2. 江苏省动物重要疫病与人兽共患病防控协同创新中心, 扬州 225009;
3. 扬州大学农业科技发展研究院(国际联合实验室), 扬州 225009;
4. 兴化市合陈畜牧兽医站, 泰州 225733
2. Jiangsu Collaborative Innovation Center for Prevention and Control of Important Animal Diseases and Zoonoses, Yangzhou 225009, China;
3. Institute of Agricultural Science and Technology Development(Joint International Research Laboratory of Agriculture & Agri-Product Safety), Yangzhou University, Yangzhou 225009, China;
4. Hechen Animal Husbandry and Veterinary Station of Xinghua, Taizhou 225733, China
镉(cadmium,Cd)是一种广泛存在于自然界的重金属,可通过消化、呼吸、皮肤等循环系统进入动物机体,半衰期长达20~30年[1]。动物摄入超过一定剂量的镉,可导致肾、肝、肺、骨骼和生殖器官损伤,并对免疫系统、心血管系统等产生毒性效应,进而引起多种疾病[2]。骨是镉毒性作用的靶器官之一,骨组织中的成骨细胞(osteoblast, OB)是确保骨组织Ca沉积与骨形成的关键细胞,而任何形式的OB损伤都可造成骨代谢稳态失衡,进而引起骨质疏松症等疾病。
骨髓间充质干细胞(bone marrow mesenchymal stem cells,BMSCs)向OB分化的过程中,存在一系列缜密的基因调控网络。其中,RUNX2(runt related transcription factor 2,RUNX2)属于Runt相关转录因子家族,不仅可以诱导BMSCs向OB分化,还可通过屏蔽与不同细胞系相关的基因区域来抑制非OB的分化;骨钙素(osteocalcin,OCN)作为OB成熟的标志,其基因启动子区的OB特异性顺式调控元件与RUNX2结合;碱性磷酸酶(alkaline phosphatase,ALP)在OB和OB前体细胞(osteoblast precursor cells,OBP)中均有表达;转录因子osterix(OSX) 是高度保守的锌指结构域,与骨组织发育密切相关,是OB分化的重要调节因子,和RUNX2依次作用于BMSCs[3]。研究发现,镉在一定程度上能够促进破骨细胞前体细胞(osteoclast precursor cells,OCP)向破骨细胞(osteoclast,OC)的分化,并对BMSCs向OB的分化具有抑制作用[4-6]。
葛根素(puerarin,Pur)是从中草药葛根中分离的异黄酮类衍生物,主要存在于豆科植物野葛或甘葛藤的根部,具有保护骨骼的作用,其治疗骨质疏松症的疗效已被报道[7]。葛根素具有促进OB增殖, 提高OB活性的作用[8]。Wnt/β-catenin通路对OB分化和骨形成有重要作用,镉可通过抑制Wnt/β-catenin通路导致OB分化受损,而葛根素可通过Wnt/β-catenin通路降低骨流失[5, 9-10]。葛根素对镉所致成骨发育的影响尚未阐明。因此,本研究旨在通过体内试验,研究镉暴露对大鼠股骨胫骨OB分化的抑制作用和葛根素对其的缓解效应及机制,为临床上葛根素防治镉暴露所致的骨组织成骨发育损伤提供理论基础。
1 材料与方法 1.1 实验动物清洁级21日龄雄性Sprague-Dawley(SD)大鼠40只,购自江苏大学比较医学中心。
1.2 主要试剂葛根素(Sigma,USA);BCA蛋白浓度测定试剂盒(上海碧云天生物技术有限公司,中国);蛋白酶抑制剂(苏州新赛美生物科技有限公司,中国);Wnt3A、LEF1、TCF1鼠源单抗(Santa Cruz Biotechnology (Shanghai) Co., Ltd.,USA);β-actin、β-catenin鼠源单抗(Cell signaling technology,Lnc,USA);羊抗鼠-HRP偶联抗体(Jackson ImmunoResearch Ltd.,USA);反转录试剂及荧光定量试剂(南京诺唯赞生物科技股份有限公司,中国);醋酸镉(cadmium acetate,CdAc2)及其他试剂均为国产分析纯。
