2. 北京生泰尔科技股份有限公司,北京 102600
2. Beijing Center Biology Co. Ltd., Beijing 102600, China
产后奶牛受分娩影响,干物质采食量下降,泌乳启动,能量需求极速升高,极易引起能量负平衡,诱发多种产后疾病[1]。瘤胃微生物区系维系着瘤胃内环境的稳态和对饲料的消化能力,因此其变化与奶牛产后能量负平衡密切相关[2-4]。短链脂肪酸(short chain fatty acids, SCFAs)是瘤胃微生物发酵产物,约占总挥发性脂肪酸的95%,反刍动物约75%的能量由其提供[5],另外SCFAs还参与了肠道免疫和炎症反应[6-8]。在亚洲,中医药被广泛用于治疗畜禽疾病已有数千年的历史,中药口服后其活性成分往往不是直接被宿主吸收,而是被肠道菌群利用和转化后,形成新的活性成分进入体内发挥药理作用[9]。归芪益母复方制剂是经典方剂归芪益母散的新剂型,出自《牛经备要医方》,能补气活血,可用于治疗产后气虚血虚之证。本课题组前期研究发现,归芪益母复方制剂能提高奶牛产后免疫机能和产奶量[10],但其作用机制目前尚未见相关报道。因此,本试验采用16S rRNA高通量测序和GC-MS/MS靶向代谢组学技术研究了归芪益母复方制剂对产后奶牛瘤胃微生物菌群丰度及短链脂肪酸含量的调节作用,旨在从瘤胃微生物发酵角度阐明该方剂对产后奶牛的保健机制。
1 材料与方法 1.1 试验分组和瘤胃液样品采集从宁夏某奶牛养殖场选取2~3胎次和体况评分相近的19头临产健康荷斯坦奶牛,随机分为试验组(G组,n=9)与对照组(B组,n=10)。试验组每头奶牛产后每日灌服500 mL归芪益母复方制剂(黄芪∶当归∶益母草=2∶2∶1,由爱迪森(北京) 生物科技有限公司制备,每1 mL相当于原生药0.5 g),对照组每日灌服等体积饮用水,连续6 d。试验期两组奶牛均采用相同全混合日粮(TMR)饲喂。产后第7天晨饲前按参考文献[11]方法采集瘤胃液,-80 ℃保存。
1.2 瘤胃微生物菌群种类与数量测定从瘤胃液样品中提取瘤胃微生物总DNA(十二烷基硫酸钠法),采用16S rRNA法扩增V3~V4区域,引物为515F(5′-GTGCCAGCMGCCGCGGTAA-3′)和806R(3′-GGACTACHVGGGTWTCTAAT-5′),扩增产物在NovaSeq6000平台测序,原始Tags数据(Raw Tags)经过滤、去除嵌合体后得到有效数据(Effective Tags),Uparse软件对其聚类为OTUs(97%的一致性),采用Mothur方法与SILVA132数据库对OTUs进行物种注释。Alpha和Beta多样性采用Qiime软件分析,R软件绘制物种累积箱形图和主坐标分析图,T-test进行Alpha多样性指数组间差异分析。
1.3 瘤胃短链脂肪酸GC-MS/MS靶向代谢组学分析样本解冻后混匀,取50 μL于1.5 mL离心管,加100 μL 36%磷酸溶液混匀,加150 μL含内标的MTBE溶剂,冰浴超声5 min;4 ℃条件下12 000 r·min-1离心10 min;取上清液90 μL至进样瓶中,-20 ℃保存。
使用7890A-5975C气相色谱-质谱联用仪(Agilent,美国,配备有电子轰击(EI)离子源和四级杆质量分析仪),配合乙酸、丙酸、丁酸、异丁酸、异戊酸、戊酸、己酸标准品进行GC-MS/MS检测分析。检测参数设置如下:采用Agilent DB-FFAP色谱柱,1.2 mL·min-1柱流速,2 μL进样量,95 ℃保持1 min后25 ℃·min-1升至100 ℃,再以17 ℃·min-1升至130 ℃,保持0.4 min;以25 ℃·min-1升至200 ℃,保持3.5 min。以标准品的浓度为横坐标,标准品与内标的峰面积比值为纵坐标考察线性,建立标准曲线,得到各物质的线性回归方程。利用Agilent MassHunter定性定量软件进行数据处理,并通过SPSS 25.0对数据进行T检验求出P-value,计算差异倍数值(Fold Change),使用Origin 2019b软件作图。
