2. 重庆三峡职业学院,重庆 404155
2. Chongqing Three Gorges Vocational College, Chongqing 404155, China
隐孢子虫(Cryptosporidium)是一种重要的专性细胞内寄生的顶复门原虫,主要寄生于宿主的胃肠道上皮细胞,引起腹泻为主的胃肠道疾病[1-2]。免疫功能正常的个体感染后通常表现为自限性腹泻,而免疫功能缺陷个体(如HIV患者和器官移植患者)感染后会出现长期慢性腹泻和严重的消化不良,甚至死亡[3]。隐孢子虫也是导致发展中国家儿童腹泻的第二大原因,据报道,在南亚和撒哈拉以南的非洲等欠发达地区,每年两岁以内儿童的隐孢子虫感染人数高达470万例[4-6]。但是,目前尚无有效防控隐孢子虫病的疫苗,美国食品药品监督管理局(Food and Drug Administration,FDA)批准的可用于治疗隐孢子虫病的唯一一种药物——硝唑尼特对于免疫功能缺陷个体治疗无效,且不能用于治疗最易感的婴幼儿群体(< 1岁)[7-8]。考虑到隐孢子虫病的严重程度与宿主状态密切相关,解析宿主和隐孢子虫的相互作用对于制定有效防控隐孢子病的策略至关重要。
环状RNA(circular RNA,circRNA)是一类无5′和3′自由末端的共价闭环非编码RNA,对RNase R耐受,比线性RNA更为稳定[9]。circRNA具有多种功能,包括充当miRNA海绵、作为蛋白质和mRNA的调节因子以及翻译成蛋白质等,其中,研究较多的是充当竞争性内源RNA(competitive endogenous RNA,ceRNA)发挥miRNA海绵作用[9-12]。据报道,细菌、病毒以及寄生虫等病原感染均可导致宿主circRNA的差异表达,且circRNA在宿主应对病原感染的反应中发挥着重要的调控作用[13-15]。作者前期利用微阵列芯片技术检测微小隐孢子虫(C. parvum)感染的HCT-8细胞circRNAs表达谱发现,显著上调的circRNA ciRS-7可通过海绵吸附miR-1270调节NF-κB信号通路促进C. parvum在HCT-8细胞中的增殖[16]。生物信息学分析发现,ciRS-7可能与22个miRNAs存在靶向互作关系[16]。研究表明,miR-219a-5p可通过调控炎症和多种细胞功能(如细胞活力、细胞增殖和细胞凋亡等)在肠易激综合征、肝病和多种癌症中发挥重要作用[17-20]。在非小细胞肺癌(NSCLC)中,ciRS-7可通过靶向miR-219a-5p调节细胞活力、迁移和侵袭以及细胞凋亡影响NSCLC的进展[20]。基于此,本研究探讨了ciRS-7与miR-219a-5p在HCT-8细胞中的靶向关系,以及ciRS-7/miR-219a-5p轴对C. parvum在HCT-8细胞中增殖的影响及其机制。
1 材料与方法 1.1 细胞和虫株人结肠癌HCT-8细胞由广东省农业科学院动物卫生研究所兽医寄生生物学研究室馈赠;C. parvum卵囊由河南农业大学动物医学院张龙现教授团队赠送,保存于西北农林科技大学寄生虫学实验室。
1.2 细胞培养HCT-8细胞在含10%胎牛血清的1640培养基中以37 ℃和5% CO2的培养条件进行培养。
1.3 C. parvum卵囊的传代与纯化为保证卵囊活力,卵囊需每3个月在新生犊牛体内进行扩繁传代,扩繁得到的卵囊经氯化铯密度梯度离心纯化处理后,重悬于添加100 U·mL-1青霉素、100 μg·mL-1链霉素和0.25 μg·mL-1两性霉素B的1×PBS中,置4 ℃冰箱保存备用。
1.4 体外感染模型将细胞均匀地接入细胞培养板中培养24 h。待细胞汇合度达到80%后,按照卵囊∶细胞=(2~10)∶1比例进行感染,感染时使用无血清RPMI 1640培养基,4 h后更换为含10%胎牛血清的1640培养基继续培养[21]。
1.5 总RNA提取和反转录使用Trizol试剂(艾科瑞,湖南)提取细胞总RNA,Nano-100微量分光光度计(奥盛,杭州)测定RNA的浓度。用于miRNA表达分析的模板按照Mir-X miRNA First-Strand Synthesis Kit说明书对总RNA进行反转录,反应体系为10 μL,包括0.25~0.80 μg cDNA、5 μL mRQ Buffer(2×)、1.25 μL mRQ Enzyme,剩余部分用DEPC水补齐;反应程序为37 ℃ 1 h,85 ℃ 5 min。
用于其它基因表达分析的模板按照Evo M-MLV反转录试剂盒(艾科瑞,湖南)说明书对总RNA进行反转录,先去除基因组DNA,反应体系为10 μL,包括1 μg cDNA、2 μL 5×gDNA Clean Buffer、1 μL gDNA Clean Reagent,剩余部分用DEPC水补齐;反应程序为42 ℃ 2 min。然后反转录成cDNA,向上一步反应物中加入1 μL RT Primer Mix、1 μL Evo M-MLV RTase Enzyme Mix、4 μL 5× RTase Reaction Buffer Mix I以及4 μL DEPC水;反应程序为37 ℃ 15 min,85 ℃ 5 s。
