2. 黑龙江省实验动物与比较医学重点实验室,哈尔滨 150030
2. Heilongjiang Key Laboratory of Experimental Animals and Comparative Medicine, Harbin 150030, China
硒作为必需的微量元素,广泛存在于动物组织中,硒能确保人体正常的生理机能,平衡人体的抗氧化状态[1],在维持人体正常的免疫系统中也起着非常重要的作用[2]。硒缺乏会导致脾、法氏囊等免疫器官发生损伤[3-4]。硒缺乏可通过线粒体介导的途径引起心肌氧化应激,诱导细胞凋亡[5],也可以通过诱导内质网应激引起细胞凋亡[6]。细胞凋亡是由基因控制的细胞自主有序的死亡,大量的研究表明,硒可以调控细胞凋亡[7-8]。Toll样受体4(TLR4)作为toll样受体家族成员,在体内多种细胞中都有表达,如心肌细胞[9]、免疫细胞[10]等,被激活后可以触发免疫炎性反应,保护机体免受感染挑战,增强获得性免疫应答能力[11]。TLR2和TLR3主要在健康胸腺中表达[12],而TLR4在胸腺炎和胸腺瘤中表达上调[13]。髓系二分化88(MyD88)和诱导IFN-β的主要含适配器蛋白(TRIF)是TLR4传输信号的两个适配器[14],MyD88通路能激活NF-κB,进一步促进炎症因子的产生[15],NF-κB也可以调控细胞凋亡[16],激活促凋亡因子的产生。Gao等[17]研究发现,成纤维细胞生长因子21(FGF21)通过抑制TLR4/MyD88/NF-κB信号通路来抑制炎症和细胞凋亡。有研究表明,上调miR-140-5p可通过抑制TLR4/MyD88/NF-κB信号通路[18],抑制KOA大鼠滑膜细胞的炎症反应和凋亡,从而保护滑膜损伤。黄芪甲苷通过抑制TLR4/NF-κB信号通路保护心肌细胞免于凋亡[19]。有研究表明,硒缺乏会导致胸腺组织发生损伤[20],并且不同的外界因素都可能导致胸腺发生细胞凋亡[21-22]。但关于硒通过TLR4信号通路对胸腺细胞凋亡影响的研究较少,所以硒缺乏引起胸腺细胞凋亡过程中是否激活Toll样受体信号转导通路是人们感兴趣的问题,因此本研究复制鸡硒缺乏模型,观察胸腺病理损伤情况,检测胸腺组织中TLR4信号转导通路与细胞凋亡相关因子的表达。并在MSB1细胞中建立体外硒缺乏模型,通过干扰和过表达TLR4基因后,检测MSB1细胞中TLR4信号转导通路及细胞凋亡相关因子的表达水平。旨在探讨硒缺乏是否激活Toll样受体信号转导通路及如何参与鸡胸腺细胞凋亡损伤,阐明缺硒性免疫器官损伤的发病机制。
1 材料与方法 1.1 动物和饮食从中国哈尔滨益农家禽有限公司购买了160只1日龄健康肉鸡。将鸡随机分为缺硒组(L组)和对照组(C组),每组80只。L组饲喂含硒0.004 mg·kg-1的自制低硒日粮,低硒日粮组成见表 1,C组饲喂含硒0.2 mg·kg-1的全配方饲料(在硒缺乏日粮中添加Na2SeO3)。给肉鸡提供一个适宜的环境,并有标准的光照控制(每天12 h)。每天进行检查,观察鸡的活动、运动、生长、异常和压力。基础日粮为在中国黑龙江省硒缺乏地区生产的玉米和豆粕。分别在第15、25、35、45、55天的不同时间点采集胸腺组织样本。部分胸腺组织在4%甲醛溶液中固定,另一部分在-80 ℃下保存。
|
|
表 1 低硒组日粮组成 Table 1 Diet composition of the selenium deficiency group |
胸腺组织样品用10 mL·L-1福尔马林溶液固定至少1周。采用常规方法制作病理石蜡切片(5 μm),并用苏木精和伊红(H & E)染色。在光学显微镜(日本Nikon)下观察其病理变化。
1.3 透射电镜检查固定胸腺组织经脱水、饱和、包埋,切片,用醋酸铀和柠檬酸铅染色。在透射电子显微镜(H7650,日本东京)下观察试验结果。
1.4 细胞培养、处理和转染MSB1细胞在10% FBS(美国BioInd)的RPMI-1640培养基中培养。MSB1细胞在含有50 mL·L-1 CO2 37 ℃的温箱中培养。将密度为2×106的MSB1细胞接种到24孔板中。将细胞随机分为6组:正常MSB1细胞(C组)、缺硒MSB1细胞(L组)、siRNA阴性对照处理后缺硒培养的MSB1细胞(L+NCsiRNA)、siChTLR4处理后缺硒培养的MSB1细胞(L+siChTLR4)、pCMV-HA-N空质粒处理后缺硒培养的MSB1细胞(L+pCMV-HA),pCMV-HA-TLR4质粒处理后缺硒培养的MSB1细胞(L+pCMV-HA-TLR4)。C组通常在含有10% FBS的RPMI-1640培养基中培养。L组中的细胞在37 ℃下孵育4~6 h后变成含有1% FBS(BioInd,USA),10 μg·mL-1胰岛素(Solarbio,北京)和5 μg·mL-1转铁蛋白(Solarbio,北京)RPMI-1640培养液中培养5 d被视为低硒条件。以Lipofiter(上海汉恒)为转染试剂并严格按照说明书要求将空质粒和TLR4质粒分别转入L+pCMV-HA-N组和L+pCMV-HA-TLR4组的细胞中,其中,pCMV-HA-TLR4以pCMV-HA-N为载体、以HA为标签,融合了TLR4目的蛋白的重组表达质粒(上海生工)。L+NCsiRNA组和L+siChTLR4组采用RNAFit(上海汉恒)作为转染试剂,按照说明书将siRNA转入细胞中,ChTLR4基因siRNA序列上游引物:5′-GGAAGAGAUGUAAGUUUCAT-3′,下游引物:5′-UGAAACUUACAUCUCUUCCTT-3′,其他条件与L组相同。