1.3 主要仪器组织研磨机SN: 1001682(天根生化科技有限公司,中国);NanoDrop2000(Thermo Fisher,USA);Milli-Q Biocel型超纯水系统(Merck Millipore,USA);电子天平;ABI7500 qRT-PCR仪(ABI,USA);PROTEAH电泳仪(BIO-RAD,USA);Tanon-5200全自动化学发光成像分析系统(上海天能科技有限公司,中国);5810R高速冷冻离心机(Eppendorf,Germany);PinAAcle 900F火焰原子吸收光谱(PerkinElmer,美国)。
1.4 镉注射浓度及葛根素饲喂量的筛选1.4.1 筛选镉注射浓度 选用不同浓度CdAc2(1.25、2.5、5、10及15 mg·kg-1BW)进行预试验。每日腹腔注射,连续注射1周,选择不良反应较轻的剂量进行后续试验。
1.4.2 筛选葛根素饲喂量 葛根素的饲喂量参考张年宝等[11]研究中的注射剂量,根据大鼠体重进行换算,在当前研究中以每千克体重200 mg进行计算,并作为本研究中葛根素的饲喂量。
1.5 实验动物分组与染毒将大鼠笼养于清洁与安静的实验动物饲养室,饲养温度(22±1)℃。自由饮食1周后随机分为4组,每组10只,分别为对照组(CON)、镉(Cd)组、葛根素(Pur)组及镉+葛根素(Cd+Pur)组。葛根素组、镉+葛根素组大鼠每日灌服葛根素(200 mg·kg-1BW),对照组、镉组大鼠每日灌服相同剂量的纯净水,连续处理5周。从第5周开始,对照组与葛根素组大鼠每日腹腔注射生理盐水,镉组、镉+葛根素组大鼠每天腹腔注射醋酸镉(2.5 mg·kg-1BW),连续处理1周。饲喂5周后,对所有大鼠禁食禁水6 h,腹腔注射2%戊巴比妥钠溶液1 mL,麻醉后颈椎脱臼法处死大鼠,分离股骨与胫骨。
1.6 原子吸收光谱法测定大鼠骨骼镉、钙、磷含量取各组大鼠骨骼样品500 mg(湿重),60 ℃烘干过夜,微波消解法对样品进行消解[12],用超纯水定容至5 mL,按照标准参照物质(SRM、1598及NIST),使用火焰原子吸收光谱测定骨组织中镉、Ca及P含量。
1.7 免疫印迹法检测大鼠股骨胫骨中相关蛋白含量1.7.1 大鼠股骨胫骨总蛋白制备 取各组大鼠股骨胫骨500 mg,液氮研磨至小块状后用组织研磨机、RIPA裂解蛋白法制备总蛋白样品。股骨胫骨呈粉末状后加入RIPA裂解液冰上裂解30 min(RIPA中添加蛋白酶抑制剂,终浓度为1 mmol·L-1)。4 ℃,12 000 r·min-1离心10 min,吸取上清,BCA法测定总蛋白浓度,将各组蛋白样品浓度调为一致,添加5×SDS Loading Buffer,混匀,煮沸10 min,-20 ℃保存。
1.7.2 Western blot检测相关蛋白表达量 将蛋白样品加入凝胶泳道110V恒压电泳90 min。使用250 mA恒流,湿转法转PVDF膜90 min。将膜转移至5%脱脂乳溶液(TBST溶液稀释),室温封闭2 h。按照蛋白大小剪条带,分别加入β-catenin抗体、Wnt3A抗体、LEF1抗体、TCF1抗体、GAPDH抗体于摇床孵育过夜。TBST洗膜3次,每次10 min,再加入相应二抗室温孵育2 h。TBST洗膜3次,每次10 min。利用ECL化学发光试剂显影于Tanon-5200全自动化学发光成像分析系统。Image J软件分析相关条带灰度值。
1.8 qRT-PCR检测大鼠成骨相关基因表达量1.8.1 引物设计 根据NCBI查询到的基因全序列,用Blast设计引物,序列如表 1,GAPDH为内参。
|
|
表 1 目的基因引物序列 Table 1 Primers sequence of target genes |
1.8.2 qRT-PCR检测相关基因表达 取各组大鼠股骨胫骨500 mg,用组织研磨机结合TRIzol法提取RNA样品。每组样品加入1 mL TRIzol,静置5 min后加入0.2 mL三氯甲烷,剧烈震荡15 s,室温静置5 min;4 ℃ 12 000 r·min-1离心10 min,将上层无色水相转移至含有0.5 mL异丙醇的1.5 mL EP管,混匀,室温静置10 min,4 ℃ 12 000 r·min-1离心10 min;各管底出现的白色沉淀即RNA,弃去上清并加入1 mL 75%乙醇轻轻摇晃,4 ℃ 12 000 r·min-1离心5 min;弃去上清,晾干至RNA透明,加无酶水溶解,根据Prime ScriptTM RT Reagent Kits试剂盒说明进行反转录,SYBR premix EX TapTM Kits试剂盒通过ABI 7500实时荧光定量PCR仪进行qRT-PCR反应,采用2-△△CT法计算基因相对表达量。