1.6 实时定量聚合酶链反应(qRT-PCR)miRNA的qRT-PCR反应体系包括2 μL cDNA、12.5 μL TB GreenⓇ Premix Ex TaqTM Ⅱ(Tli RNaseH Plus)、0.5 μL ROX Dye(50×)、0.5 μL miRNA-specific forward primer、0.5 μL mRQ3’primer、9 μL ddH2O;反应程序:95 ℃ 10 min;95 ℃ 15 s,退火30 s,40个循环。采用2-ΔΔCt方法进行计算miRNA的相对表达水平,以u6基因作为内参对照[22]。
其他基因的qRT-PCR反应体系包括5 μL cDNA、1 μL Primer mix、4 μL DEPC水和10 μL 2×SYBR Green Mix;反应程序:95 ℃ 10 min;95 ℃ 10 s,退火30 s,72 ℃ 32 s,40个循环。采用2-ΔΔCt方法进行计算基因的相对表达水平,以GAPDH基因作为内参对照[21]。引物信息见表 1。
|
|
表 1 qRT-PCR引物信息 Table 1 Information for primers used for qRT-PCR |
按照Lipofectamine 2000试剂说明书分别将pcDNA3.1(+)-ciRS7 Exon质粒(Addgene,美国)及其对照pcDNA3.1(+) 质粒(实验室保存)、ciRS-7干扰RNA(si-ciRS-7)及其对照si-control(锐博,广州)、miR-219a-5p mimics及其对照mimics control(吉玛,上海)、miR-219a-5p inhibitor及其对照inhibitor control(吉玛,上海)转染至HCT-8细胞中。
1.8 双荧光素酶报告载体的构建1.8.1 野生型载体的构建 通过在线工具starBase v3.0(http://starbase.sysu.edu.cn/index.php)预测到ciRS-7序列上存在的miR-219a-5p的结合位点,以此设计包含结合位点在内的ciRS-7野生型(ciRS-7-WT)片段的PCR扩增引物(表 2),于引物的上下游分别添加保护碱基和Sac I和Sal I限制性内切酶位点;经PCR扩增得到ciRS-7-WT的扩增产物,然后将扩增产物回收纯化;连接至pMDTM19-T(上海生工)载体上,16 ℃过夜连接;将pMDTM19-T-ciRS-7-WT连接产物转化至大肠杆菌JM-109感受态细胞中;于LB固体培养基(含0.1% Amp)中,37 ℃培养箱内过夜培养后挑取菌落;以菌液为模板进行PCR扩增;对阳性菌液进行扩大培养,提取pMDTM19-T-ciRS-7-WT重组质粒;将pMDTM19-T-ciRS-7-WT质粒和pmirGLO进行双酶切,得到线性化的pmirGLO质粒和ciRS-7-WT片段连接后按照上述操作步骤,最终得到mirGLO-ciRS-7-WT重组质粒[23];进行测序和双酶切鉴定;将构建成功的pmirGLO-ciRS-7-WT重组质粒置于-20 ℃保存备用。
|
|
表 2 包含miR-219a-5p结合位点的野生型ciRS-7的引物信息 Table 2 Primer information of wild-types for ciRS-7 containing the binding sites of miR-219a-5p |
1.8.2 突变型载体的构建 将ciRS-7和miR-219a-5p的结合位点突变后,包含ciRS-7和miR-219a-5p突变结合位点在内的ciRS-7突变序列(ciRS-7-MUT)由北京擎科生物科技有限公司合成,在突变序列的头尾分别添加保护碱基及SacⅠ和SalⅠ限制性内切酶位点;合成的突变序列直接连接到pmirGLO质粒上;扩大培养后提取pmirGLO-ciRS-7-MUT重组质粒;进行测序和双酶切鉴定;将构建成功的pmirGLO-ciRS-7-MUT重组质粒置于-20 ℃保存备用。
1.9 双荧光素酶报告基因试验使用96孔细胞培养板培养细胞;按照Lipofectamine 2000试剂说明书将pmirGLO-ciRS-7-WT重组质粒(或pmirGLO-ciRS-7-MUT重组质粒)或pmirGLO空质粒和miRNA mimics(或mimics control)转染至HCT-8细胞中,培养48 h[23]。培养结束后,按照高灵敏性双荧光素酶检测试剂盒说明书进行荧光素酶活性检测;首先利用1×Luc-Lysis Ⅱ Buffer裂解细胞;再利用Spark多功能酶标仪(Tecan,瑞士),以Fluc工作液检测萤火虫荧光素酶的发光值,Rluc工作液检测海肾荧光素酶发光值;最后计算出萤火虫荧光素酶和海肾荧光素酶的发光比值。