1.5 流式细胞术根据试剂盒说明书,使用双AnnexinV/碘化丙啶(PI)细胞凋亡检测试剂盒分析细胞凋亡。MSB1细胞1 000 r离心5 min,在室温黑暗下,用AnnexinV-FITC和PI染色15 min。然后,用流式细胞术(FZCSARIA,中国)和FlowJo7.6.1软件检测细胞凋亡率。
1.6 AO/EB染色采用吖啶橙/溴化乙锭(AO/EB)检测活细胞和凋亡细胞。MSB1细胞在24孔板上培养,用PBS洗涤,用AO/EB染色5 min。然后在荧光显微镜(日本Nikon)下拍摄MSB1细胞。
1.7 总RNA的提取和qRT-PCR检测按照产品说明书,使用总RNA试剂盒Ⅱ(天根生物技术有限公司,北京)从胸腺组织中提取总RNA。qRT-PCR在LightCycler@480系统(罗氏,瑞士)上进行。引物序列详见表 2。采用2-ΔΔCt的方法计算靶基因的相对表达量,并与管家基因β-actin(用于mRNA)的表达量进行比较。
|
|
表 2 鸡qPCR的基因序列 Table 2 Gene-special primers used for qPCR in chicken |
用蛋白酶抑制剂和RIPA缓冲液提取胸腺组织和细胞总蛋白。等量的蛋白质样品用12% SDPS-PAGE分离,并电转移到硝酸纤维素膜上。转印后,用5%脱脂牛奶在37 ℃下封闭2 h。随后,将硝酸纤维素膜在4 ℃的一抗中孵育过夜。用TTBS洗涤后,在室温下,进行二次抗体孵育2 h,然后用TTBS洗涤。该膜采用ECL超灵敏发光溶液进行曝光。利用ImageJ软件对波段强度进行量化。本研究使用的抗体:ChTLR4 (1∶100)(东北农业大学病理解剖实验室制备的多克隆抗体),MyD88 (1∶500,万类,沈阳),NF-κB (1∶500,万类,沈阳)和Caspase-3 (1∶500,万类,沈阳),Caspase-9 (1∶500,万类,沈阳),Bax (1∶500,万类,沈阳),Bcl-2 (1∶500,万类,沈阳),GADPH (1∶1 000,Abmart,上海),HRP标记的山羊抗兔IgG (1∶2000,ZSGB-B)。
1.9 统计分析采用SPSS (17版; SPSS,Chicago,IL,USA)进行统计学显著性分析。用GraphPad (版本7.0,GraphPad Software inc.,San Diego,CA,USA)进行统计学分析,P < 0.05具有统计学意义。在每种情况下至少进行3个独立的试验,并以“平均值(SEM)±标准误差”表示数据。
2 结果 2.1 硒缺乏鸡胸腺形态学观察2.1.1 硒缺乏鸡胸腺病理组织学观察结果 鸡胸腺组织形态学观察结果显示,C组鸡胸腺皮质髓质界限清晰,淋巴细胞排列整齐。L组鸡皮质髓质的淋巴细胞数量减少、细胞排列紊乱、皮质髓质充血、核碎裂,广泛的局灶性坏死,随着日龄的增加,缺硒组胸腺均出现损伤,并且在35日龄最为严重。
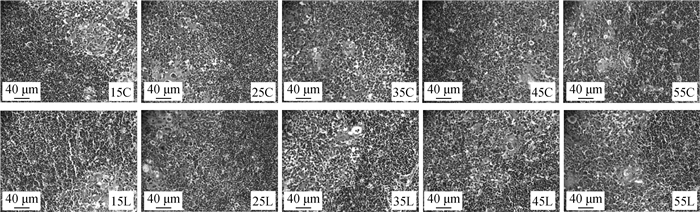
|
C.对照组;L.缺硒组;15~55. 15~55日龄的鸡 C. Control group; L. Selenium deficiency group; 15~55. 15~55-day-old chickens 图 1 不同日龄硒缺乏鸡胸腺组织病理形态 Fig. 1 Histopathological morphology of thymus tissue in selenium-deficient chickens at different ages |
2.1.2 硒缺乏鸡胸腺组织透射电镜观察结果 电镜结果显示,C组鸡胸腺组织淋巴细胞排列紧密,核质比大、核膜光滑,细胞结构完整(图 2a)。L组鸡胸腺组织淋巴细胞间出现裂隙,体积缩小,细胞碎裂,核染色质边集,线粒体肿胀,嵴断裂(图 2b)。
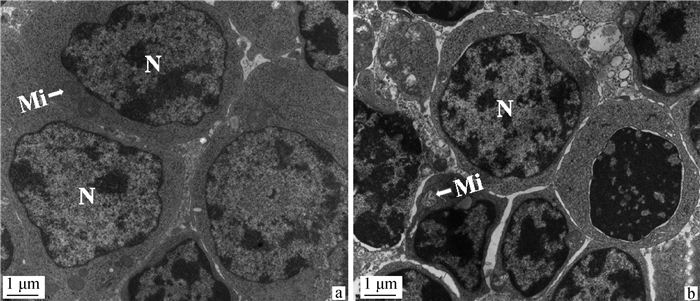
|
