卵泡是卵巢的基本功能单位,其生长发育对雌性哺乳动物的生殖进程起决定作用[1]。卵泡的生长发育是一个高度精确、有序且具有周期性的过程[2],从原始卵泡的激活开始,逐渐生长发育成熟直至选择为优势卵泡或闭锁,此过程伴随着卵母细胞和周围体细胞的生长,其中颗粒细胞(granulosa cells, GCs)的生长发育对这一过程的影响尤为重要[3]。
GCs的增殖和凋亡是卵泡成熟或闭锁的重要因素和必要条件[4],GCs的增殖可以促进卵泡发育成熟,而GCs凋亡则引发卵泡的闭锁[5],同时,GCs还是卵巢中类固醇激素生成的主要来源,卵泡液中的类固醇激素维持卵巢正常的生理功能,其主要包括雌二醇(estrogen, E2)和孕酮(progesterone, P4)[6]。哺乳动物约有99%卵泡发育停滞或闭锁,只有1%的卵泡可以发育成熟并排卵,卵泡的成熟或闭锁对于雌性哺乳动物维持健康的生殖系统动态平衡十分重要[7],因此,长期以来,卵泡的发育和闭锁机制一直是生殖生物学的研究热点。
microRNA(miRNA)广泛参与原始卵泡的形成、卵泡的募集、选择和闭锁等雌性哺乳动物的生殖过程[8]。miRNA主要通过抑制靶基因的表达进而调节GCs增殖和凋亡[9],例如:miR-181被证实通过减少PCNA的积累和靶向ACVRIA来抑制GCs的增殖[10];而过表达miR-320则能抑制小鼠GCs的增殖[11];同时,long non-coding RNA tcons-00814106通过海绵介导miR-1343调控猪GCs增殖和凋亡[12]。此外,miRNA对于类固醇激素合成也发挥重要的调节作用,例如:miR-133b可以直接靶向FOXL2从而抑制FOXL2介导的StAR和CYP19A1的转录,从而促进E2的生成[13];而miR-378通过靶向CYP19A1抑制E2的合成[14];miR-375参与调控CRH的表达进而抑制GCs合成E2[15]。
最近研究报道,miR-495参与调控腔前卵泡的生长发育[16]。同时,与非妊娠期相比,妊娠早期miR-495高表达,暗示其可能参与早期妊娠的建立[17]。而在绵羊繁殖进程中,miR-495-3p通过直接或间接方式影响促性腺激素释放激素的活性和释放[18]。但是,miR-495-3p调控卵巢卵泡发育的分子机制尚不明确。为了揭示miR-495-3p在山羊卵巢GCs中的功能及其调控机制,本研究转染miR-495-3p mimics和inhibitor,分析其对山羊卵巢GCs凋亡、增殖、周期、类固醇激素分泌及相关基因表达的影响。
1 材料与方法 1.1 试验主要试剂DMEM/F12培养基、胎牛血清FBS(HyClone,美国),FSHR一抗(稀释比例1∶200,葆光生物,中国),生物素标记二抗(稀释比例1∶20,葆光生物,中国),辣根酶标记的工作液、DAB工作液、CCK-8、BCA试剂盒(葆光生物,中国),EndoFectinTM(莱博斯,中国),Opti-DMEM(HyClone,美国),RNAiso(Invitrogen,美国),PrimeScriptTM RT Master Mix、MiR-XTM miRNA FirstStrand Synthesis(TaKaRa,中国),Annexin V-PE(Biolegend,中国),7-AAD(Biolegend,中国),PI/RnaseA(SeaBiotech,中国),ELISA试剂盒(博诺恒,中国),细胞裂解液(Solarbio,中国)。
1.2 卵巢采集与细胞培养及转染本试验严格按照国际动物福利合作委员会(ICCAW)和西南大学动物实验伦理委员会(西南大学〔2017〕7号)要求。从西南大学羊场选取3~4月龄的大足黑山羊母羊,屠宰后收集新鲜卵巢,置于37 ℃的生理盐水中1 h内转运至实验室。山羊卵巢GCs收集培养方式同Wang等[19]的报道,GCs培养于DMEM/F12培养基中,补充10%胎牛血清,置于细胞培养箱中37 ℃、5% CO2的条件培养。将miR-495-3p mimics、miR-495-3p mimics NC、miR-495-3p inhibitor、miR-495-3p inhibitor NC(序列见表 1)分别转染至GCs中,每组3个重复,细胞转染依据制造商说明书:5 μL miR-495-3p mimics/NC、inhibitor /NC(100 nmol·L-1)或EndoFectinTMMax转染试剂用Opti-DMEM分别稀释至150 μL,室温静置5 min。将稀释的miR-495-3p mimics/NC或inhibitor /NC溶液以及EndoFectinTMMax溶液轻柔混合,室温静置20 min,向6孔板中逐滴添加RNA-EndoFectinTM复合物,在CO2培养箱中37 ℃孵育24~48 h。
|
|
表 1 所设计的miRNA序列 Table 1 The designed miRNA sequences |
将贴壁生长的细胞消化后制成单细胞悬浮液,转入放有细胞爬片的12孔板中,培养24 h,用4%的多聚甲醛固定细胞爬片后用0.5%的Triton X-100溶液通透30 min,随后用5% 的BSA溶液室温封闭30 min。结束封闭的细胞爬片滴加用1%BSA溶液稀释的FSHR一抗,置于37 ℃,5% CO2培养箱中孵育2 h。无菌PBS洗3次(每次5 min),滴加用1%BSA溶液稀释的生物素标记二抗,置于37 ℃,5% CO2培养箱中孵育30 min。最后,细胞爬片与辣根酶标记的工作液以及新配制的DAB工作液共孵育。FSHR阳性细胞膜被染成棕色,细胞核染成蓝色,利用倒置显微镜采集图像。