1.10 蛋白质免疫印迹(Western blot)RIPA裂解液提取细胞总蛋白样品;按照30 μg·孔-1样品上样量进行SDS-PAGE凝胶电泳;电泳结束后将胶上的蛋白转印到PVDF膜上;转膜结束后,利用5%的脱脂奶粉溶液封闭PVDF膜,室温1 h;4 ℃过夜孵育LC3B(1∶1 000,Abways,上海)、p62(1∶1 000,Abways,上海)和GAPDH(1∶500 000,ABclonal,武汉) 抗体;次日孵育HRP标记二抗(1∶5 000,生工,上海);孵育结束后进行ECL发光成像;Image-Pro Plus 6.0软件进行蛋白灰度分析。
1.11 统计分析利用Student’s t和IBM SPSS statistics 22.0检验方法进行统计学差异分析,采用Graphpad Prism V 6.07作图;*.P < 0.05表示差异显著,**.P < 0.01表示差异极显著,***.P < 0.001表示差异极极显著。
2 结果 2.1 C. parvum感染对HCT-8细胞自噬的影响利用Western blot检测C. parvum感染HCT-8细胞不同时间点的自噬相关蛋白LC3B-Ⅱ和p62的表达情况。结果表明,与未感染对照相比,LC3B-Ⅱ和p62蛋白质的表达量在C. parvum感染HCT-8细胞8、12、24、36和48 h均显著(P < 0.05)或极显著(P < 0.01)与极极显著(P < 0.001)升高(图 1)。

|
A. Western bolt分析LC3B-Ⅱ和p62蛋白质在C. parvum感染HCT-8细胞中的表达;B. 对A图中LC3B-Ⅱ的蛋白质相对表达水平的灰度分析结果;C. 对A图中p62的蛋白质相对表达水平的灰度分析结果。*.P < 0.05, **.P < 0.01, ***.P < 0.001,下同 A. Western bolt analysis of the protein levels of LC3B-Ⅱ and p62 in HCT-8 cells infected with C. parvum; B. Densitometry analysis of the relative protein levels of LC3B-Ⅱ in figure A; C. Densitometry analysis of the relative protein levels of p62 in figure A. *.P < 0.05, **.P < 0.01, ***.P < 0.001, the same as below 图 1 LC3B-Ⅱ和p62在C. parvum感染HCT-8细胞中的蛋白质相对表达水平 Fig. 1 Relative protein levels of LC3B-Ⅱ and p62 in HCT-8 cells infected with C. parvum |
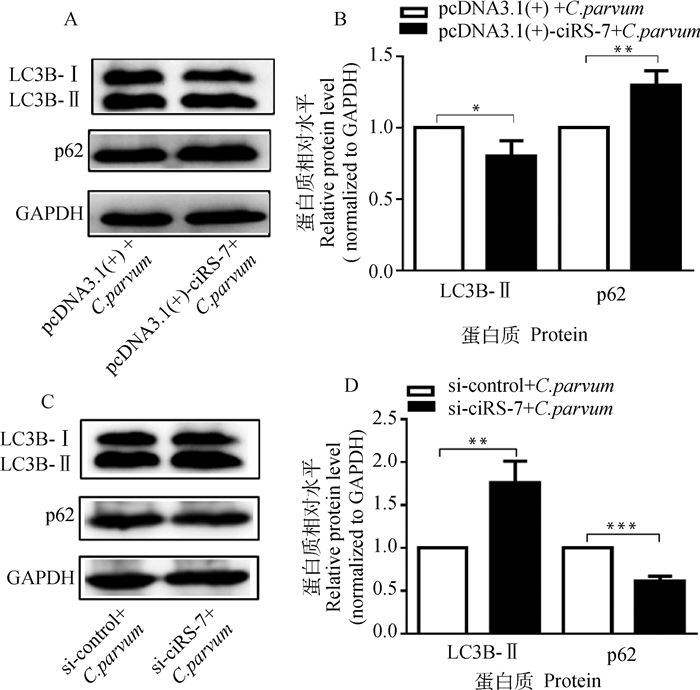
为分析ciRS-7对C. parvum感染诱发的HCT-8细胞自噬的影响,通过过表达或干扰ciRS-7的表达后,检测C. parvum感染HCT-8细胞中LC3B-Ⅱ和p62的蛋白质表达水平。结果显示,过表达ciRS-7显著降低了C. parvum感染HCT-8细胞中的LC3B-Ⅱ的蛋白质表达水平,但增加了p62的蛋白质表达水平(图 2A、2B);而干扰ciRS-7的表达显著促进了C. parvum感染HCT-8细胞中的LC3B-Ⅱ的蛋白质表达水平,但抑制了p62的蛋白质表达水平(图 2C、2D)。
|
A. Western bolt分析转染pcDNA3.1(+)-ciRS-7质粒对感染细胞中的LC3B-Ⅱ和p62蛋白质表达的影响;B. 对A图中LC3B-Ⅱ和p62的蛋白质相对表达水平的灰度分析;C. Western bolt分析转染si-ciRS-7对感染细胞中的LC3B-Ⅱ和p62蛋白质表达的影响;D. 