a. C组胸腺的超微结构变化(比例尺: 1 μm);b. L组胸腺的超微结构变化(比例尺: 1 μm);N. 细胞核;Mi. 线粒体 a. Ultrastructural changes of the thymus in the C group (scale bar: 1 μm); b. Ultrastructural changes of the thymus in the L group(scale bar: 1 μm); N. Nucleus; Mi. Mitochondria 图 2 35日龄鸡胸腺的超微病理变化 Fig. 2 Ultramicropathological changes in the thymus of 35-day-old chickens |
2.2.1 硒缺乏鸡胸腺组织中TLR4、MyD88和NF-κB的mRNA和蛋白表达结果 qRT-PCR和Western blot结果显示,与15日龄对照组相比,在L组胸腺中TLR4的mRNA相对表达在15日龄无明显变化(P>0.05,图 3a),在25~55日龄极显著升高(P < 0.01,图 3a);MyD88和NF-κB的mRNA相对表达在15~55日龄极显著升高(P < 0.01,图 3b, c)。与15日龄对照组相比,L组胸腺中TLR4、MyD88和NF-κB的蛋白相对表达在15~55日龄极显著增加(P < 0.01,图 3 d~g)。
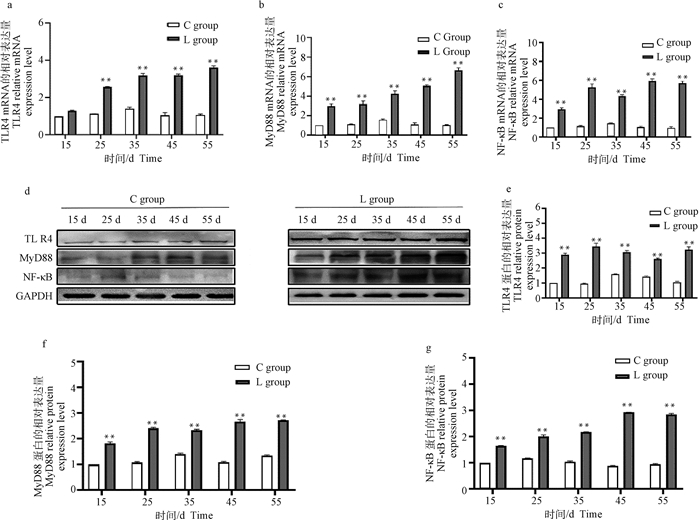
|
a~c.胸腺组织中TLR4、MyD88和NF-κB mRNA的表达水平;d.胸腺组织Western blot结果;e~g.胸腺组织中TLR4、MyD88和NF-κB的灰度值分析。与对照组相比,*.P < 0.05,**.P < 0.01。下同 a-c. The mRNA expression levels of TLR4, MyD88 and NF-κB in thymus tissue; d. Western blot results in thymus tissue; e-g. Gray value analysis of TLR4, MyD88 and NF-κB in thymus tissue. Compared with the control group, *.P < 0.05, **.P < 0.01. The same as below 图 3 鸡胸腺组织中TLR4、MyD88和NF-κB mRNA和蛋白的相对表达 Fig. 3 The mRNA and protein relative expression of TLR4, MyD88 and NF-κB in the thymus of chickens |
2.2.2 硒缺乏鸡胸腺组织中凋亡相关基因的mRNA和蛋白表达 qRT-PCR和Western blot结果显示,与15日龄C组相比,L组胸腺中Caspase-3、Caspase-9、Bax mRNA相对表达在15~55日龄均极显著增加(P < 0.01, 图 4a~c),Bcl-2在第15~55日龄极显著下降(P < 0.01,图 4d)。与15日龄C组相比,L组胸腺中Caspase-3、Caspase-9和Bax的蛋白相对表达在15~55日龄极显著增加(P < 0.01, 图 4f~h);Bcl-2蛋白相对表达在15~55日龄极显著降低(P < 0.01,图 4i)。
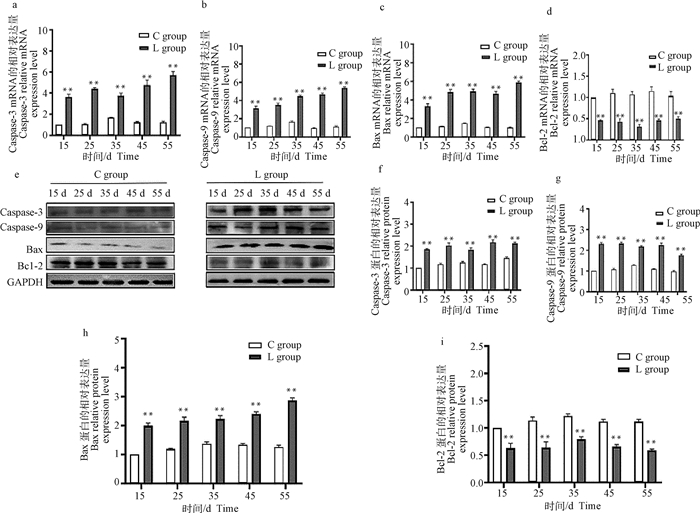
|
a~d. 胸腺组织凋亡相关基因mRNA表达量;e.胸腺组织Western blot结果;f~i.胸腺组织中凋亡相关基因表达蛋白的灰度值分析 a-d. The mRNA expression levels of apoptosis-related genes in thymus tissue; e. Western blot results in thymus tissue; f-i. Gray value analysis of proteins of apoptosis-related genes in thymus tissue 图 4 鸡胸腺凋亡相关基因mRNA和蛋白相对表达量 Fig. 4 The mRNA and protein relative expression of apoptosis-related genes in the thymus of chickens |
AO/EB染色结果(图 5a)显示,核染色质着绿色并呈正常结构的是活细胞;核染色质着绿色呈固缩状或圆珠状的是早期凋亡细胞;核染色质着橘红色并呈正常结构是非凋亡的死亡细胞;核染色质为橘红色并呈固缩状或圆珠状是晚期凋亡细胞。AO/EB染色定量结果显示,与C组相比,L组凋亡细胞数极显著增加(P < 0.01);与L组相比,L+NCsiRNA组凋亡细胞数无明显变化(P>0.05),L+siChTLR4组凋亡细胞数极显著降低(P < 0.01),L+pCMV-HA-N组凋亡细胞数无明显变化(P>0.05),L+pCMV-HA-TLR4组凋亡细胞数极显著升高(P < 0.01),详见图 5b。