1.9 数据与分析应用SPSS 21.0软件对数据进行显著性t-检验和单因素方差(one-way ANOVA)分析,结果以“平均值(Mean)±标准差(SD)”表示,*P < 0.05表示差异显著,**P < 0.01表示差异极显著。
2 结果 2.1 镉浓度筛选各浓度醋酸镉(CdAc2)腹腔注射大鼠结果显示,1.25 mg·kg-1BW组无明显不良反应,2.5 mg·kg-1BW组大鼠精神沉郁、饮食欲下降,个别大鼠出现腹泻症状,5 mg·kg-1BW组精神沉郁且饮食欲下降,被毛粗乱,不良症状较2.5 mg·kg-1BW组严重,10 mg·kg-1BW组饮食欲下降且所有大鼠于第3日死亡,15 mg·kg-1BW组所有大鼠于第2日死亡。选择轻微不良反应组(2.5 mg·kg-1BW)进行后续试验。
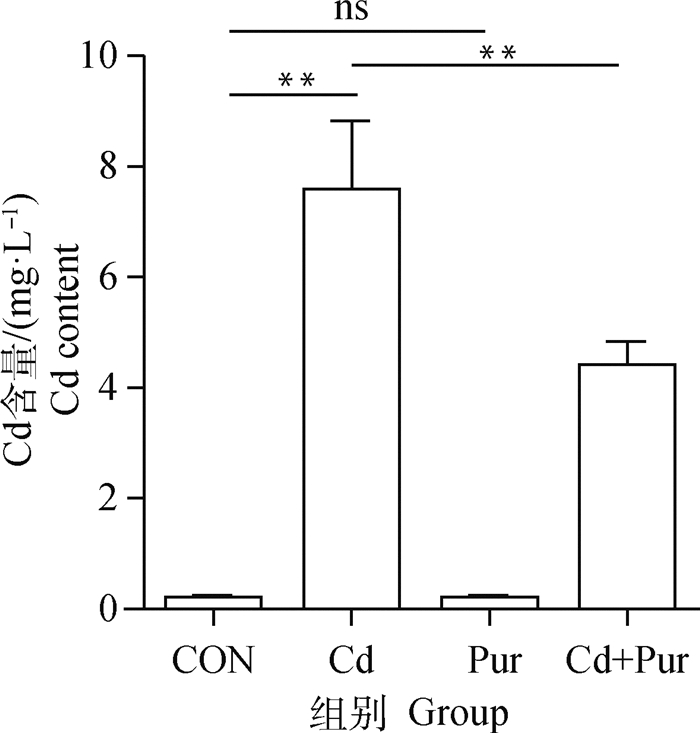
2.2 镉暴露及葛根素处理对大鼠股骨胫骨中镉含量的影响由大鼠股骨胫骨镉含量检测结果显示(图 1),对照组与葛根素组镉含量较低,两组间无明显差异。与对照组相比,镉组股骨胫骨中镉含量极显著升高(P < 0.01);与镉组相比,镉+葛根素组中镉含量极显著降低(P < 0.01)。
|
*差异显著(P<0.05);**差异极显著(P<0.01);ns表示无显著差异,下同 * Indicate significant difference (P < 0.05);** Indicate extremely significant difference (P < 0.01); ns indicate no significant difference, the same as below 图 1 SD大鼠股骨胫骨中镉含量 Fig. 1 Content of cadmium in femur and tibia of SD rats |
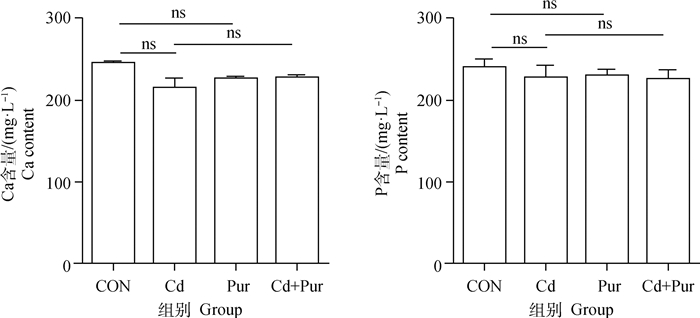
大鼠股骨胫骨中Ca和P含量检测结果显示(图 2),各组大鼠股骨胫骨中Ca和P含量无显著性差异(P>0.05)。
|
图 2 镉与葛根素对SD大鼠股骨胫骨中Ca和P含量的影响 Fig. 2 Effects of Cadmium and puerarin on Ca and P content in femur and tibia of SD rat |
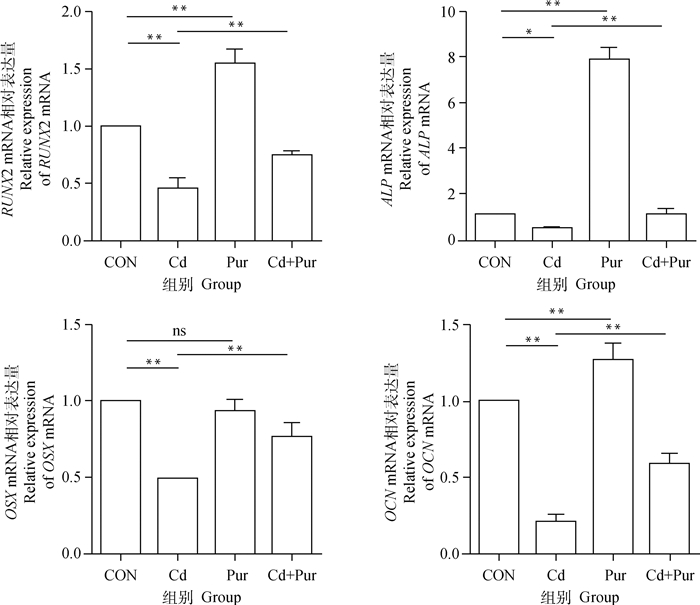
qRT-PCR检测大鼠股骨胫骨中成骨相关基因RUNX2、ALP、OCN和OSX的mRNA水平。结果如图 3,与对照组相比,镉组大鼠股骨胫骨中RUNX2、OCN、OSX mRNA转录水平极显著降低(P < 0.01),ALP mRNA转录水平显著降低(P < 0.05);葛根素组大鼠股骨胫骨中RUNX2、ALP、OCN mRNA转录水平极显著升高(P < 0.01),OSX mRNA表达水平无显著差异(P>0.05)。与镉组相比,镉+葛根素组大鼠股骨胫骨中RUNX2、OCN、OSX和ALP mRNA转录水平均极显著升高(P < 0.01)。
|
图 3 镉与葛根素对SD大鼠股骨胫骨中成骨相关基因转录的影响 Fig. 3 Effects of cadmium and puerarin on the transcription of osteogenic-related genes in femur and tibia of SD rat |
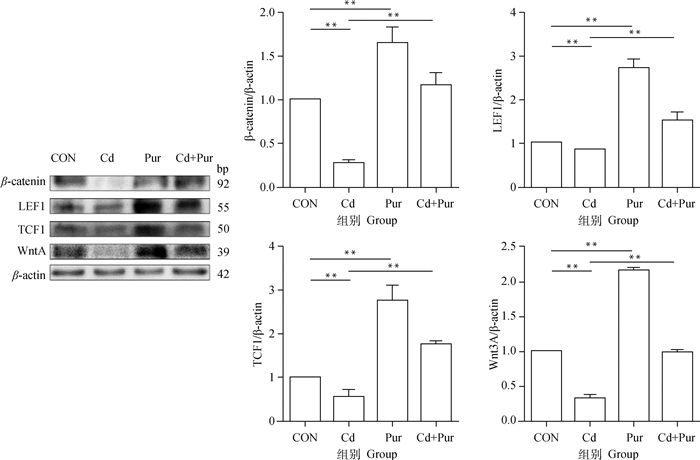
蛋白免疫印迹结果显示(图 4),与对照组相比,镉组大鼠股骨胫骨中Wnt/β-catenin通路相关蛋白表达水平极显著降低(P < 0.01);葛根素组大鼠股骨胫骨中Wnt/β-catenin通路相关蛋白表达水平极显著升高(P < 0.01)。与镉组相比,镉+葛根素组大鼠股骨胫骨中Wnt/β-catenin通路相关蛋白表达水平均极显著升高(P < 0.01)。
|
图 4 镉与葛根素对SD大鼠股骨胫骨中Wnt/β-catenin通路相关蛋白表达的影响 Fig. 4 Effects of cadmium and puerarin on the expression of Wnt/β-catenin pathway related proteins in femur and tibia of SD rats |
镉是广泛存在于环境中的有毒重金属,可对肾产生损伤作用,致肾小管对Ca和(或)P重吸收障碍,血清中Ca和(或)P缺乏,继而动员骨中Ca和(或)P进入血液,造成骨丢失及骨质疏松[13-15]。张翔[16]研究发现,低剂量镉暴露20周,可导致骨中的Ca和(或)P沉积下调。本研究显示,与对照组相比,镉组大鼠股骨胫骨Ca和P沉积减少,但无显著性差异,这可能与镉作用时间较短有关。