对C图中LC3B-Ⅱ和p62的蛋白质相对表达水平的灰度分析 A. Western bolt analysis of the impact on the protein levels of LC3B-Ⅱ and p62 in infected cell transfected with pcDNA3.1(+)-ciRS-7 plasmid; B. Densitometry analysis of the relative protein levels of LC3B-Ⅱ and p62 in figure A; C. Western bolt analysis of the impact on the protein levels of LC3B-Ⅱ and p62 in infected cell transfected with si-ciRS-7; D. Densitometry analysis of the relative protein levels of LC3B-Ⅱ and p62 in figure C 图 2 ciRS-7对C. parvum感染HCT-8细胞中的LC3B-Ⅱ和p62蛋白质表达的影响 Fig. 2 The impact of ciRS-7 on the protein levels of LC3B-Ⅱ and p62 in HCT-8 cells infected with C. parvum |
利用qRT-PCR检测到ciRS-7的靶miRNA miR-219a-5p的表达在C. parvum感染HCT-8细胞24 h时显著下调(图 3A);过表达ciRS-7显著抑制了C. parvum感染HCT-8细胞中miR-219a-5p的表达,而干扰ciRS-7表达的作用相反(图 3A);双荧光素酶报告基因试验分析发现,在HCT-8细胞中,miR-219a-5p mimics显著降低了pmirGLO-ciRS-7-WT载体的荧光素酶活性,且不影响pmirGLO-ciRS-7-MUT载体和pmirGLO空载体的荧光素酶活性;上述结果表明,在C. parvum感染的HCT-8细胞中,ciRS-7对miR-219a-5p存在靶向调节作用(图 3B、3C)。

|
A. 转染pcDNA3.1(+)-ciRS-7质粒或si-ciRS-7对感染细胞中miR-219a-5p表达的影响;B. miR-219a-5p和ciRS-7的预测结合位点;C. 双荧光素酶报告基因试验验证miR-219a-5p和ciRS-7的结合作用 A. The impact on the miR-219a-5p levels in infected cells transfected with pcDNA3.1(+)-ciRS-7 plasmid or si-ciRS-7; B. The predicted binding sites of miR-219a-5p and ciRS-7; C. Validation of the binding capacity of miR-219a-5p and ciRS-7 via dual luciferase reporter assays 图 3 C. parvum感染HCT-8细胞中ciRS-7和miR-219a-5p的关系验证结果 Fig. 3 Validation of the relationship between ciRS-7 and miR-219a-5p in HCT-8 cells infected with C. parvum |
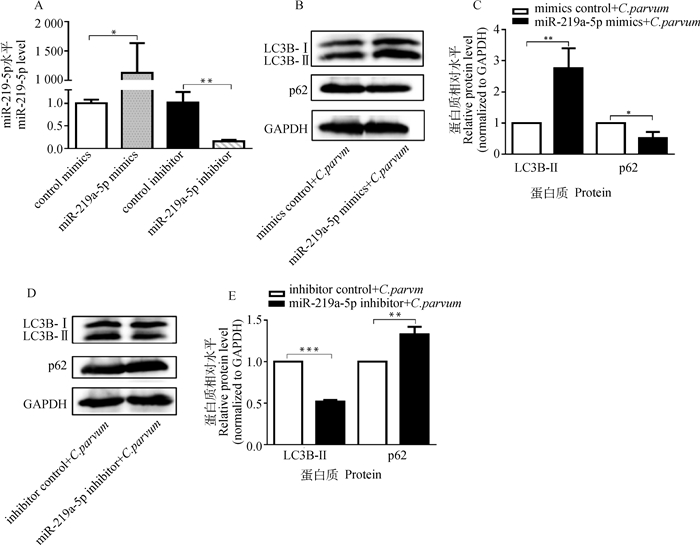
为了检测miR-219a-5p对C. parvum感染诱发HCT-8细胞自噬的影响,通过转染miR-219a-5p mimics或miR-219a-5p inhibitor后,检测C. parvum感染HCT-8细胞中LC3B-Ⅱ和p62的蛋白质表达水平。结果显示,转染miR-219a-5p mimics显著增加了HCT-8细胞中miR-219a-5p的表达量,而转染miR-219a-5p inhibitor显著降低其表达量(图 4A);miR-219a-5p mimics显著促进了C. parvum感染HCT-8细胞中的LC3B-Ⅱ的蛋白质表达水平,但抑制了p62的蛋白质表达水平(图 4B、4C);而miR-219a-5p inhibitor显著抑制了C. parvum感染HCT-8细胞中的LC3B-Ⅱ的蛋白质表达水平,但促进了p62的蛋白质表达水平(图 4D、4E)。
|
A. miR-219a-5p mimics和miR-219a-5p inhibitor的效果鉴定;B. Western bolt分析转染miR-219a-5p mimics对感染细胞中的LC3B-Ⅱ和p62蛋白质表达的影响;C. 对B图中LC3B-Ⅱ和p62的蛋白质相对表达水平的灰度分析;D. Western bolt分析转染miR-219a-5p inhibitor对感染细胞中的LC3B-Ⅱ和p62蛋白质表达的影响;E. 