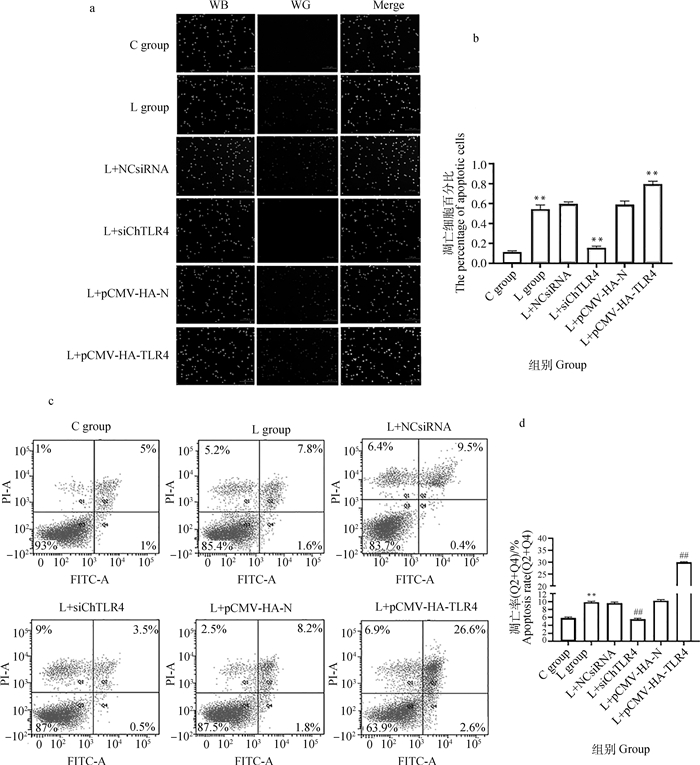
|
a. AO/EB荧光染色(比例尺,100 μm);b. Image J荧光定量强度分析AO/EB荧光染色;c. 流式细胞仪分析结果;d.流式细胞术的定量结果(n=3)。与对照组相比,*.P < 0.05, **. P < 0.001;与缺硒组相比,#.P < 0.05, ##.P < 0.001。下同 a.The AO/EB fluorescence staining (scale bar, 100 μm); b. The AO/EB fluorescence intensity quantitative analysis by Image J; c. The results of flow cytometry analysis; d. The quantitative results of flow cytometry (n=3). Compared with the control group, *. P < 0.05, **. P < 0.01; Compared with the seleniun deficiency group, #. P < 0.05, ##. P < 0.01. The same as below 图 5 MSB1细胞凋亡检测结果 Fig. 5 Cell apoptosis assay results for MSB1 cells |
流式细胞术结果(图 5c)显示,采用AnnexinV-FITC/PI双染色法检测MSB1细胞的凋亡率,流式细胞术定量结果显示,与C组相比,L组凋亡率极显著增加(P < 0.01),与L组相比,L+NCsiRNA组凋亡率变化不明显(P>0.05),L+siChTLR4组凋亡率极显著降低(P < 0.01),L+pCMV-HA-N组凋亡率变化不明显(P>0.05),L+pCMV-HA-TLR4组凋亡率极显著升高(P < 0.01),详见图 5d。
2.4 硒缺乏诱导MSB1细胞TLR4信号转导分子及凋亡相关基因的mRNA和蛋白的表达2.4.1 TLR4干扰和过表达效果 qRT-PCR和Western blot结果(图 6)显示,与C组相比,L组TLR4的mRNA和蛋白表达极显著增加(P < 0.01),与L组相比,L+NCsiRNA组和L+pCMV-HA-N组TLR4 mRNA和蛋白表达水平未发生明显改变(P>0.05),L+siChTLR4组TLR4 mRNA和蛋白表达水平极显著降低(P < 0.01),L+pCMV-HA-TLR4组TLR4 mRNA和蛋白表达水平极显著升高(P < 0.01)。
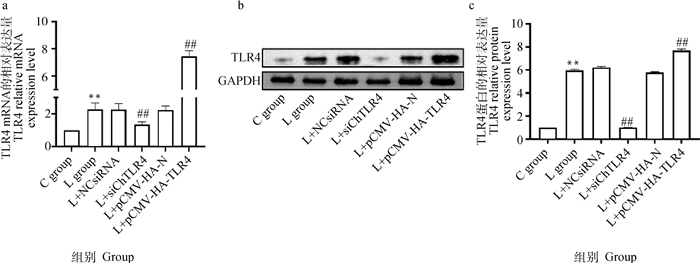
|
a.MSB1细胞中TLR4的mRNA表达水平;b. MSB1细胞Western blot检测结果;c. TLR4的灰度值分析 a. The mRNA expression levels of TLR4 in MSB1 cells; b. Western blot results in MSB1 cells; c. Gray value analysis of TLR4 图 6 MSB1细胞中TLR4的mRNA和蛋白的相对表达 Fig. 6 The mRNA and protein relative expression of ChTLR4 in MSB1 cells |
2.4.2 干扰和过表达TLR4后对MSB1细胞中信号转导分子及凋亡相关基因的影响 qRT-PCR和Western blot结果(图 7)显示,与C组相比,L组MyD88、NF-κB、Caspase-3、Caspase-9、Bax的mRNA和蛋白表达水平显著增加(P < 0.05),L组Bcl-2的mRNA和蛋白表达水平极显著降低(P < 0.01)。与L组相比,L+siChTLR4组的MyD88、NF-κB、Caspase-3、Caspase-9、Bax的mRNA和蛋白表达极显著降低(P < 0.01),Bcl-2的mRNA和蛋白表达水平极显著升高(P < 0.01),与L组相比,L+pCMV-HA-TLR4组的MyD88、NF-κB、Caspase-3、Caspase-9、Bax mRNA和蛋白表达极显著升高(P < 0.01),Bcl-2的mRNA和蛋白表达水平极显著降低(P < 0.01)。

|