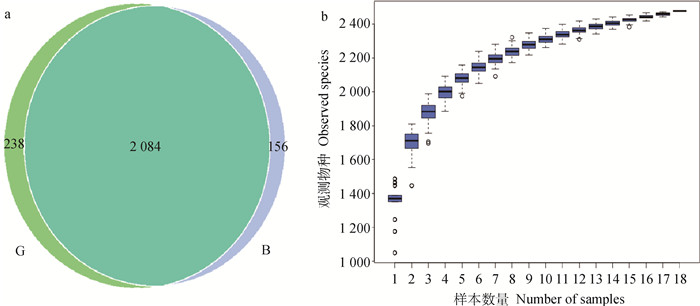
2 结果 2.1 奶牛产后各组瘤胃样本的测序与物种注释B组和G组瘤胃液原始序列数量、过滤和去嵌合体后的有效序列及长度、优化比例详见表 1。两组样本总OTU数目为2 478,其中B组和G组检测到的OTU数分别是2 240和2 322个,共有OTU数为2 084,占总OTU数的82.65%(图 1a)。物种累积曲线已趋于平缓,说明样本数据量充足,可以反映产后7 d奶牛瘤胃微生物种类(图 1b)。
|
|
表 1 产后奶牛瘤胃液样本序列信息 Table 1 Sequence information of rumen fluid of postpartum dairy cows |
|
图 1 OTU韦恩图(a)和物种累积箱形图(b) Fig. 1 OTU Venn diagram (a) and species accumulation boxplot (b) |
由图 2a可知,在门水平上,B组与G组的优势菌群都是厚壁菌门(Firmicutes)和拟杆菌门(Bacteroidota),B组中厚壁菌门占比60.19%,拟杆菌门占比33.57%;G组中厚壁菌门占比61.06%,拟杆菌门占比31.44%。在属水平上(图 2b),两组的优势菌属均为普雷沃氏菌属(Prevotella)、克里斯滕森菌属(Christensenellaceae)和瘤胃球菌科_NK4A214菌属(NK4A214_group),其中B组各菌属平均相对丰度分别为13.62%、12.95%和17.14%,G组平均相对丰度分别为13.24%、13.40%和17.02%。

|
图 2 基于门水平(a)和属水平(b)的物种分布图 Fig. 2 Species distribution map based on phylum level (a) and genus level (b) |
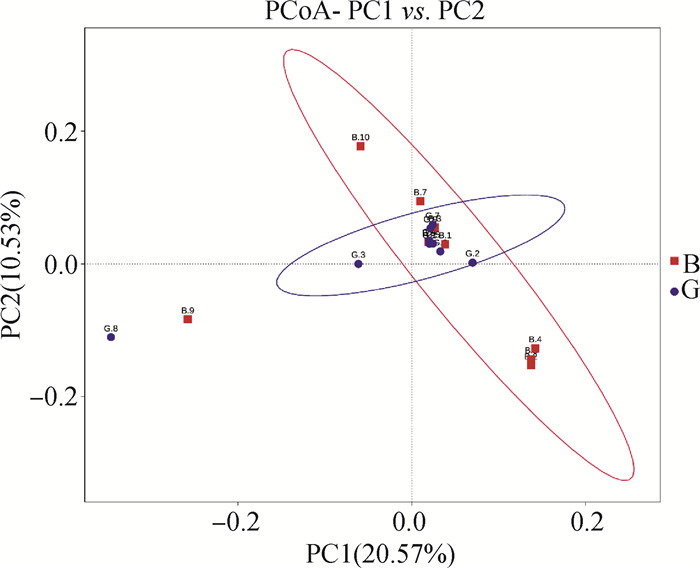
由表 2可知,G组奶牛Alpha多样性指数中Chao1和ACE较B组显著升高(P<0.05),二者均是评估群落内OTU数目的指数,说明灌服归芪益母复方制剂后奶牛瘤胃菌群总数显著升高。Beta多样性分析中PCoA可视化了两组瘤胃样品微生物群落的差异,可以看出,B组和G组有明显夹角,表明灌服归芪益母复方制剂明显改变了产后奶牛瘤胃菌群构成(图 3)。
|
|
表 2 瘤胃样品Alpha多样性指数 Table 2 Alpha diversity index of rumen samples |
|
图 3 两组瘤胃样品PCoA图 Fig. 3 PCoA images of two groups of rumen samples |
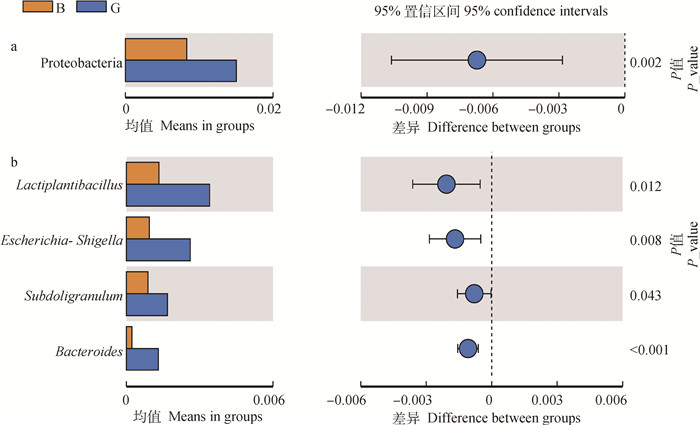
T检验考察了B组和G组在门水平和属水平上的差异物种,在门水平上,G组变形菌相对丰度极显著高于B组(P < 0.01);在属水平上,G组拟杆菌属(Bacteroides)和Escherichia-Shigella属的相对丰度极显著高于B组(P < 0.01),乳杆菌属(Lactiplantibacillus)和Subdoligranulum属显著高于B组(P < 0.05),见图 4。
|
图 4 两组奶牛在门水平(a)和属水平(b)的组间差异物种 Fig. 4 Differences in species at phylum level (a) and genus level (b) between the two groups of cows |
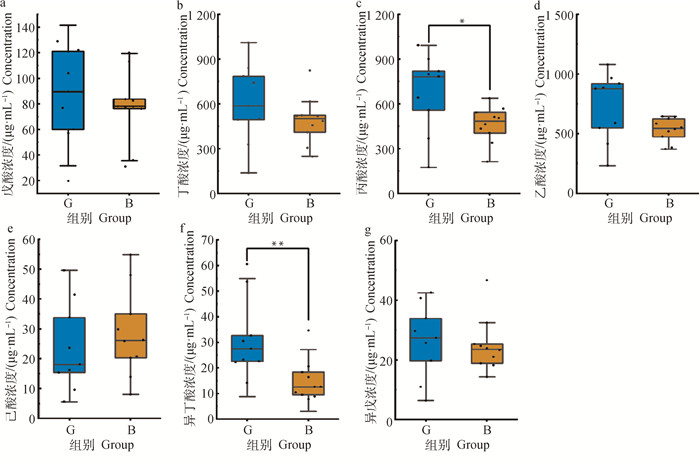