对D图中LC3B-Ⅱ和p62的蛋白质相对表达水平的灰度分析 A. Efficiency identification of miR-219a-5p mimics and miR-219a-5p inhibitor; B. Western bolt analysis of the impact on the protein levels of LC3B-Ⅱ and p62 in infected cells transfected with miR-219a-5p mimics; C. Densitometry analysis of the relative protein levels of LC3B-Ⅱ and p62 in figure B; D. Western bolt analysis of the impact on the protein levels of LC3B-Ⅱ and p62 in infected cells transfected with miR-219a-5p inhibitor; E. Densitometry analysis of the relative protein levels of LC3B-Ⅱ and p62 in figure D 图 4 miR-219a-5p对C. parvum感染HCT-8细胞中的LC3B-Ⅱ和p62蛋白质表达的影响 Fig. 4 The impact of miR-219a-5p on the protein levels of LC3B-Ⅱ and p62 in HCT-8 cells infected with C. parvum |
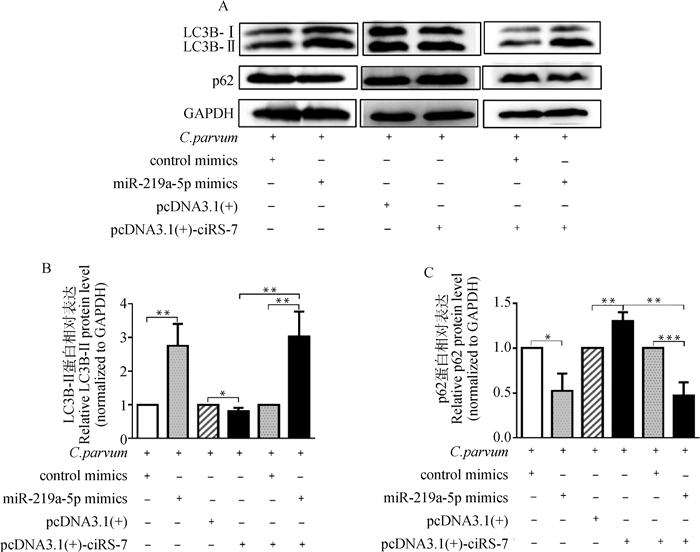
为确定ciRS-7是否通过靶向miR-219a-5p调节C. parvum感染诱发的HCT-8细胞自噬,通过共转染pcDNA3.1(+)-ciRS-7质粒和miR-219a-5p mimics后,检测C. parvum感染HCT-8细胞中LC3B-Ⅱ和p62的蛋白质表达水平。结果显示,在C. parvum感染的HCT-8细胞中,miR-219a-5p mimics逆转了过表达ciRS-7对LC3B-Ⅱ的蛋白质表达水平的抑制作用和p62的蛋白质表达水平的促进作用(图 5)。
|
A. Western bolt分析转染miR-219a-5p mimics或/和pcDNA3.1(+)-ciRS-7质粒对感染细胞中的LC3B-Ⅱ和p62蛋白质表达的影响;B. 对A图中LC3B-Ⅱ的蛋白质相对表达水平的灰度分析;C. 对A图中p62的蛋白质相对表达水平的灰度分析 A. Western bolt analysis of the impacts on the protein levels of LC3B-Ⅱ and p62 in infected cells transfected with miR-219a-5p mimics or/and pcDNA3.1(+)-ciRS-7 plasmid; B. Densitometry analysis of the relative protein levels of LC3B-Ⅱ in figure A; C. Densitometry analysis of the relative protein levels of p62 in figure A 图 5 转染miR-219a-5p mimics或/和pcDNA3.1(+)-ciRS-7质粒对C. parvum感染HCT-8细胞中的LC3B-Ⅱ和p62蛋白质表达的影响 Fig. 5 The impacts on the protein levels of LC3B-Ⅱ and p62 in C. parvum-infecting HCT-8 cells transfected with miR-219a-5p mimics or/and pcDNA3.1(+)-ciRS-7 plasmid |
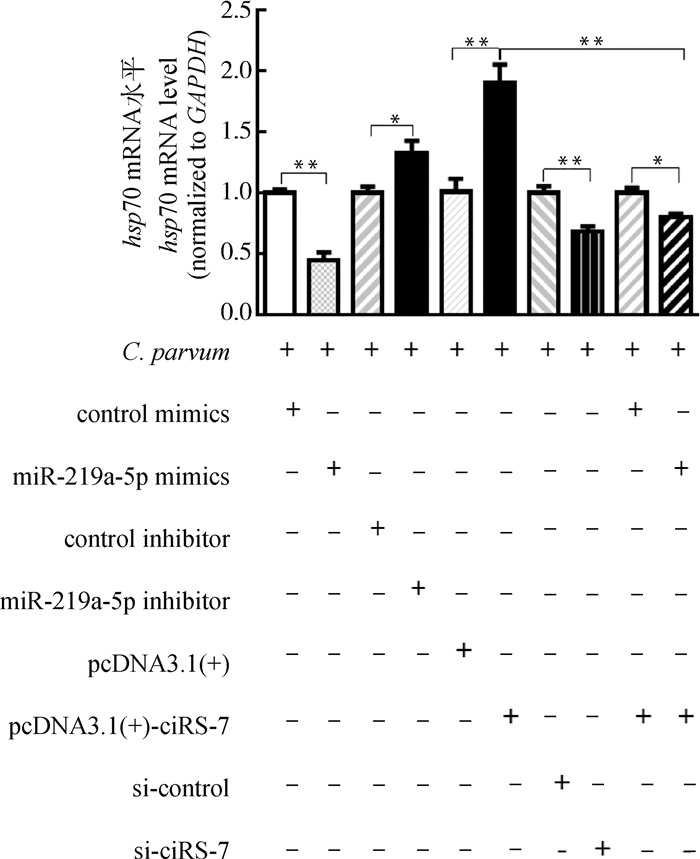
由于自噬在清除寄生虫感染的防御反应中发挥了重要作用,本研究利用qRT-PCR检测了ciRS-7/miR-219a-5p轴对C. parvum在HCT-8细胞中增殖的影响。结果表明,miR-219a-5p mimics显著降低了感染细胞中C. parvum hsp70基因的mRNA表达水平,而miR-219a-5p inhibitor显著升高了感染细胞中C. parvum hsp70基因的mRNA表达水平(图 6)。进一步研究发现,miR-219a-5p mimics逆转了过表达ciRS-7对感染细胞中C. parvum hsp70基因的mRNA表达水平的促进作用(图 6)。
|
图 6 ciRS-7/miR-219a-5p轴对HCT-8细胞中C. parvum hsp70基因的mRNA表达的影响 Fig. 6 The impacts of ciRS-7/miR-219a-5p axis on the miRNA levels of the C. parvum hsp70 gene in HCT-8 cells |