a~f.MSB1细胞中MyD88、NF-κB、Caspase-3、Caspase-9、Bax和Bcl-2的mRNA表达水平;g. MSB1细胞Western blot检测结果;h~m. MSB1细胞中MyD88、NF-κB、Caspase-3、Caspase-9、Bax和Bcl-2的灰度值分析。 a-f. The mRNA expression levels of MyD88, NF-κB, Caspase-3, Caspase-9, Bax, Bcl-2 in MSB1 cells; g. Western blot results in MSB1 cells; h-m. Gray value analysis of MyD88, NF-κB, Caspase-3, Caspase-9, Bax, Bcl-2 图 7 MSB1细胞中MyD88、NF-κB、Caspase-3、Caspase-9、Bax和Bcl-2的mRNA和蛋白相对表达 Fig. 7 The mRNA and protein relative expression of MyD88, NF-κB, Caspase-3, Caspase-9, Bax, Bcl-2 in MSB1 cells |
胸腺组织结构的正常才能确保机体发挥正常的功能,胸腺组织损伤会影响机体的免疫功能。正常的胸腺组织被膜和胸腺小叶结构完整,皮质髓质界限清晰,淋巴细胞密集且完整[24]。有研究表明,硒缺乏会导致鸡胸腺小叶结构紊乱、有轻度的萎缩,出现坏死,并伴有少量的出血,淋巴细胞排列疏松无规则[25]。还有研究表明,硒缺乏会导致胸腺皮质区域变薄,胸腺小叶的髓质明显扩张,网状上皮细胞明显增加。胸腺小叶的皮质髓质中的淋巴细胞明显减少,并且分布稀疏。本试验结果表明,硒缺乏影响鸡胸腺组织生长发育,导致胸腺组织发生损伤,淋巴细胞数量减少、细胞排列紊乱、皮质髓质充血、核碎裂,广泛的局灶性坏死。
在超微结构观察中,硒缺乏会显著增加淋巴细胞变性坏死的数量,然而这些淋巴细胞会被网状的巨噬细胞吞噬。通过超微病理学观察,亦见胸腺网状细胞线粒体发生退行性变,进一步证明了网状细胞结构和功能的损伤可能导致胸腺的萎缩以及淋巴细胞减少[27]。硒缺乏组胸腺淋巴细胞外周血和中央淋巴细胞染色质异常,淋巴细胞的细胞膜不完整,细胞内的线粒体肿胀。本试验结果表明,硒缺乏组鸡胸腺组织淋巴细胞间出现裂隙,体积缩小,细胞碎裂,核染色质边集,线粒体肿胀,嵴断裂。
3.2 硒缺乏对细胞凋亡的影响在缺硒状态下,抗氧化酶(TRXR和GPX)活性降低,ROS产生,信号级联的氧化还原调节紊乱,导致促炎细胞因子如IL-1、IL-6、IL-10、TNF-α和COX-2的表达增加,导致炎症和凋亡增加。有报道Gpx3、Gpx4、Dio1、Txnrd1、Txnrd2、Txnrd3、SelS、Selpb基因与促凋亡Caspase-3基因呈强负相关,与抗凋亡Bcl-2基因呈强正相关。硒蛋白W(SelW)通过抑制炎症和细胞凋亡来保护免疫器官免受氧化应激的影响[31],硒蛋白K(SelK)通过调控内质网应激启动未折叠蛋白反应,以限制ROS的产生,从而防止过度自噬介导的降解和凋亡死亡。硒化合物(亚硒酸盐、硒纳米颗粒、硒代蛋氨酸、硒二谷胱甘肽、亚硒酸等)的生物学效应,均取决于浓度,低浓度硒化合物只调节细胞内氧化还原,而高浓度硒诱导氧化应激和凋亡。硒纳米粒子(NPs)引起Caspases-3、Caspases-8和Caspases-9的剂量依赖性激活,并增加FADD表达,以及过度产生活性氧ROS,导致线粒体功能障碍,从而促进细胞凋亡。以上结果均提示,不同存在形式的硒均与细胞凋亡密切相关。在本研究中,通过检测缺硒组鸡胸腺组织中凋亡相关因子(Caspase-3、Caspase-9、Bax、Bcl-2)mRNA和蛋白水平,发现呈促凋亡趋势变化,又在体外通过流式细胞术和AO/EB染色检测低硒条件下MSB1细胞的凋亡情况,发现MSB1细胞出现明显的凋亡,本研究的结果和以上的研究发现相对一致,所以得出硒缺乏导致胸腺发生细胞凋亡。
3.3 TLR4信号转导通路对细胞凋亡的影响当TLR与配体结合后,TLR的TIR发生变构,MyD88的C端区域与TIR结合,N端通过死亡结构域(DD)募集同样含有死亡作用域的IRAK,导致IRAK自身磷酸化,磷酸化的IRAK从MyD88上脱离并与TRAF6结合,TRAF6活化丝裂激活蛋白激酶家族成员中的NF-κB诱导激酶(NIK),NIK自身磷酸化激活IκKs,导致IκB的泛素化并从IκB/NF-κB复合物中释放,NF-κB被激活,而NF-κB能通过上调促凋亡因子Bax,进而激活细胞凋亡[37]。当使用siRNA敲除TLR4基因后,发现可以抑制Caspase-3、Caspase-8、Caspase-9、Bax的激活从而防止小鼠心肌凋亡,还有研究发现,成纤维细胞生长因子21(FGF21)也通过抑制TLR4/MyD88/NF-κB信号通路来抑制炎症和细胞凋亡,还有研究通过分别下调TLR4/MyD88/NF-κB信号通路相关分子,来抑制KOA大鼠滑膜细胞炎症反应和凋亡,从而保护滑膜损伤。以上研究结果发现,TLR4可能通过MyD88/NF-κB信号通路介导细胞凋亡,为验证这个结论,本研究体外试验中,分别干扰和过表达TLR4后,通过检测信号通路(TLR4/MyD88/NF-κB)和凋亡相关因子(Caspase-3、Caspase-9、Bax、Bcl-2)的表达,发现分别干扰和过表达TLR4后会调控MyD88/NF-κB信号通路以及凋亡相关因子的改变。综上所述,表明TLR4/MyD88/NF-κB信号通路参与细胞凋亡。