各短链脂肪酸的回归方程见表 3,根据回归方程,计算出了各组奶牛瘤胃短链脂肪酸的含量(表 4)。由图 5可知,灌服归芪益母复方制剂后,G组除己酸外其余6种短链脂肪酸含量均高于B组,且丙酸的含量显著高于B组(P < 0.05);异丁酸的含量极显著高于B组(P < 0.01)。
|
|
表 3 短链脂肪酸含量的标准曲线线性回归方程 Table 3 Standard curve linear regression equation of the content of fatty acids |
|
|
表 4 奶牛瘤胃短链脂肪酸含量 Table 4 Short-chain fatty acids concentration in rumen of dairy cows |
|
a~g.分别为戊酸、丁酸、丙酸、乙酸、己酸、异丁酸和异戊酸浓度。*.表示差异显著(P < 0.05);**.表示差异极显著(P < 0.01) a-g. Represent valeric acid, butyric acid, propionic acid, acetic acid, caproic acid, isobutyric acid and isovaleric acid concentrations, respectively. *. Means the difference is significant (P < 0.05); **. Means the difference is extremely significant (P < 0.01) 图 5 两组奶牛短链脂肪酸含量箱形图和散点图 Fig. 5 Box plot and scatter plot of the concentration of short-chain fatty acids in two groups of dairy cows |
瘤胃是由多种微生物组成的复杂生态系统,这些微生物可发酵饲料中的粗纤维,产生短链脂肪酸,为宿主提供能量,并介导了肠道免疫和炎症反应[12-13]。归芪益母方出自《牛经备要医方》,具有补气生血、活血祛瘀功效,主治产后气血虚弱和血瘀证。但其作用机制的现代科学内涵还有待于进一步探索。众多研究已证实,奶牛产后食欲下降和泌乳启动所导致的能量负平衡,易诱发各种围产期疾病[14]。鉴于此,本试验从瘤胃微生物及其代谢产物短链脂肪酸的角度揭示该方对产后奶牛的保健机制。
3.1 归芪益母复方制剂对产后奶牛瘤胃变形菌门和乳杆菌属菌群的影响Alpha和Beta多样性结果显示,归芪益母复方制剂使产后奶牛瘤胃菌群种类和数量发生明显改变,其中变形菌门极显著升高。变形菌等兼性厌氧菌可以通过消耗氧气、改变pH、降低氧化还原电位以及产生二氧化碳使环境更适于厌氧菌的定植与生存[15-16],由此推测,归芪益母复方制剂有利于瘤胃厌氧环境的维持,对产后奶牛瘤胃厌氧菌的定植有促进作用。
本研究结果进一步显示,归芪益母复方制剂能明显增高产后奶牛瘤胃乳杆菌属的相对丰度。乳杆菌属是瘤胃中降解淀粉的主要菌属,具有改善肠道菌群结构[17],维持肠道健康[18],调节免疫[19]和减少甲烷产生[20]等作用。它通过分解淀粉产生乳酸、短链脂肪酸和细菌素等物质抑制病原菌的增殖[21],对乳房炎具有良好的疗效[22]。另外乳酸杆菌NK318.1可诱导分化肠道巨噬细胞,并能扩大抗炎巨噬细胞[23]。赵洁等[24]发现,乳房炎奶牛肠道乳酸杆菌数量较健康奶牛明显降低。本课题前期研究发现,归芪益母复方制剂能够增强产后奶牛免疫机能,降低乳中体细胞数[10],可能还与其增加乳杆菌属丰度密切相关。