细胞自噬在宿主抗寄生虫感染的防御反应中起重要的作用。宿主通过自噬的降解作用可清除入侵细胞的寄生虫,如细胞受到弓形虫感染时通过启动自噬来破坏弓形虫的纳虫空泡膜(parasitophorous vacuole membrane,PVM),从而清除弓形虫的速殖子[24];伯氏疟原虫(Plasmodium berghei)早期感染细胞内脂化的LC3B-Ⅱ直接结合在PVM上,并通过募集自噬受体p62、NBR1和NDP52以及泛素等来清除疟原虫[25]。寄生虫也可通过抑制细胞自噬、诱导细胞的不完全自噬甚至利用宿主的自噬来促进自身的增殖,如弓形虫可以通过激活EGFR/Akt通路,抑制LC3在其周围的积累,从而逃避宿主自噬对其的清除[26];伯氏疟原虫的UIS3蛋白通过结合PVM上的LC3来抑制肝细胞的自噬,以促进自身的存活[27];克氏锥虫(Trypanoma cruzi)感染时可通过诱导细胞发生不完全自噬来逃避宿主的自噬清除作用[28];利什曼原虫(Leishmania spp.)感染可以双相和时间依赖性的方式抑制(感染早期)或诱导(感染后期)宿主自噬来促进自身的生长和发育[29]。蒋衡[30]研究发现C. parvum在感染12~24 h时诱导了HCT-8细胞的不完全自噬,与本研究的结果相似,作者发现C. parvum感染HCT-8细胞8~48 h均显著诱导了细胞的不完全自噬。但与Priyamvada等[31]的研究结果存在差异,他们发现C. parvum感染24 h时可显著诱导Caco-2细胞的完全自噬,这种差异可能是由于不同的C. parvum亚型、感染方法以及细胞模型所致。
前人研究证实,circRNA可通过调节自噬相关蛋白和自噬相关途径来直接激活或抑制自噬,同时circRNA可通过海绵吸附miRNAs来间接调控自噬[32]。食管鳞状细胞癌(esophageal cell squamous carcinoma,ESCC)细胞中显著上调的ciRS-7可通过靶向miR-1299上调EGFR的表达,诱导EGFR下游Akt-mTOR通路的激活,从而抑制饥饿或雷帕霉素诱导的ESCC细胞自噬[33]。在骨关节炎(osteoarthritis,OA)中,ciRS-7/miR-7轴可调节OA引起的软骨降解和自噬抑制过程,过表达ciRS-7或干扰miR-7的表达时可诱导OA软骨细胞的自噬并缓解软骨降解,反之,干扰ciRS-7或增加miR-7的表达时则会抑制OA软骨细胞的自噬并加重软骨降解[34]。在本研究中,过表达ciRS-7显著抑制了C. parvum感染诱发的HCT-8细胞自噬,而干扰ciRS-7的表达显著促进了C. parvum感染诱发的HCT-8细胞自噬,表明ciRS-7正调控C. parvum感染诱发的HCT-8细胞自噬。此外,ciRS-7的靶miRNA miR-219a-5p在C. parvum感染的HCT-8细胞中显著下调,过表达ciRS-7显著降低了C. parvum感染HCT-8细胞中的miR-219a-5p的表达,而干扰ciRS-7的表达显著增加了C. parvum感染HCT-8细胞中的miR-219a-5p的表达,并且通过双荧光素酶报告基因试验进一步证实了ciRS-7和miR-219a-5p在HCT-8细胞中存在结合活性,表明在C. parvum感染HCT-8细胞中,ciRS-7可靶向调节miR-219a-5p。进一步研究发现,miR-219a-5p mimics可显著促进C. parvum感染诱发的HCT-8细胞自噬,而miR-219a-5p inhibitor则显著抑制C. parvum感染诱发的HCT-8细胞自噬,且miR-219a-5p mimics可逆转过表达ciRS-7时对C. parvum感染诱发的细胞自噬的抑制作用。综上表明,在C. parvum感染期间,ciRS-7可通过靶向miR-219a-5p调控C. parvum感染诱发的HCT-8细胞自噬。
目前,基于基因水平检测C. parvum在体内和体外感染模型中荷虫量的靶标分子主要是18S rRNA基因和hsp70基因,其中,hsp70基因已用于检测C. parvum在肠上皮细胞中的荷虫量,且已证实了基于hsp70基因和18S rRNA基因的qRT-PCR方法检测C. parvum的肠上皮细胞中的荷虫量时的趋势一致[35-37]。蒋衡[30]利用C. parvum 18S rRNA基因的mRNA表达水平代表C. parvum在HCT-8细胞中的虫体数量,发现具有抑制自噬作用的miR-30a可显著增加C. parvum在HCT-8细胞中的虫体数量,而具有促自噬作用的miR-26a则显著降低C. parvum在HCT-8细胞中的虫体数量。在本研究中,基于C. parvum hsp70基因得到的结果与之相似,作者发现,ciRS-7通过抑制C. parvum感染诱发的细胞自噬来促进C. parvum在HCT-8细胞中的增殖,而miR-219a-5p通过促进C. parvum感染诱发的细胞自噬来抑制C. parvum在HCT-8细胞中的增殖,且ciRS-7可通过靶向miR-219a-5p抑制C. parvum感染诱发的HCT-8细胞自噬,从而促进C. parvum在HCT-8细胞中的增殖。