4 结论硒缺乏通过TLR4/MyD88/NF-κB信号通路介导鸡胸腺细胞凋亡,并进一步诱导鸡胸腺损伤。
| [1] |
ROMAN M, JITARU P, BARBANTE C. Selenium biochemistry and its role for human health[J]. Metallomics, 2014, 6(1): 25-54. DOI:10.1039/C3MT00185G |
| [2] |
HE S P, YU Q F, HE Y J, et al. Dietary resveratrol supplementation inhibits heat stress-induced high-activated innate immunity and inflammatory response in spleen of yellow-feather broilers[J]. Poult Sci, 2019, 98(12): 6378-6387. DOI:10.3382/ps/pez471 |
| [3] |
ZHANG R L, GUO R, LIU Q, et al. Selenium deficiency via the TLR4/TRIF/NF-κB signaling pathway leading to inflammatory injury in chicken spleen[J]. Biol Trace Elem Res, 2021, 199(2): 693-702. DOI:10.1007/s12011-020-02173-0 |
| [4] |
ZHANG R L, LIU Q, GUO R, et al. Selenium deficiency induces autophagy in chicken bursa of fabricius through ChTLR4/MyD88/NF-κB pathway[J]. Biol Trace Elem Res, 2021, 200(7): 3303-3314. |
| [5] |
ZHANG L W, GAO Y H, FENG H Q, et al. Effects of selenium deficiency and low protein intake on the apoptosis through a mitochondria-dependent pathway[J]. J Trace Elem Med Biol, 2019, 56: 21-30. DOI:10.1016/j.jtemb.2019.06.019 |
| [6] |
ZHENG Y Y, ZHANG B, GUAN H Y, et al. Selenium deficiency causes apoptosis through endoplasmic reticulum stress in swine small intestine[J]. Biofactors, 2021, 47(5): 788-800. DOI:10.1002/biof.1762 |
| [7] |
KATAOKA K, TOKUTOMI Y, YAMAMOTO E, et al. Apoptosis signal-regulating kinase 1 deficiency eliminates cardiovascular injuries induced by high-salt diet[J]. J Hypertens, 2011, 29(1): 76-84. DOI:10.1097/HJH.0b013e32833fc8b0 |
| [8] |
SAITOH M, NISHITOH H, FUJⅡ M, et al. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1[J]. EMBO J, 1998, 17(9): 2596-2606. DOI:10.1093/emboj/17.9.2596 |
| [9] |
KRISHNAN J, SELVARAJOO K, TSUCHIYA M, et al. Toll-like receptor signal transduction[J]. Exp Mol Med, 2007, 39(4): 421-438. DOI:10.1038/emm.2007.47 |
| [10] |
TAKEDA K, AKIRA S. Toll-like receptors[M]//Current Protocols in Immunology. New York: John Wiley & Sons, Inc., 2015.