3.2 归芪益母复方制剂对产后奶牛瘤胃拟杆菌属菌群及其代谢产物丙酸和异丁酸的影响拟杆菌属含有大量的碳水化合物活性酶基因,具有很强的聚糖利用能力[25],能从饮食中解构植物纤维、宿主黏蛋白层和糖胺聚糖,并利用多糖产生乙酸盐和丙酸盐,为宿主提供能量[26]。Smith等[27]研究发现,给GF小鼠提供乙酸盐和丙酸盐后,可激活GPR43上调Foxp3表达从而刺激结肠Treg的扩增,以增加IL-10的表达,提高免疫功能。除此之外,Yioshida等[28]发现,拟杆菌属的菌株可抑制肠道微生物产生脂多糖,降低促炎性免疫反应。Aktar等[29]也确认拟杆菌属的菌株对宿主免疫T细胞的功能有促进作用。其代谢物乙酸不但能提供能量,还能维持肠道稳态,抑制病原菌生长和炎症反应[30],丙酸能降低β-羟丁酸浓度, 减少尿酮的产生并预防脂肪肝[31],并且在缓解炎症和提高免疫方面有效[32]。本研究结果表明,归芪益母复方制剂可使产后奶牛瘤胃拟杆菌属丰度及其代谢产物丙酸含量显著升高,乙酸略有升高。本课题组前期研究发现,归芪益母复方制剂能提高产后奶牛宿主外周淋巴细胞的调节作用[33]。由此可见,灌服归芪益母复方制剂有利于预防产后奶牛能量负平衡,提高免疫力,减少产后炎症疾病的发生。
拟杆菌和变形菌门,尤其是拟杆菌与氨基酸代谢有关,可产生异丁酸等支链脂肪酸[34]。异丁酸属于异位酸的一种,是瘤胃内瘤胃球菌和产琥珀酸丝状杆菌等纤维分解菌生长因子,当异丁酸含量较低时就会限制这些微生物的生长,影响营养物质消化与代谢[35]。饲粮中补充异丁酸可改善泌乳奶牛瘤胃发酵、泌乳性能和生长发育[36],提高肉牛瘤胃总挥发性脂肪酸浓度、纤维分解菌数量和饲料纤维消化率[37]。本研究结果显示,产后奶牛灌服归芪益母复方制剂后,随着变形菌门和拟杆菌属菌群数量的增加,其瘤胃代谢物异丁酸含量也极显著升高,说明归芪益母复方制剂可能对产后奶牛瘤胃纤维分解菌菌群的恢复和营养物质消化有积极影响。
3.3 归芪益母复方制剂对产后奶牛瘤胃Subdoligranulum属菌群及其代谢产物丁酸的影响Van Hul等[38]研究发现,Subdoligranulum丰度与脂联素(ADPN)和高密度脂蛋白胆固醇(HDL-C)水平呈正相关。Takahashi等[39]发现,围产期健康奶牛血液HDL-C水平明显高于脂肪肝和泌乳热奶牛。ADPN具有改善肝糖脂代谢紊乱的作用,并且可以通过降低TNF-α和IL-6的释放以达到抗炎的作用[40-41]。另外,Subdoligranulum可以产生丁酸,发挥抗炎的作用[42-43]。Van Immerseel等[44]和Kaakoush等[45]研究指出,炎症性肠病与Subdoligranulum等丁酸生成菌的丰度降低有关。Schulthess等[46]发现,丁酸能抑制组蛋白去乙酰化酶3 (HDAC3),分化巨噬细胞,增强抗菌功能。本研究表明,灌服归芪益母复方制剂后奶牛瘤胃Subdoligranulum丰度明显增加,其代谢产物丁酸的含量也有升高趋势。陶柱萍等[30]也发现,黄芪有效成分黄芪苷可以升高Butyricimonas, Roseburia和Subdoligranulum等产丁酸菌的丰度。据此推测,归芪益母复方制剂可通过增加产后奶牛瘤胃Subdoligranulum的丰度发挥调节肝糖脂代谢紊乱和抗炎作用。
除此之外,本研究还发现,变形菌门下Escherichia-Shigella属在灌服归芪益母复方制剂后显著升高,由于本研究所采用的SLIVE数据库将Escherichia和Shigella归为同一个属[47],很难在种水平用16S rRNA技术进行鉴别区分,故其生理意义尚不清楚。
4 结论本试验采用16S rRNA高通量测序和GC-MS/MS靶向代谢组学技术,研究了灌服归芪益母复方制剂对产后奶牛瘤胃微生物菌群及其发酵产物短链脂肪酸的调节作用,结果显示归芪益母复方制剂能显著提高产后奶牛瘤胃变形菌门、乳杆菌属、拟杆菌属和Subdoligranulum属微生物菌群丰度,并能显著提高瘤胃短链脂肪酸丙酸和异丁酸的含量。研究结果从瘤胃微生物菌群及短链脂肪酸角度揭示了归芪益母方对产后奶牛的保健机制。
| [1] |