1.4 CCK-8检测CCK-8检测细胞活性,在96孔板中培养或转染GCs(1×104个·孔-1),转染后放入CO2培养箱培养,设置3个时间梯度(24、36、48 h),每个梯度4个重复。每孔加入10 μL的CCK-8试剂,置于CO2细胞培养箱中孵育2 h。随后使用酶标仪检测混合物在450 nm处的吸光度,计算细胞增殖活力。
1.5 细胞凋亡及细胞周期检测转染36 h后收集GCs,利用缓冲液重悬细胞,调节细胞密度为107个·mL-1,在流式管中加入100 μL细胞悬液,随后分别加入5 μL Annexin V-PE和5 μL 7-AAD混合均匀,室温避光孵育15 min,使用流式细胞术检测细胞凋亡。转染36 h后收集GCs,每个样本加入500 μL提前配置好的PI/RNA染色工作液(PI∶RnaseA=9∶1),室温避光孵育1 h,利用流式细胞术检测细胞周期,用Modfit软件进行数据分析。
1.6 ELISA检测细胞转染36 h后,收集细胞上清液50 μL,使用酶联免疫吸附试验(ELISA)试剂盒检测E2和P4水平。根据试剂盒说明书,使用酶标仪在450 nm波长下测定不同处理组的吸光度。通过对标准曲线的线性回归,计算出相应的样品浓度。
1.7 qRT-PCR检测基因表达用RNAiso试剂从GCs中提取总RNA,用Nanodrop1000评估RNA的纯度和浓度。利用PrimeScriptTM RT Master Mix将mRNA逆转录成cDNA。根据MiR-XTM miRNA FirstStrand Synthesis说明书要求,miRNA逆转录为cDNA。根据TaKaRa公司TB Green® Premix Ex TaqTM Ⅲ(Tli RNaseH Plus)进行qRT-PCR,反应体系为15 μL:TB Green Premix Ex Taq Ⅱ(Tli RNaseH Plus)(2×)7.5 μL,PCR Forward Primer(10 μmol·L-1) 0.6 μL,PCR Reverse Primer(10 μmol·L-1)0.6 μL,RT反应液(cDNA溶液)1.2 μL,灭菌水5.1 μL。反应条件为:第一阶段预变性95 ℃ 30 s,循环一次;第二阶段PCR反应95 ℃ 5 s,60 ℃ 30 s,循环40次,熔解曲线根据引物不同设置为65~95 ℃,每0.5 ℃读取一次Ct值。相对表达量采用2-ΔΔCT法计算。编码基因表达量归一化为β-actin的相对表达量,miRNA表达量归一化为U6的相对表达量。所有qRT-PCR反应均为4个重复,所用引物见表 2。
|
|
表 2 qRT-PCR引物序列 Table 2 The primers sequence of qRT-PCR |
细胞转染后36 h,用含1%蛋白酶抑制剂及磷酸酶抑制剂的细胞裂解液裂解细胞,混合物4 ℃离心,收集细胞总蛋白,BCA试剂盒测定蛋白浓度。总蛋白提取液在12% SDS PAGE凝胶上分离,并在硝化纤维素膜上印迹。用5%脱脂牛奶在4 ℃的TBST缓冲液中封闭2 h,结束后将膜与一抗4 ℃孵育过夜,随后二抗孵育2 h,用ECL显影液(A液∶B液=1∶1)显影,Image LAS-4000系统检测化学发光,Image J软件分析蛋白灰度。
1.9 统计分析利用Prism-6软件进行统计分析,多个数据的比较用单因素方差(One-Way ANOVA)分析,两组数据比较用独立t检验,试验重复3次以上,数据结果采用“平均值±标准误(Mean±SEM)”表示,P<0.05表示差异显著,P>0.05表示差异不显著。
2 结果 2.1 山羊卵巢GCs的分离鉴定及miR-495-3p转染效率验证山羊卵巢GCs最初分离培养呈现圆点状,培养24 h后贴壁生长,呈现长梭形(图 1A)。为了检测本研究分离培养的GCs纯度,利用GCs中特异性表达的FSHR来鉴定GCs。染色后可观察到GCs膜被染成棕色,细胞核染成蓝色(图 1B)。为研究miR-495-3p在卵巢GCs中的作用,将miR-495-3p mimics、mimics NC、miR-495-3p inhibitor和inhibitor NC转染到GCs中,分别在24、36和48 h观察细胞转染情况(图 1C),并用qRT-PCR验证其转染效率(图 1D)。结合细胞荧光信号,转染miR-495-3p mimics 36 h时miR-495-3p表达量最高(P<0.01)。因此,后续试验转染时间为36 h。由于miRNA inhibitor的干扰原理,未检测到miR-495-3p表达量变化。

|
A. 贴壁生长的山羊卵巢GCs;B. 细胞免疫组化鉴定山羊卵巢GCs;C.转染miR-495-3p mimics、mimics NC、miR-495-3p inhibitor和inhibitor NC细胞荧光图(24、36、48 h);D. 转染36 h后,qRT-PCR检测miR-495-3p mRNA表达量:ns表示差异不显著,*表示差异显著P<0.05,**表示差异极显著P<0.01 A. Goat ovary GCs that grow adherently; B. Cellular immunohistochemistry to identify goat ovarian GCs; C. Fluorescence images of cells transfected with miR-495-3p mimics, mimics NC, miR-495-3p inhibitor and inhibitor NC (24, 36, 48 h); D. 36 h after transfection, the expression of miR-495-3p mRNA was detected by qRT-PCR: ns indicates that the difference is not significant, * indicates the significant difference P < 0.05, ** indicates the extremely significant difference P < 0.01 图 1 卵巢GCs的分离鉴定及miR-495-3p转染 Fig. 1 Isolation and identification of ovarian GCs and miR-495-3p transfection |