|
| [11] |
刘晴. ChTLR4/MyD88/NF-κB信号通路介导硒缺乏雏鸡法氏囊自噬发生的研究[D]. 哈尔滨: 东北农业大学, 2021. LIU Q. ChTLR4/MyD88/NF-κB signaling pathway mediated autophagy in selenium-deficient chicken bursa of Fabricius[D]. Harbin: Northeast Agricultural University, 2021. (in Chinese) |
| [12] |
ZAREMBER K A, GODOWSKI P J. Tissue expression of human Toll-like receptors and differential regulation of Toll-like receptor mRNAs in leukocytes in response to microbes, their products, and cytokines[J]. J Immunol, 2002, 168(2): 554-561. DOI:10.4049/jimmunol.168.2.554 |
| [13] |
BERNASCONI P, BARBERIS M, BAGGI F, et al. Increased toll-like receptor 4 expression in thymus of myasthenic patients with thymitis and thymic involution[J]. Am J Pathol, 2005, 167(1): 129-139. DOI:10.1016/S0002-9440(10)62960-4 |
| [14] |
MIAO Z R, MIAO Z Y, WANG S C, et al. Exposure to imidacloprid induce oxidative stress, mitochondrial dysfunction, inflammation, apoptosis and mitophagy via NF-kappaB/JNK pathway in grass carp hepatocytes[J]. Fish Shellfish Immunol, 2022, 120: 674-685. DOI:10.1016/j.fsi.2021.12.017 |
| [15] |
BISWAS S K, LOPEZ-COLLAZO E. Endotoxin tolerance: new mechanisms, molecules and clinical significance[J]. Trends Immunol, 2009, 30(10): 475-487. DOI:10.1016/j.it.2009.07.009 |
| [16] |
任改艳, 孙阿宁, 张晶晶, 等. NF-κB在细胞凋亡中调节作用的研究进展[J]. 中国药理学与毒理学杂志, 2015, 29(2): 323-327. REN G Y, SUN A N, ZHANG J J, et al. Advances in roles of NF-κB in regulating pathways of apoptosis[J]. Chinese Journal of Pharmacology and Toxicology, 2015, 29(2): 323-327. DOI:10.3867/j.issn.1000-3002.2015.02.26 (in Chinese) |
| [17] |
GAO J, LIU Q H, LI J L, et al. Fibroblast growth factor 21 dependent TLR4/MYD88/NF-κB signaling activation is involved in lipopolysaccharide-induced acute lung injury[J]. Int Immunopharmacol, 2020, 80: 106219. DOI:10.1016/j.intimp.2020.106219 |
| [18] |
HUANG X Q, QIAO F, XUE P. The protective role of microRNA-140-5p in synovial injury of rats with knee osteoarthritis via inactivating the TLR4/Myd88/NF-κB signaling pathway[J]. Cell Cycle, 2019, 18(18): 2344-2358. DOI:10.1080/15384101.2019.1647025 |
| [19] |
ZHOU M Q, SHAO L, WU J, et al. Dihydromyricetin protects against lipopolysaccharide-induced cardiomyocyte injury through the toll-like receptor-4/nuclear factor-κB pathway[J]. Mol Med Rep, 2017, 16(6): 8983-8988. DOI:10.3892/mmr.2017.7742 |
| [20] |
杨子江. 低硒日粮对肉鸡免疫器官中硒蛋白与细胞因子表达的影响[D]. 哈尔滨: 东北农业大学, 2016. YANG Z J. Effects of diet with low selenium on the selenoproteins and cytokines expressions in broilers immune organs[D]. Harbin: Northeast Agricultural University, 2016. (in Chinese) |
| [21] |
梁娜, 彭西, 吴邦元, 等. 亚硒酸钠对黄曲霉毒素B1致雏鸡胸腺细胞死亡受体通路活化的缓解作用研究[J]. 四川农业大学学报, 2018, 36(4): 527-534. LIANG N, PENG X, WU B Y, et al. Protective effects of dietary sodium selenite on the activation of death receptor pathway in thymus of the chicks exposed to AFB1[J]. Journal of Sichuan Agricultural University, 2018, 36(4): 527-534. (in Chinese) |
| [22] |
郭凤英, 李培锋, 贾俊涛. 牛磺胆酸对小鼠胸腺细胞凋亡影响的研究[J]. 湖北农业科学, 2017, 56(19): 3706-3707, 3713. GUO F Y, LI P F, JIA J T. The studies on taurocholic acid influencing mice thymocyte apoptosis[J]. Hubei Agricultural Sciences, 2017, 56(19): 3706-3707, 3713. DOI:10.14088/j.cnki.issn0439-8114.2017.19.026 (in Chinese) |
| [23] |
XU S, XIAOJING L, XINYUE S, et al. Pig lung fibrosis is active in the subacute CdCl2 exposure model and exerts cumulative toxicity through the M1/M2 imbalance[J]. Ecotoxicol Environ Saf, 2021, 225: 112757. DOI:10.1016/j.ecoenv.2021.112757 |