研究发现,镉可以促进人BMSCs向成脂细胞分化,抑制其向OB分化,间接造成骨密度下降,导致骨质疏松症等疾病[17]。骨代谢平衡是OC行使的骨吸收和OB行使的骨重建共同维持[18],其中,OB具有形成骨骼、调节细胞外骨基质成分的构成和使Ca及P沉积的功能。OB起源于BMSCs,在转录因子RUNX2和OSX的调控下向成骨祖细胞定向分化,最终分化为骨细胞[3]。RUNX2是促进骨形成的关键调控因子,为OB分化的早期标志物,并在骨形成中起关键作用[19]。ALP是BMSCs向OB分化的早期标志物,可分解磷酸酯维持矿化所需的无机磷酸盐,其活性水平可以反映OB分化的程度[20-21]。OCN参与胚胎骨形成,并在骨重建过程中被激活,是OB成熟的重要标志[22]。此外,OSX是RUNX2调控OB分化过程中的下游靶点,可激活COL1A1(collagen type I alpha 1 chain,COL1A1)和OCN的转录,实现骨形成[23]。骨质疏松症主要是由BMSCs向OB分化失调所致。本研究结果显示,与对照组相比,镉组大鼠OB相关基因RUNX2及OCN的表达极显著降低,与Ma等[6]研究结果一致;OSX表达极显著降低,ALP显著降低,与Wu等[5]结果一致。此外,王怡等[24]研究发现,低浓度镉对体外OB有一定的毒性作用。Al-Ghafari等[25]研究发现,镉可直接作用于人OB,主要通过降低ATP含量,抑制线粒体活性和有氧呼吸来破坏细胞生物功能。这些研究表明,镉可对体内外OB的分化及骨矿化产生一定的抑制作用。
葛根素是一种从野葛的根中提取的植物激素,其化学结构和雌激素类似,具有一定的抗骨质疏松作用。为防止雌性大鼠体内不同水平雌激素的干扰,本研究选用了雄性SD大鼠。研究发现,葛根素具有促进OB分化的作用,可通过ERK1/2和p38-MAPK通路促进BMSCs向OB的分化[26]。葛根素和锌可抑制骨髓基质细胞成脂分化,间接促进其向OB分化,从而发挥抗骨质疏松作用[27]。此外,葛根素亦可与雌二醇协同作用,增加骨质疏松性骨折大鼠的骨密度[28]。本试验中,与对照组相比,葛根素组大鼠OB相关基因(RUNX2、OCN和ALP)的表达极显著升高;与镉组相比,镉+葛根素组骨中镉含量显著下降,且成骨相关基因(RUNX2、OCN、OSX与ALP)表达极显著升高,表明葛根素可促进OB分化,并减轻镉在股骨胫骨中的沉积。
另外,研究表明,Wnt/β-catenin信号通路可参与骨骼的生长,调控成骨祖细胞向OB分化[19, 29-30]。Wnt家族由许多高度保守的基因组成,其中Wnt3A可与卷曲蛋白结合后激活Wnt/β-catenin信号通路,在脊椎动物中,β-catenin可发挥核转录激活因子作用,在胞质内聚集发生核位移后,与TCF1和LEF1互作并介导Wnt信号传导及其下游基因转录[31-32]。在临床应用研究方面,葛根素可通过Wnt/β-catenin信号通路减轻来曲唑(一种非甾体芳香化酶抑制剂)引起的大鼠骨流失[10],葛根素可通过Wnt/β-catenin信号通路抑制人BMSCs成脂分化[33]。本研究中,与对照组相比,镉组大鼠股骨胫骨中Wnt/β-catenin信号通路相关蛋白(β-catenin、LEF1、TCF1与Wnt3A)的表达均极显著下调,与Wu等[5]报道一致。且本研究中葛根素组大鼠股骨胫骨中Wnt/β-catenin信号通路蛋白的表达极显著升高;与镉组相比,镉+葛根素组可使大鼠股骨胫骨中Wnt/β-catenin信号通路相关蛋白的表达均极显著上调,说明葛根素通过Wnt/β-catenin通路缓解镉暴露对大鼠股骨胫骨中OB分化的抑制作用。
4 结论综上所述,镉暴露可增加大鼠股骨胫骨中的镉沉积,并对OB分化造成一定的损伤,该过程涉及Wnt/β-catenin信号通路。此外,葛根素对镉抑制大鼠股骨胫骨中OB分化具有一定的缓解效应。
| [1] |
AKERSTROM M, BARREGARD L, LUNDH T, et al. The relationship between cadmium in kidney and cadmium in urine and blood in an environmentally exposed population[J]. Toxicol Appl Pharmacol, 2013, 268(3): 286-293. DOI:10.1016/j.taap.2013.02.009 |
| [2] |