为探究miR-495-3p对GCs凋亡的影响,转染miR-495-3p mimics、mimics NC、miR-495-3p inhibitor和inhibitor NC 36 h后,利用流式细胞术检测细胞凋亡率。结果显示,与mimics NC组相比,miR-495-3p mimics极显著升高细胞凋亡率(图 2A、2B、2E,P < 0.01);而与inhibitor NC组相比,miR-495- 3p inhibitor极显著下调细胞凋亡率(图 2C~E,P < 0.01)。qRT-PCR检测凋亡相关基因相对表达水平,结果显示,与mimics NC组相比,miR-495-3p mimics显著上调BAX mRNA相对表达水平(P < 0.05),极显著下调BCL2 mRNA相对表达水平(P < 0.01);与inhibitor NC组相比,miR-495-3p inhibitor极显著下调BAX mRNA相对表达水平(P < 0.001),但对BCL2的表达无显著影响(图 2F)。Western blot检测蛋白表达水平结果与qRT-PCR结果趋势相一致(图 2G、2H)。以上结果表明,过表达miR-495-3p可以促进GCs凋亡,而抑制miR-495-3p可以阻碍细胞凋亡。
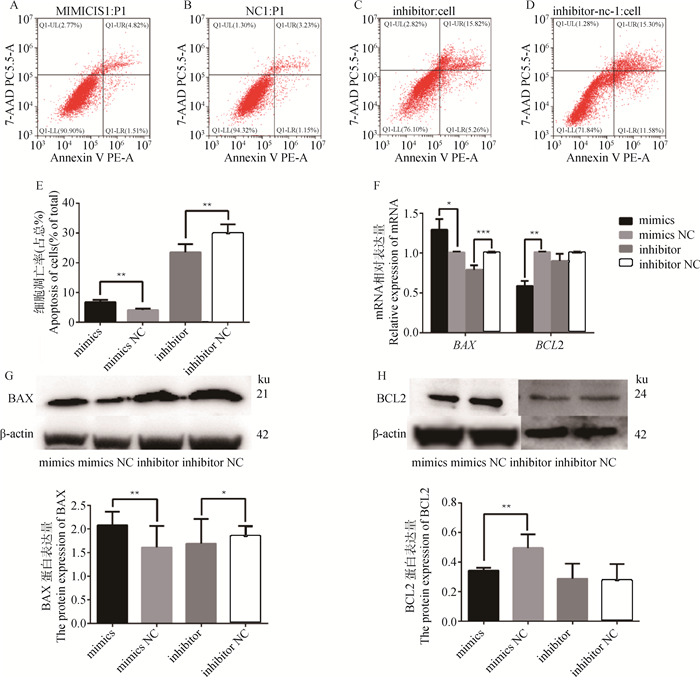
|
A、B、C、D. 流式细胞术检测GCs转染miR-495-3p mimics、mimics NC、inhibitor、inhibitor NC 36 h后的细胞凋亡:UL表示死亡细胞,UR表示晚期凋亡细胞,LR表示早期凋亡细胞,LL表示活的非凋亡细胞;E. 柱状图显示凋亡细胞的百分比;F. qRT-PCR检测细BAX、BCL2 mRNA相对表达量;G、H. Western blot检测BAX、BCL2的蛋白相对表达量。*. P<0.05,**. P<0.01,***. P<0.001,下同 A, B, C, D. Flow cytometry was used to detect the apoptosis of GCs on 36 h after transfection of miR-495-3p mimics, mimics NC, inhibitor and inhibitor NC: UL stands for dead cells, UR stands for late apoptotic cells, LR stands for early apoptotic cells, and LL stands for live non-apoptotic cells; E. The histogram shows the percentage of apoptotic cells; F. qRT-PCR detects the relative expression of BAX and BCL2 mRNA; G, H. Western blot was used to detect the relative expression of BAX and BCL2 proteins. *. P < 0.05, **. P < 0.01, ***. P < 0.001, the same as below 图 2 miR-495-3p促进卵巢GCs的凋亡 Fig. 2 miR-495-3p promotes the apoptosis of ovarian GCs |
用CCK-8检测不同转染时间细胞的增殖活力,分析miR-495-3p对细胞增殖的影响。结果显示,转染24 h时,过表达或抑制miR-495-3p对细胞的增殖活力无显著影响(P>0.05);转染36 h时,与mimics NC组相比,miR-495-3p mimics显著降低细胞增殖活力(P < 0.05),而inhibitor组无显著变化;转染48 h时,与对照组相比miR-495-3p mimics组和miR-495-3p inhibitor组显著(P < 0.05)或极显著(P < 0.001)降低细胞增殖活力(图 3A),表明随着转染时间的增加,细胞的增殖活力逐渐下降,并且细胞增殖结果与miR-495-3p在GCs中的转染结果一致(图 1D)。qRT-PCR结果显示,过表达或抑制miR-495-3p对增殖标志基因PCNA mRNA的相对表达水平无显著影响(图 3B,P>0.05),但Western blot结果发现,过表达miR-495-3p显著降低PCNA的蛋白表达水平(图 3C,P < 0.05)。综上表明,过表达miR-495-3p可以抑制GCs增殖。
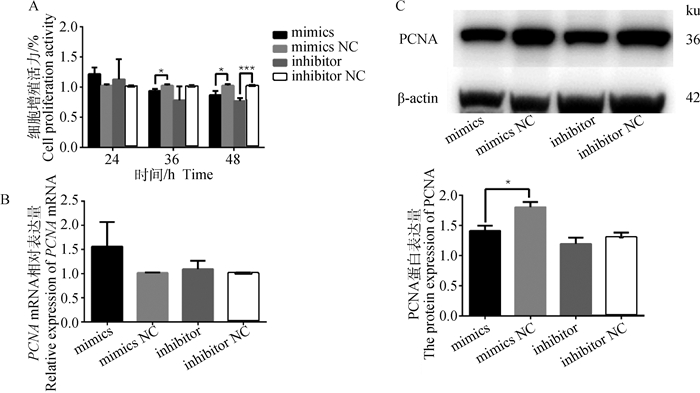
|
