1371/journal.pone.0239151 |
| [36] |
NAGYOVA E, NEMCOVA L, MLYNARCIKOVA A, et al. Lapatinib inhibits meiotic maturation of porcine oocyte-cumulus complexes cultured in vitro in gonadotropin-supplemented medium[J]. Fertil Steril, 2013, 99(6): 1739-1748. DOI:10.1016/j.fertnstert.2012.12.040 |
| [37] |
LIU W, XIN Q L, WANG X, et al. Estrogen receptors in granulosa cells govern meiotic resumption of pre-ovulatory oocytes in mammals[J]. Cell Death Dis, 2017, 8(3): e2662. DOI:10.1038/cddis.2017.82 |
| [38] |
KUROWSKA P, MLYCZYŃSKA E, ESTIENNE A, et al. Expression and impact of vaspin on in vitro oocyte maturation through MAP3/1 and PRKAA1 signalling pathways[J]. Int J Mol Sci, 2020, 21(24): 9342. DOI:10.3390/ijms21249342 |
(编辑 郭云雁)