EDER J M, GORDEN P J, LIPPOLIS J D, et al. Lactation stage impacts the glycolytic function of bovine CD4(+) T cells during ex vivo activation[J]. Sci Rep, 2020, 10(1): 40-45. DOI:10.1038/s41598-019-56429-4 |
| [2] |
XUE Z, LIU N, WANG Y, et al. Combining orchardgrass and alfalfa: Effects of forage ratios on in vitro rumen degradation and fermentation characteristics of silage compared with hay[J]. Animals Basel, 2019, 10(1): 5-9. |
| [3] |
BREDE M, ORTON T, PINIOR B, et al. PacBio and Illumina MiSeq amplicon sequencing confirm full recovery of the bacterial community after subacute ruminal acidosis challenge in the RUSITEC System[J]. Front Microbiol, 2020, 7(11): 13-18. |
| [4] |
VICNI J L, REEVES W R, SWARTHOUT J T, et al. Glyphosate in livestock: feed residues and animal health[J]. J ANIM SCI, 2019, 11(97): 4509-4518. |
| [5] |
SUN M, WU W, LIU Z, et al. Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases.[J]. J GASTROENTEROL, 2017, 52(1): 1-8. DOI:10.1007/s00535-016-1242-9 |
| [6] |
LUO D, PENG Z, YANG L, et al. Niacin protects against butyrate-induced apoptosis in rumen epithelial cells[J]. Oxid Med Cell Longev, 2019, 10(13): 217-228. |
| [7] |
NEUBAUER V, PETRI R M, HUMER E, et al. Starch-rich diet induced rumen acidosis and hindgut dysbiosis in dairy cows of different lactations[J]. Animals Basel, 2020, 10(10): 17-27. |
| [8] |
MA J, SHAH A M, WANG Z, et al. Comparing the gastrointestinal barrier function between growth-retarded and normal yaks on the Qinghai-Tibetan Plateau[J]. PeerJ, 2020, 3(8): 9-15. |
| [9] |
LIN T L, LU C C, LAI W F, et al. Role of gut microbiota in identification of novel TCM-derived active metabolites[J]. Protein Cell, 2021, 12(5): 394-410. DOI:10.1007/s13238-020-00784-w |
| [10] |