A.CCK8检测miR-495-3p mimics、mimics NC、inhibitor和inhibitor NC处理组的细胞活力;B. qRT-PCR检测细胞增殖标志基因PCNA的mRNA表达;C. Western blot检测增殖标志基因PCNA蛋白的表达 A. CCK8 detect the cell viability of miR-495-3p mimics, mimics NC, inhibitor and inhibitor NC treatment groups; B. qRT-PCR detect the mRNA expression of the cell proliferation marker gene PCNA; C. Western blot was used to detect the protein expression of PCNA 图 3 miR-495-3p抑制GCs的增殖 Fig. 3 miR-495-3p inhibits the proliferation of GCs |
为明确miR-495-3p影响细胞增殖的具体分裂时期,利用流式细胞术检测细胞周期分布,结果发现过表达miR-495-3p极显著降低了G1期比率(P < 0.01),显著增加G2/M期比率(P < 0.05),对S期没有显著影响(图 4A、4B、4E),而抑制miR-495-3p对细胞周期没有显著影响(图 4C、4D、4E,P>0.05)。qRT-PCR检测细胞周期相关基因的相对表达水平,与对照组相比,发现过表达miR-495-3p显著降低了CDK4(P < 0.001)和CCND1(P < 0.05)的mRNA水平,但其对CCNE1没有显著影响(P>0.05);抑制miR-495-3p极显著降低CCNE1的mRNA水平(P < 0.01),而对CDK4和CCND1的mRNA水平无显著影响(图 4F)。结果表明,过表达miR-495-3p引起细胞周期阻滞在G2/M期。
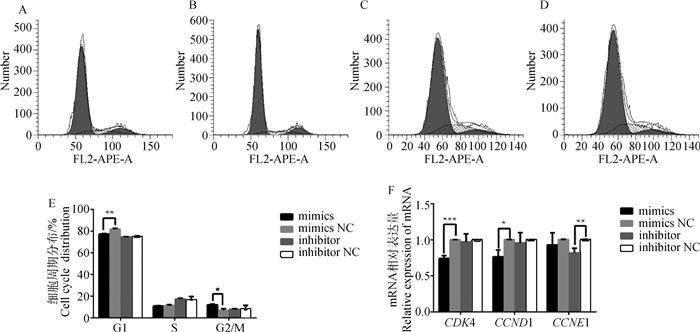
|
A、B、C、D. 流式细胞术检测miR-495-3p mimics、mimics NC、inhibitor、inhibitor NC处理组的细胞周期;E. 柱状图显示细胞周期分布的百分比;F. qRT-PCR检测与细胞周期相关基因(CDK4、CCND1和CCNE1)的mRNA表达 A, B, C, D. Flow cytometry detect the cell cycle of miR-495-3p mimics, mimics NC, inhibitor, inhibitor NC treatment groups; E. The histogram shows the percentage of cell cycle distribution; F. qRT-PCR detects the mRNA expression of genes related to cell cycle (CDK4, CCND1 and CCNE1) 图 4 miR-495-3p阻滞GCs的细胞周期进程 Fig. 4 miR-495-3p blocks the cell cycle progression of GCs |
利用ELISA试剂盒检测GCs培养液上清中的E2和P4含量。结果表明,过表达或抑制miR-495-3p均显著促进E2(P < 0.01)和P4(P < 0.001,P < 0.05)的分泌(图 5A、5B)。qRT-PCR和Western blot检测类固醇激素合成相关基因和蛋白的相对表达水平,与对照组相比,过表达miR-495-3p显著升高3β-HSD(P < 0.001)、CYP11A1(P < 0.01)和CYP19A1(P < 0.05)的mRNA和蛋白表达水平(图 5C、5D、5F、5G),但对StAR无显著影响(图 5C、5E,P>0.05)。抑制miR-495-3p极显著增加CYP11A1的mRNA和蛋白的表达水平(图 5C、5F,P < 0.01),极显著降低StAR的mRNA和蛋白表达水平(图 5C&E,P < 0.001),极显著降低3β-HSD的mRNA表达水平(P < 0.01),但对蛋白的表达无显著影响(图 5C、5D),同时,抑制miR-495-3p对CYP19A1的mRNA和蛋白表达水平并无显著影响(图 5C、5G,P>0.05)。
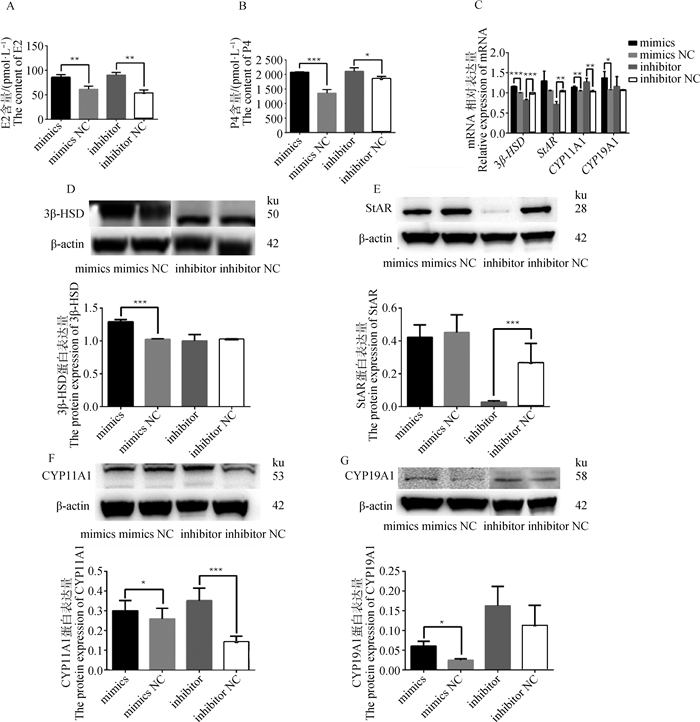
|
A、B. ELISA检测miR-495-3p mimics、mimics NC、inhibitor和inhibitor NC处理组上清液中E2和P4含量;C. qRT-PCR检测类固醇激素合成相关基因的mRNA表达量;D、E、F、G. Western blot检测类固醇激素合成相关基因3β-HSD、StAR、CYP11A1、CYP19A1的蛋白表达量 A, B. ELISA was used to detect the contents of E2 and P4 in the supernatant of miR-495-3p mimics, mimics NC, inhibitor and inhibitor NC treatment groups; C. qRT-PCR detection of mRNA expression of steroid hormone synthesis-related genes; D, E, F, G. Western blot was used to detect the protein expression of steroid hormone synthesis-related genes 3β-HSD, StAR, CYP11A1 and CYP19A1 图 5 miR-495-3p促进GCs类固醇激素分泌 Fig. 5 miR-495-3p promotes the secretion of steroid hormones in GCs |