张妮娅, 齐德生. 镉对畜禽的毒害作用及防制[J]. 饲料工业, 2002, 23(12): 37-39. ZHANG N Y, QI D S. Toxic effect of cadmium on livestock and poultry and its control[J]. Feed Industry, 2002, 23(12): 37-39. DOI:10.3969/j.issn.1001-991X.2002.12.014 (in Chinese) |
| [3] |
HOJO H, OHBA S. Gene regulatory landscape in osteoblast differentiation[J]. Bone, 2020, 137: 115458. DOI:10.1016/j.bone.2020.115458 |
| [4] |
WANG Y, FU Y X, GU J H, et al. Cadmium induces the differentiation of duck embryonic bone marrow cells into osteoclasts in vitro[J]. Vet J, 2014, 200(1): 181-185. DOI:10.1016/j.tvjl.2014.02.004 |
| [5] |
WU L, WEI Q Z, LV Y J, et al. Wnt/β-Catenin pathway is involved in cadmium-induced inhibition of osteoblast differentiation of bone marrow mesenchymal stem cells[J]. Int J Mol Sci, 2019, 20(6): 1519. DOI:10.3390/ijms20061519 |
| [6] |
MA Y G, RAN D, CAO Y, et al. The effect of P2X7 on cadmium-induced osteoporosis in mice[J]. J Hazard Mater, 2021, 405: 124251. DOI:10.1016/j.jhazmat.2020.124251 |
| [7] |
王国波, 罗波, 苟印尧, 等. 葛根素联合雌二醇对大鼠骨质疏松性骨折愈合的影响[J]. 中国临床药理学杂志, 2020, 36(14): 2052-2055. WANG G B, LUO B, GOU Y Y, et al. Effect of puerarin combined with estradiol on the healing of osteoporotic fracture in rats[J]. The Chinese Journal of Clinical Pharmacology, 2020, 36(14): 2052-2055. (in Chinese) |
| [8] |
方志辉, 张天锋. 葛根素对骨质疏松大鼠成骨细胞增殖和活性的影响及其作用机制[J]. 广西医学, 2020, 42(20): 2680-2684. FANG Z H, ZHANG T F. Effect of puerarin on osteoblast proliferation and activity and its action mechanism in rats with osteoporosis[J]. Guangxi Medical Journal, 2020, 42(20): 2680-2684. (in Chinese) |
| [9] |
ZHOU Y H, LIN J Y, SHAO J, et al. Aberrant activation of Wnt signaling pathway altered osteocyte mineralization[J]. Bone, 2019, 127: 324-333. DOI:10.1016/j.bone.2019.06.027 |
| [10] |
韩俊, 蒋现永, 叶恒, 等. 葛根素通过OPG/RANKL及Wnt/β-catenin信号通路介导对来曲唑引起骨流失的保护作用[J]. 中国骨质疏松杂志, 2020, 26(11): 1604-1608. HAN J, JIANG X Y, YE H, et al. Puerarin protects bone loss caused by letrozole via OPG/RANKL and Wnt/β-catenin signaling pathway[J]. Chinese Journal of Osteoporosis, 2020, 26(11): 1604-1608. DOI:10.3969/j.issn.1006-7108.2020.11.008 (in Chinese) |