郭启勇, 陶金忠, 吴心华, 等. 归芪益母口服液对产后奶牛免疫机能和生产性能的影响[J]. 中国兽医学报, 2020, 40(10): 2013-2019. GUO Q Y, TAO J Z, WU X H, et al. Effect of Guiqi Yimu oral liquid on immune function and production performance of postpartum dairy cows[J]. Chinese Journal of Veterinary Science, 2020, 40(10): 2013-2019. DOI:10.16303/j.cnki.1005-4545.2020.10.21 (in Chinese) |
| [11] |
张瑞雪, 刘欣, 徐晓锋, 等. 奶牛分娩前后瘤胃代谢物变化规律及其代谢通路研究[J]. 畜牧兽医学报, 2021, 52(11): 3137-3148. ZHANG R X, LIU X, XU X F, et al. Study on the changes of rumen metabolites and metabolic pathways in dairy cows before and after parturition[J]. Acta Veterinaria et Zootechnica Sinica, 2021, 52(11): 3137-3148. DOI:10.11843/j.issn.0366-6964.2021.011.015 (in Chinese) |
| [12] |
HUWS S A, CREVEY C J, OYAMA L B, et al. Addressing global ruminant agricultural challenges through understanding the rumen microbiome: Past, present, and future[J]. Front Microbiol, 2018, 9(25): 21-26. |
| [13] |
NATHANI N M, PATE A K, MOTAPALL C S, et al. Effect of roughage on rumen microbiota composition in the efficient feed converter and sturdy Indian Jaffrabadi buffalo (Bubalus bubalis)[J]. BMC Genomics, 2015, 12(16): 11-16. |
| [14] |
ESPOSITO G, IRONS P C, WEBB E C, et al. Interactions between negative energy balance, metabolic diseases, uterine health and immune response in transition dairy cows[J]. Anim Reprod Sci, 2014, 144(3-4): 60-71. DOI:10.1016/j.anireprosci.2013.11.007 |
| [15] |
CHOW W, LEE Y K. Mucosal interactions and gastrointestinal microbiota[J]. Gastrointestinal microbiology, 2006, 6: 123-136. |
| [16] |
CAMBRIDGE M W. Microbial inhabitants of humans: Their ecology and role in health and disease[M]. Cambridge University Press, 2005.