miRNA在卵巢中的表达十分丰富,并且对卵巢的生理功能起着重要的调节作用[20]。在本研究中,过表达miR-495-3p促进GCs凋亡,这一结果与其它miRNA在卵巢GCs中的研究结果类似。Tu等[21]报道miR-10家族在人、大鼠和小鼠中均可诱导GCs凋亡,相反,Zhang等[22]发现,miR-21-5p靶向Smad7抑制猪卵巢GCs凋亡,表明miRNA对GCs凋亡具有复杂的调节作用。miR-144[23]和miR-29[24]在促进卵巢GCs凋亡的同时伴有BAX、BCL2和caspase3等凋亡基因的表达量变化,通常细胞凋亡途径分为内部线粒体途径和死亡受体介导的外部凋亡途径,其中内部凋亡途径主要受BCL2家族蛋白调控,而外部凋亡途径的主要调控因子为效应凋亡蛋白酶caspase3[25]。本试验证实了miR-495-3p能够引起BAX和BCL2表达量的变化,表明miR-495-3p可能通过线粒体凋亡途径促进GCs凋亡。
GCs的增殖对卵泡发育成熟起着至关重要的作用。本研究发现,miR-495-3p呈时间依赖性地抑制GCs增殖并下调PCNA蛋白水平,过表达miR-495-3p将细胞周期阻滞在G2/M期。在肿瘤细胞中,CDK的表达变化导致细胞周期紊乱[26],抑制CDK4可以影响细胞G1期阻滞,显著抑制细胞增殖[27],而在骨肉瘤的研究中,过表达miR-495-3p能够降低CDK4、CDK6、CCND1和CCNA2表达水平从而抑制骨肉瘤细胞增殖[28]。因此,miR-495-3p通过抑制CDK4和CCND1的表达可能阻滞了细胞周期进程,但是miR-495-3p具体通过何种途径影响GCs周期有待进一步研究。
哺乳动物在整个发情周期,卵泡会经历形态和生理功能变化,这些变化是由卵巢中GCs分泌的类固醇激素E2和P4调控介导。有研究表明,哺乳动物体内E2浓度越高,体内卵泡数量和体积会逐渐增加[29],一般来说,E2合成量取决于两种情况: 一是参与E2合成酶的活性; 二是分泌E2细胞的数量[30],并且研究发现,将E2合成过程中的关键酶CYP19A1敲除后,会使小鼠出现E2分泌不足从而影响卵泡的发育[31]。本研究中,同抑制miR-143[32]或miR-764-3p[33]可以增加小鼠卵巢GCs中E2分泌的结果相似,过表达miR-495-3p后,在mRNA和蛋白水平均显著增加E2合成限速酶CYP19A1的表达量。此外,P4能够调节卵泡的生长,影响排卵及卵子的质量,因此P4是评价卵巢功能的重要指标之一[34],在P4合成过程中,StAR将胆固醇从线粒体外运送到线粒体内,随后CYP11A1促进胆固醇向孕烯醇酮的转化,孕烯醇酮在3β-HSD的作用下生成P4[35],CYP11A1作用的胆固醇向孕烯醇酮转化过程是P4合成的限速步骤[36]。本研究结果显示,过表达miR-495-3p可上调3β-HSD和CYP11A1表达,这一结果说明过表达miR-495-3p可以通过上调3β-HSD和CYP11A1促进胆固醇到孕烯醇酮再到P4的合成过程。值得注意的是,本试验发现,抑制miR-495-3p显著降低StAR蛋白表达,这将导致胆固醇转运至线粒体膜内的量减少,进而限制P4的合成,但试验结果却发现miR-495-3p促进P4合成,这可能由于抑制miR-495-3p提高了P4合成限速酶CYP11A1的表达,进而促进GCs分泌P4。相似的,在猪卵巢GCs中,抑制miR-21-5p可以抑制E2和促进P4分泌;抑制miR-130a-3p促进山羊卵巢GCs中E2和P4的分泌[37];miR-101-3p增加奶山羊GCs培养液中E2和P4的含量[38],这表明,miRNA调控GCs合成孕酮的机制可能比预想的更为复杂。
4 结论本研究结果表明,miR-495-3p促进山羊卵巢GCs凋亡、抑制其增殖,将细胞周期阻滞在G2/M期,刺激类固醇激素的分泌。表明miR-495-3p在山羊卵泡发育中具有重要的调控作用。
| [1] |
GUO S H, QUAN S, ZOU S Y. Roles of the notch signaling pathway in ovarian functioning[J]. Reprod Sci, 2021, 28(10): 2770-2778. DOI:10.1007/s43032-021-00610-6 |
| [2] |
TU F, PAN Z X, YAO Y, et al. MiR-34a targets the inhibin beta B gene, promoting granulosa cell apoptosis in the porcine ovary[J]. Genet Mol Res, 2014, 13(2): 2504-2512. DOI:10.4238/2014.January.14.6 |
| [3] |
MATZUK M M, BURNS K H, VIVEIROS M M, et al. Intercellular communication in the mammalian ovary: oocytes carry the conversation[J]. Science, 2002, 296(5576): 2178-2180. DOI:10.1126/science.1071965 |
| [4] |
AERTS J M J, BOLS P E J. Ovarian follicular dynamics: a review with emphasis on the bovine species.Part I: folliculogenesis and pre-antral follicle development[J]. Reprod Domest Anim, 2010, 45(1): 171-179. DOI:10.1111/j.1439-0531.2008.01302.x |