4 结论本研究揭示了C. parvum感染诱发HCT-8细胞的不完全自噬,以及宿主circRNA ciRS-7通过靶向抑制miR-219a-5p的表达来抑制C. parvum感染诱发的HCT-8细胞的自噬,以促进C. parvum在HCT-8细胞中的增殖。
| [1] |
CHEN X M, KEITHLY J S, PAYA C V, et al. Cryptosporidiosis[J]. N Engl J Med, 2002, 346(22): 1723-1731. DOI:10.1056/NEJMra013170 |
| [2] |
LIU A Q, GONG B Y, LIU X H, et al. A retrospective epidemiological analysis of human Cryptosporidium infection in China during the past three decades (1987-2018)[J]. PLoS Negl Trop Dis, 2020, 14(3): e0008146. DOI:10.1371/journal.pntd.0008146 |
| [3] |
KARABEY M, CAN H, ÖNER T Ö, et al. Cryptosporidium spp. during chemotherapy: a cross-sectional study of 94 patients with malignant solid tumor[J]. Ann Saudi Med, 2021, 41(5): 293-298. DOI:10.5144/0256-4947.2021.293 |
| [4] |
KOTLOFF K L, NATARO J P, BLACKWELDER W C, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study[J]. Lancet, 2013, 382(9888): 209-222. DOI:10.1016/S0140-6736(13)60844-2 |
| [5] |
CHECKLEY W, WHITE A C Jr, JAGANATH D, et al. A review of the global burden, novel diagnostics, therapeutics, and vaccine targets for cryptosporidium[J]. Lancet Infect Dis, 2015, 15(1): 85-94. DOI:10.1016/S1473-3099(14)70772-8 |
| [6] |
SOW S O, MUHSEN K, NASRIN D, et al. The burden of Cryptosporidium diarrheal disease among children < 24 months of age in moderate/high mortality regions of Sub-Saharan Africa and South Asia, utilizing data from the global enteric multicenter study (GEMS)[J]. PLoS Negl Trop Dis, 2016, 10(5): e0004729. DOI:10.1371/journal.pntd.0004729 |
| [7] |
ABUBAKAR I, ALIYU S H, ARUMUGAM C, et al. Prevention and treatment of cryptosporidiosis in immunocompromised patients[J]. Cochrane Database Syst Rev, 2007(1): CD004932. |
| [8] |
LOVE M S, CHOY R K M. Emerging treatment options for cryptosporidiosis[J]. Curr Opin Infect Dis, 2021, 34(5): 455-462. DOI:10.1097/QCO.0000000000000761 |
| [9] |
JECK W R, SHARPLESS N E. Detecting and characterizing circular RNAs[J]. Nat Biotechnol, 2014, 32(5): 453-461. DOI:10.1038/nbt.2890 |
| [10] |