| [11] |
张年宝, 程慧珍, 崔卫东, 等. 葛根素对肾性高血压大鼠的降压作用及对肾组织ANGⅡ的影响[J]. 中药药理与临床, 2010, 26(2): 26-29. ZHANG N B, CHENG H Z, CUI W D, et al. Antihypertensive effect of puerarin in renal hypertensive rats and its effect on level of angiotensin Ⅱ in renal tissue[J]. Pharmacology and Clinics of Chinese Materia Medica, 2010, 26(2): 26-29. (in Chinese) |
| [12] |
李燕群. 原子吸收光谱法在重金属铅镉分析中的应用进展[J]. 冶金分析, 2008, 28(6): 33-41. LI Y Q. Development and application of atomic absorption spectrometry in analysis of lead and cadmium[J]. Metallurgical Analysis, 2008, 28(6): 33-41. DOI:10.3969/j.issn.1000-7571.2008.06.007 (in Chinese) |
| [13] |
LIU F, LI Z F, WANG Z Y, et al. Role of subcellular calcium redistribution in regulating apoptosis and autophagy in cadmium-exposed primary rat proximal tubular cells[J]. J Inorg Biochem, 2016, 164: 99-109. DOI:10.1016/j.jinorgbio.2016.09.005 |
| [14] |
GE Z L, DIAO H P, JI X L, et al. Gap junctional intercellular communication and endoplasmic reticulum stress regulate chronic cadmium exposure induced apoptosis in HK-2 cells[J]. Toxicol Lett, 2018, 288: 35-43. DOI:10.1016/j.toxlet.2018.02.013 |
| [15] |
GE J, ZHANG C, SUN Y C, et al. Cadmium exposure triggers mitochondrial dysfunction and oxidative stress in chicken (Gallus gallus) kidney via mitochondrial UPR inhibition and Nrf2-mediated antioxidant defense activation[J]. Sci Total Environ, 2019, 689: 1160-1171. DOI:10.1016/j.scitotenv.2019.06.405 |
| [16] |
张翔. 低剂量镉联合高脂饮食对小鼠骨骼和糖脂代谢的影响及维生素D保护作用的研究[D]. 苏州: 苏州大学, 2019. ZHANG X. Effects of combined exposure to low-dose cadmium and high-fat diet on bone quality and glucose-lipid metabolism and protective effect of vitamin D in mice[D]. Suzhou: Soochow University, 2019. (in Chinese) |
| [17] |