| [5] |
HE Y M, DENG H H, JIANG Z L, et al. Effects of melatonin on follicular atresia and granulosa cell apoptosis in the porcine[J]. Mol Reprod Dev, 2016, 83(8): 692-700. DOI:10.1002/mrd.22676 |
| [6] |
XU D J, HE H S, JIANG X H, et al. SIRT2 plays a novel role on progesterone, estradiol and testosterone synthesis via PPARs/LXRα pathways in bovine ovarian granular cells[J]. J Steroid Biochem Mol Biol, 2019, 185: 27-38. DOI:10.1016/j.jsbmb.2018.07.005 |
| [7] |
ZHOU J W, PENG X W, MEI S Q. Autophagy in ovarian follicular development and atresia[J]. Int J Biol Sci, 2019, 15(4): 726-737. DOI:10.7150/ijbs.30369 |
| [8] |
MAALOUF S W, LIU W S, PATE J L. MicroRNA in ovarian function[J]. Cell Tissue Res, 2016, 363(1): 7-18. DOI:10.1007/s00441-015-2307-4 |
| [9] |
ZHOU J L, YAO W, LIU K Q, et al. MicroRNA let-7 g regulates mouse granulosa cell autophagy by targeting insulin-like growth factor 1 receptor[J]. Int J Biochem Cell Biol, 2016, 78: 130-140. DOI:10.1016/j.biocel.2016.07.008 |
| [10] |
ZHANG Q, SUN H X, JIANG Y, et al. MicroRNA-181a suppresses mouse granulosa cell proliferation by targeting activin receptor ⅡA[J]. PLoS One, 2013, 8(3): e59667. DOI:10.1371/journal.pone.0059667 |
| [11] |
YIN M M, WANG X R, YAO G D, et al. Transactivation of MicroRNA-320 by MicroRNA-383 regulates granulosa cell functions by targeting E2F1 and SF-1 Proteins[J]. J Biol Chem, 2014, 289(26): 18239-18257. DOI:10.1074/jbc.M113.546044 |
| [12] |
HU H Y, FU Y F, ZHOU B, et al. Long non-coding RNA TCONS_00814106 regulates porcine granulosa cell proliferation and apoptosis by sponging miR-1343[J]. Mol Cell Endocrinol, 2021, 520: 111064. DOI:10.1016/j.mce.2020.111064 |
| [13] |
DAI A Y, SUN H X, FANG T, et al. MicroRNA-133b stimulates ovarian estradiol synthesis by targeting Foxl2[J]. FEBS Lett, 2013, 587(15): 2474-2482. DOI:10.1016/j.febslet.2013.06.023 |
| [14] |
XU S Y, LINHER-MELVILLE K, YANG B B, et al. Micro-RNA378 (miR-378) regulates ovarian estradiol production by targeting aromatase[J]. Endocrinology, 2011, 152(10): 3941-3951. DOI:10.1210/en.2011-1147 |
| [15] |
余雏琳. miR-375介导CRH对猪卵巢颗粒细胞E2合成的负调控[D]. 北京: 中国农业大学, 2018. YU C L. MiR-375 mediates CRH signaling pathway in inhibiting E2 synthesis in porcine granulosa cell[D]. Beijing: China Agricultural University, 2018. (in Chinese) |
| [16] |