|
| [17] |
SPLICHAL I, DONOVAN S M, SPLICHAL O Z, et al. Colonization of germ-free piglets with commensal Lactobacillus amylovorus, Lactobacillus mucosae, and probiotic E. coli Nissle 1917 and their interference with Salmonella typhimurium[J]. Microorganisms, 2019, 7(8): 273. DOI:10.3390/microorganisms7080273 |
| [18] |
SANDERS M E, MERENSTEI D J, REID G, et al. Probiotics and prebiotics in intestinal health and disease: From biology to the clinic[J]. Nat Rev Gastro Hepat, 2019, 16(10): 605-616. DOI:10.1038/s41575-019-0173-3 |
| [19] |
GARCIA-CASTILLO V, KOMATSU R, CLUA P, et al. Evaluation of the immunomodulatory activities of the probiotic strain Lactobacillus fermentum UCO-979C[J]. Front Immunol, 2019, 10(13): 13-17. |
| [20] |
MIZRAHI I, WALACE R J, MORAIS S. The rumen microbiome: Balancing food security and environmental impacts[J]. Nat Rev Microbiol, 2021, 19(9): 553-566. DOI:10.1038/s41579-021-00543-6 |
| [21] |
LEBEER S, BRON P A, MARCO M L, et al. Identification of probiotic effector molecules: Present state and future perspectives[J]. Curr Opin Biotechnol, 2018, 2(49): 217-223. |
| [22] |
FERNANDEZ L, AROYO R, ESPINOSA I, et al. Probiotics for human lactational mastitis[J]. Benef Microbes, 2014, 5(2): 169-183. DOI:10.3920/BM2013.0036 |
| [23] |
HUANG Y, QI H, ZHANG Z, et al. Gut REG3γ-Associated Lactobacillus induces anti-inflammatory macrophages to maintain adipose tissue homeostasis[J]. Front Immunol, 2017, 4(8): 10-16. |
| [24] |
赵洁, 马晨, 席晓敏, 等. PCR-DGGE法分析健康奶牛和乳房炎患牛肠道微生物菌群的多样性[J]. 中国奶牛, 2014(17): 1-5. ZHAO J, MA C, XI X M, et al. Diversity analysis of intestinal microflora from healthy cow and mastitis cow by PCR-DGGE[J]. China Dairy Cattle, 2014(17): 1-5. (in Chinese) |
| [25] |
POLLET R M, MARTIN L M, KOROPATKIN N M. TonB-dependent transporters in the Bacteroidetes: Unique domain structures and potential functions[J]. Mol Microbiol, 2021, 115(3): 490-501. DOI:10.1111/mmi.14683 |
| [26] |
MAGNE F, GOTTELAND M, GAUTHIER L, et al. The Firmicutes/Bacteroidetes ratio: A relevant marker of gut dysbiosis in obese patients?[J]. Nutrients, 2020, 12(5): 14-17. |
| [27] |
SMITH P M, HOWITT M R, PANIKOV N, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis[J]. Science, 2013, 341(6145): 569-573. |
| [28] |
YIOSHIDA N, EMOTO T, YAMASHITA T, et al. Bacteroides vulgatus and Bacteroides dorei reduce gut microbial lipopolysaccharide production and inhibit atherosclerosis[J]. Circulation, 2018, 138(22): 2486-2498. |
| [29] |
AKTAR R, PARKAR N, STENTZ R, et al. Human resident gut microbe Bacteroides thetaiotaomicron regulates colonic neuronal innervation and neurogenic function[J]. Gut Microbes, 2020, 11(6): 1745-1757. |
| [30] |
陶柱萍, 龙宇, 李灿委, 等. 肠道菌群在中草药抗溃疡性结肠炎中的作用[J]. 药学学报, 2021, 56(2): 391-402. TAO Z P, LONG Y, LI C W, et al. Role of gut microbiota in the treatment of ulcerative colitis with traditional Chinese medicine[J]. Acta Pharmaceutica Sinica, 2021, 56(2): 391-402. (in Chinese) |
| [31] |
DORN K, LEIBER F, SUNDRUM A, et al. A field trial on the effects of pure sodium propionate and a combination with herbal extracts on short term development of subclinical ketosis[J]. Livest Sci, 2016, 5(187): 87-95. |
| [32] |