胡友坤, 吴璐, 张样聪, 等. 氯化镉对人骨髓间充质干细胞成骨与成脂分化能力的影响[C]//中国毒理学会第九次全国毒理学大会论文集. 太原: 中国毒理学会, 2019: 1. HU Y K, WU L, ZHANG Y C, et al. Effects of cadmium chloride on osteogenic and adipogenic differentiation of human bone marrow mesenchymal stem cells[C]//The 9th National Congress of Toxicology of the Chinese Society of Toxicology. Taiyuan: Chinese Society of Toxicology, 2019: 1. (in Chinese) |
| [18] |
FENG X, MCDONALD J M. Disorders of bone remodeling[J]. Annu Rev Pathol Mech Dis, 2011, 6: 121-145. DOI:10.1146/annurev-pathol-011110-130203 |
| [19] |
KIM J M, LIN C J, STAVRE Z, et al. Osteoblast-osteoclast communication and bone homeostasis[J]. Cells, 2020, 9(9): 2073. DOI:10.3390/cells9092073 |
| [20] |
QIU J C, LI D S, MOU X N, et al. Effects of graphene quantum dots on the self-renewal and differentiation of mesenchymal stem cells[J]. Adv Healthc Mater, 2016, 5(6): 702-710. DOI:10.1002/adhm.201500770 |
| [21] |
TAO K, XIAO D M, WENG J, et al. Berberine promotes bone marrow-derived mesenchymal stem cells osteogenic differentiation via canonical Wnt/β-catenin signaling pathway[J]. Toxicol Lett, 2016, 240(1): 68-80. DOI:10.1016/j.toxlet.2015.10.007 |
| [22] |
WANG G C, ZHENG L, ZHAO H S, et al. In vitro assessment of the differentiation potential of bone marrow-derived mesenchymal stem cells on genipin-chitosan conjugation scaffold with surface hydroxyapatite nanostructure for bone tissue engineering[J]. Tissue Eng Part A, 2011, 17(9-10): 1341-1349. DOI:10.1089/ten.tea.2010.0497 |
| [23] |
CAO Y, ZHOU Z C, DE CROMBRUGGHE B, et al. Osterix, a transcription factor for osteoblast differentiation, mediates antitumor activity in murine osteosarcoma[J]. Cancer Res, 2005, 65(4): 1124-1128. DOI:10.1158/0008-5472.CAN-04-2128 |
| [24] |
王怡, 刘伟, 赵鸿雁, 等. 镉对大鼠成骨细胞OPG/RANKL表达的影响[J]. 扬州大学学报(农业与生命科学版), 2014, 35(1): 9-12. WANG Y, LIU W, ZHAO H Y, et al. Effect of cadmium on expression of OPG/RANKL in OBs[J]. Journal of Yangzhou University (Agricultural and Life Science Edition), 2014, 35(1): 9-12. (in Chinese) |
| [25] |
AL-GHAFARI A, ELMORSY E, FIKRY E, et al. The heavy metals lead and cadmium are cytotoxic to human bone osteoblasts via induction of redox stress[J]. PLoS One, 2019, 14(11): e0225341. DOI:10.1371/journal.pone.0225341 |