| [24] |
王云. 鸡胸腺发育形态学研究[D]. 武汉: 华中农业大学, 2010. WANG Y. Morphological study on the development of chicken thymus[D]. Wuhan: Huazhong Agricultural University, 2010. (in Chinese) |
| [25] |
王巧红. 硒缺乏致鸡免疫抑制机制的研究[D]. 哈尔滨: 东北农业大学, 2009. WANG Q H. Research on the mechanism of selenium deficiency-induced immunosuppression in chickens[D]. Harbin: Northeast Agricultural University, 2009. (in Chinese) |
| [26] |
PAN T R, LIU T Q, TAN S R, et al. Lower selenoprotein T expression and immune response in the immune organs of broilers with exudative diathesis due to selenium deficiency[J]. Biol Trace Elem Res, 2018, 182(2): 364-372. DOI:10.1007/s12011-017-1110-3 |
| [27] |
彭西. 日粮硒对雏鸡免疫功能影响的机理研究[D]. 雅安: 四川农业大学, 2009. PENG X. Mechanisms of the immune functional impairment caused by dietary selenium in chicks[D]. Ya'an: Sichuan Agricultural University, 2009. (in Chinese) |
| [28] |
KHOSO P A, YANG Z J, LIU C P, et al. Selenium deficiency downregulates selenoproteins and suppresses immune function in chicken thymus[J]. Biol Trace Elem Res, 2015, 167(1): 48-55. DOI:10.1007/s12011-015-0282-y |
| [29] |
LIANG Y, LIN S L, WANG C W, et al. Effect of selenium on selenoprotein expression in the adipose tissue of chickens[J]. Biol Trace Elem Res, 2014, 160(1): 41-48. DOI:10.1007/s12011-014-0024-6 |
| [30] |
WANG Q Y, HUANG J Q, ZHANG H, et al. Selenium deficiency-induced apoptosis of chick embryonic vascular smooth muscle cells and correlations with 25 selenoproteins[J]. Biol Trace Elem Res, 2017, 176(2): 407-415. DOI:10.1007/s12011-016-0823-z |
| [31] |
YU D, ZHANG Z W, YAO H D, et al. The role of selenoprotein W in inflammatory injury in chicken immune tissues and cultured splenic lymphocyte[J]. Biometals, 2015, 28(1): 75-87. DOI:10.1007/s10534-014-9804-x |
| [32] |
WANG S C, ZHAO X, LIU Q Q, et al. Selenoprotein K protects skeletal muscle from damage and is required for satellite cells-mediated myogenic differentiation[J]. Redox Biol, 2022, 50: 102255. DOI:10.1016/j.redox.2022.102255 |
| [33] |
DIWAKAR B T, KORWAR A M, PAULSON R F, et al. The regulation of pathways of inflammation and resolution in immune cells and cancer stem cells by selenium[J]. Adv Cancer Res, 2017, 136: 153-172. |
| [34] |
ROYET J. Infectious non-self recognition in invertebrates: lessons from Drosophila and other insect models[J]. Mol Immunol, 2004, 41(11): 1063-1075. DOI:10.1016/j.molimm.2004.06.009 |
| [35] |
秦洪琼. 桂枝麻黄各半汤对流感病毒FM1株感染寒环境中小鼠肺部TLR7和RLH信号通路的影响[D]. 广州: 暨南大学, 2018. QIN H Q. Effects of Guizhimahuanggebantang on the TLR7 and RLH pathway in influenza virus infected mouse lungs in a cold environment[D]. Guangzhou: Ji'nan University, 2018. (in Chinese) |
| [36] |
SHOU Y, LI N Y, LI L, et al. NF-kappaB-mediated up-regulation of Bcl-X(S) and Bax contributes to cytochrome c release in cyanide-induced apoptosis[J]. J Neurochem, 2002, 81(4): 842-852. DOI:10.1046/j.1471-4159.2002.00880.x |
| [37] |
GILL J S, WINDEBANK A J. Ceramide initiates NF kappaB-mediated caspase activation in neuronal apoptosis[J]. Neurobiol Dis, 2000, 7(4): 448-461. DOI:10.1006/nbdi.2000.0312 |
| [38] |
FEI X W, HE Y T, CHEN J, et al. The role of Toll-like receptor 4 in apoptosis of brain tissue after induction of intracerebral hemorrhage[J]. J Neuroinflammation, 2019, 16(1): 234. DOI:10.1186/s12974-019-1634-x |
(编辑 白永平)