WALKENHORST M, LEIBER F, MAESCHLI A, et al. A multicomponent herbal feed additive improves somatic cell counts in dairy cows ‐ a two stage, multicentre, placebo-controlled long-term on-farm trial[J]. J Anim Physiol An N, 2019, 104(2): 439-452. |
| [33] |
张博, 秦俊杰, 朱浩, 等. 归芪益母口服液对产后奶牛炎性因子调节作用[J]. 中兽医医药杂志, 2021, 40(04): 56-60. ZHANG B, QIN J J, ZHU H, et al. Effect of Guiqi Yimu oral liquid on the regulation of inflammatory factors in postpartum cows[J]. Journal of Traditional Chinese Veterinary Medicine, 2021, 40(04): 56-60. (in Chinese) |
| [34] |
NDOU S P, TUN H M, KIARIE E, et al. Dietary supplementation with flaxseed meal and oat hulls modulates intestinal histomorphometric characteristics, digesta- and mucosa-associated microbiota in pigs[J]. Sci Rep, 2018, 8(1): 58-68. |
| [35] |
梁艳, 刘强, 王聪, 等. 异丁酸对犊牛瘤胃及小肠黏膜基因表达的影响[J]. 动物营养学报, 2015, 15(08): 2483-2492. LIANG Y, LIU Q, WANG C, et al. Effects of isobutyrate on gene expressions of ruminal and small intestinal mucosa of calves[J]. Chinese Journal of Animal Nutrition, 2015, 15(08): 2483-2492. (in Chinese) |
| [36] |
LIU Q, WANG C, YANG W Z, et al. Effects of isobutyrate on rumen fermentation, lactation performance and plasma characteristics in dairy cows[J]. Anim Feed Sci Tech, 2009, 1(154): 58-67. |
| [37] |
WANG C, LIU Q, ZHANG Y L, et al. Effects of isobutyrate supplementation on ruminal microflora, rumen enzyme activities and methane emissions in Simmental steers[J]. J Anim Physiol An N, 2015, 99(1): 123-131. |
| [38] |
VAN HUL M, LE R T, PRIFTI E, et al. From correlation to causality: The case of Subdoligranulum[J]. Gut Microbes, 2020, 12(1): 1-13. |
| [39] |
TAKAHASHI T, MORI A, ODA H, et al. Comparison of cholesterol levels among lipoprotein fractions separated by anion-exchange high-performance liquid chromatography in periparturient Holstein-Friesian dairy cows[J]. J Vet Med Sci, 2021, 83(2): 260-266. |
| [40] |
MAKKI K, DEEHAN E C, WALTER J, et al. The impact of dietary fiber on gut microbiota in host health and disease[J]. Cell Host Microbe, 2018, 23(6): 705-715. |
| [41] |
KOH A, DE V F, KOVATCHEVA-DATCHARY P, et al. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites[J]. Cell, 2016, 165(6): 1332-1345. |
| [42] |
VINOLO M A R, RODRIGUES H G, NACHBAR R T, et al. Regulation of Inflammation by short chain fatty acids[J]. Nutrients, 2011, 3(10): 858-876. |
| [43] |
HOLMSTROM K, COLLINS M D, MOLER T, et al. Subdoligranulum variabile gen. nov., sp. nov. from human feces[J]. Anaerobe, 2004, 10(3): 197-203. |
| [44] |
VAN IMMERSEEL F, DUCATELLE R, DE V M, et al. Butyric acid-producing anaerobic bacteria as a novel probiotic treatment approach for inflammatory bowel disease[J]. J Med Microbiol, 2010, 59(2): 141-143. |
| [45] |
KAAKOUSH N O, DAY A S, HUINAO K D, et al. Microbial dysbiosis in pediatric patients with Crohn's disease[J]. J Clin Microbiol, 2012, 50(10): 3258-3266. |
| [46] |
SCHULTHESS J, PANDEY S, CAPITANI M, et al. The short chain fatty acid butyrate imprints an antimicrobial program in macrophages[J]. Immunity, 2019, 50(2): 432-445. |
| [47] |
LU J, SALZBERG S L. Ultrafast and accurate 16S rRNA microbial community analysis using Kraken 2[J]. Microbiome, 2020, 8(1): 124-133. |
(编辑 范子娟)