刘晓明. 小鼠卵巢中六种microRNAs和细胞周期检验点蛋白Chk1/2的功能和调控机制研究[D]. 武汉: 华中农业大学, 2015. LIU X M. Six microRNAs and check point Kinases1/2 (Chk1/2) pathway on mouse ovaries[D]. Wuhan: Huazhong Agricultural University, 2015. (in Chinese) |
| [17] |
GEBREMEDHN S, SALILEW-WONDIM D, HOELKER M, et al. Exploring maternal serum microRNAs during early pregnancy in cattle[J]. Theriogenology, 2018, 121: 196-203. DOI:10.1016/j.theriogenology.2018.08.020 |
| [18] |
ZHANG Z B, TANG J S, DI R, et al. Integrated hypothalamic transcriptome profiling reveals the reproductive roles of mRNAs and miRNAs in sheep[J]. Front Genet, 2020, 10: 1296. DOI:10.3389/fgene.2019.01296 |
| [19] |
WANG S J, LIU W J, WANG L K, et al. The role of Melatonin receptor MTNR1A in the action of Melatonin on bovine granulosa cells[J]. Mol Reprod Dev, 2017, 84(11): 1140-1154. DOI:10.1002/mrd.22877 |
| [20] |
贺小云, 刘秋月, 储明星. miRNA调控哺乳动物卵泡发育和卵母细胞成熟的研究进展[J]. 畜牧兽医学报, 2019, 50(11): 2175-2185. HE X Y, LIU Q Y, CHU M X. Advances in miRNA regulating mammalian follicular development and oocyte maturation[J]. Acta Veterinaria et Zootechnica Sinica, 2019, 50(11): 2175-2185. DOI:10.11843/j.issn.0366-6964.2019.11.001 (in Chinese) |
| [21] |
TU J J, YANG Y Z, HOI-HUNG A C, et al. Conserved miR-10 family represses proliferation and induces apoptosis in ovarian granulosa cells[J]. Sci Rep, 2017, 7(1): 41304. DOI:10.1038/srep41304 |
| [22] |
ZHANG X D, CHEN Y G, YANG M, et al. MiR-21-5p actions at the Smad7 gene during pig ovarian granulosa cell apoptosis[J]. Anim Reprod Sci, 2020, 223: 106645. DOI:10.1016/j.anireprosci.2020.106645 |
| [23] |
韦唯. miR-101与miR-144对奶山羊卵巢颗粒细胞调控的研究[D]. 杨凌: 西北农林科技大学, 2017. WEI W. Study of regulation about miR-101 and miR-144 on ovarian granulosa cells in dairy goat[D]. Yangling: Northwest A&F University, 2017. (in Chinese) |
| [24] |
WANG P J, LIU S J, ZHU C, et al. MiR-29 regulates the function of goat granulosa cell by targeting PTX3 via the PI3K/AKT/mTOR and Erk1/2 signaling pathways[J]. J Steroid Biochem Mol Biol, 2020, 202: 105722. DOI:10.1016/j.jsbmb.2020.105722 |
| [25] |
JIA L T, CHEN S Y, YANG A G. Cancer gene therapy targeting cellular apoptosis machinery[J]. Cancer Treat Rev, 2012, 38(7): 868-876. DOI:10.1016/j.ctrv.2012.06.008 |
| [26] |
MALUMBRES M, BARBACID M. Cell cycle, CDKs and cancer: a changing paradigm[J]. Nat Rev Cancer, 2009, 9(3): 153-166. DOI:10.1038/nrc2602 |
| [27] |
HE Z Y, DANG J, SONG A L, et al. NEAT1 promotes colon cancer progression through sponging miR-495-3p and activating CDK6 in vitro and in vivo[J]. J Cell Physiol, 2019, 234(11): 19582-19591. DOI:10.1002/jcp.28557 |