| [26] |
YANG X, YANG Y, ZHOU S, et al. Puerarin stimulates osteogenic differentiation and bone formation through the ERK1/2 and p38-MAPK signaling pathways[J]. Curr Mol Med, 2018, 17(7): 488-496. DOI:10.2174/1566524018666171219101142 |
| [27] |
LIU H, LI W, GE X Y, et al. Coadministration of puerarin (low dose) and zinc attenuates bone loss and suppresses bone marrow adiposity in ovariectomized rats[J]. Life Sci, 2016, 166: 20-26. DOI:10.1016/j.lfs.2016.09.024 |
| [28] |
牛士贞, 牛通, 倪勇, 等. 葛根素注射液联合雌二醇注射液对大鼠骨质疏松性骨折愈合的影响[J]. 中国临床药理学杂志, 2019, 35(23): 3077-3080. NIU S Z, NIU T, NI Y, et al. Effect of Puerarin injection combined with estradiol injection on healing of osteoporotic fracture in rats[J]. The Chinese Journal of Clinical Pharmacology, 2019, 35(23): 3077-3080. (in Chinese) |
| [29] |
WANG Y P, LI Y P, PAULSON C, et al. Wnt and the Wnt signaling pathway in bone development and disease[J]. Front Biosci (Landmark Ed), 2014, 19(3): 379-407. DOI:10.2741/4214 |
| [30] |
GAO X R, GE J, LI W Y, et al. LncRNA KCNQ1OT1 promotes osteogenic differentiation to relieve osteolysis via Wnt/β-catenin activation[J]. Cell Biosci, 2018, 8: 19. DOI:10.1186/s13578-018-0216-4 |
| [31] |
HECHT A, VLEMINCKX K, STEMMLER M P, et al. The p300/CBP acetyltransferases function as transcriptional coactivators of β-catenin in vertebrates[J]. EMBO J, 2000, 19(8): 1839-1850. DOI:10.1093/emboj/19.8.1839 |
| [32] |
VAN DE WETERING M, CAVALLO R, DOOIJES D, et al. Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF[J]. Cell, 1997, 88(6): 789-799. DOI:10.1016/S0092-8674(00)81925-X |
| [33] |
周睿娴, 王光义, 陈睿, 等. 葛根素抑制间充质干细胞成脂肪分化的作用及机制[J]. 西部中医药, 2020, 33(3): 34-37. ZHOU R X, WANG G Y, CHEN R, et al. Effects and mechanism of puerarin in inhibiting adipogenic differentation of mesenchymal stem cells[J]. Western Journal of Traditional Chinese Medicine, 2020, 33(3): 34-37. (in Chinese) |
(编辑 范子娟)