| [28] |
ZHAO G, ZHANG L W, QIAN D J, et al. miR-495-3p inhibits the cell proliferation, invasion and migration of osteosarcoma by targeting C1q/TNF-related protein 3[J]. Onco Targets Ther, 2019, 12: 6133-6143. DOI:10.2147/OTT.S193937 |
| [29] |
NAKANO R, NAKAYAMA T, IWAO M. Inhibition of ovarian follicle growth by a chemical antiestrogen[J]. Horm Res, 1982, 16(4): 230-236. DOI:10.1159/000179506 |
| [30] |
LI Z, JIANG J Y, YI X H, et al. MiR-18b regulates the function of rabbit ovary granulosa cells[J]. Reprod Fertil Dev, 2021, 33(5): 363-371. DOI:10.1071/RD20237 |
| [31] |
RICHARDS J S, IRELAND J J, RAO M C, et al. Ovarian follicular development in the rat: hormone receptor regulation by estradiol, follicle stimulating hormone and luteinizing hormone[J]. Endocrinology, 1976, 99(6): 1562-1570. DOI:10.1210/endo-99-6-1562 |
| [32] |
ZHANG L, ZHANG X X, ZHANG X J, et al. MiRNA-143 mediates the proliferative signaling pathway of FSH and regulates estradiol production[J]. J Endocrinol, 2017, 234(1): 1-14. DOI:10.1530/JOE-16-0488 |
| [33] |
WANG L L, LI C, LI R, et al. MicroRNA-764-3p regulates 17β-estradiol synthesis of mouse ovarian granulosa cells by targeting steroidogenic factor-1[J]. In Vitro Cell Dev Biol Anim, 2016, 52(3): 365-373. DOI:10.1007/s11626-015-9977-9 |
| [34] |
SHAO X, LIU Y L, LIU M, et al. Testosterone represses estrogen signaling by upregulating miR-22:A mechanism for imbalanced steroid hormone production in preeclampsia[J]. Hypertension, 2017, 69(4): 721-730. DOI:10.1161/HYPERTENSIONAHA.116.08468 |
| [35] |
ELUSTONDO P, MARTIN L A, KARTEN B. Mitochondrial cholesterol import[J]. Biochim Biophys Acta Mol Cell Biol Lipids, 2017, 1862(1): 90-101. |
| [36] |
LEE K A, VOLENTINE K K, BAHR J M. Two steroidogenic pathways present in the chicken ovary: theca layer prefers △5 pathway and granulosa layer prefers △4 pathway[J]. Domest Anim Endocrinol, 1998, 15(1): 1-8. DOI:10.1016/S0739-7240(97)00057-X |
| [37] |
ZHU L, JING J, QIN S Q, et al. MiR-130a-3p regulates steroid hormone synthesis in goat ovarian granulosa cells by targeting the PMEPA1 gene[J]. Theriogenology, 2021, 165: 92-98. DOI:10.1016/j.theriogenology.2021.02.012 |
| [38] |
AN X P, MA H D, LIU Y H, et al. Effects of miR-101-3p on goat granulosa cells in vitro and ovarian development in vivo via STC1[J]. J Anim Sci Biotechnol, 2020, 11: 102. DOI:10.1186/s40104-020-00506-6 |
(编辑 郭云雁)