ZHOU W Y, CAI Z R, LIU J, et al. Circular RNA: metabolism, functions and interactions with proteins[J]. Mol Cancer, 2020, 19(1): 172. DOI:10.1186/s12943-020-01286-3 |
| [11] |
YUE B L, WANG J, SONG C C, et al. Biogenesis and ceRNA role of circular RNAs in skeletal muscle myogenesis[J]. Int J Biochem Cell Biol, 2019, 117: 105621. DOI:10.1016/j.biocel.2019.105621 |
| [12] |
PATOP I L, WVST S, KADENER S. Past, present, and future of circRNAs[J]. EMBO J, 2019, 38(16): e100836. |
| [13] |
YANG R C, XU B J, YANG B, et al. Circular RNA transcriptomic analysis of primary human brain microvascular endothelial cells infected with meningitic Escherichia coli[J]. Mol Ther Nucleic Acids, 2018, 13: 651-664. DOI:10.1016/j.omtn.2018.10.013 |
| [14] |
ZHANG Y, ZHANG H, AN M H, et al. Crosstalk in competing endogenous RNA networks reveals new circular RNAs involved in the pathogenesis of early HIV infection[J]. J Transl Med, 2018, 16(1): 332. DOI:10.1186/s12967-018-1706-1 |
| [15] |
YU H L, MI C H, WANG Q, et al. Comprehensive analyses of circRNA expression profiles and function prediction in chicken cecums after Eimeria tenella infection[J]. Front Cell Infect Microbiol, 2021, 11: 628667. DOI:10.3389/fcimb.2021.628667 |
| [16] |
YIN Y L, LIU T L, YAO Q, et al. Circular RNA ciRS-7 affects the propagation of Cryptosporidium parvum in HCT-8 cells by sponging miR-1270 to activate the NF-κB signaling pathway[J]. Parasit Vectors, 2021, 14(1): 238. DOI:10.1186/s13071-021-04739-w |
| [17] |
MAHURKAR-JOSHI S, RANKIN C R, VIDRLOCK E J, et al. The colonic mucosal microRNAs, microRNA-219a-5p, and microRNA-338-3p are downregulated in irritable bowel syndrome and are associated with barrier function and MAPK signaling[J]. Gastroenterology, 2021, 160(7): 2409-2422.e19. DOI:10.1053/j.gastro.2021.02.040 |
| [18] |
XIAO Y, ZHANG S H, LI Q, et al. miR-219a-5p ameliorates hepatic ischemia/reperfusion injury via impairing TP53BP2[J]. Dig Dis Sci, 2019, 64(8): 2177-2186. DOI:10.1007/s10620-019-05535-4 |
| [19] |
ZHU X T, CHEN L, LIN J H. miR-219a-5p represses migration and invasion of osteosarcoma cells via targeting EYA2[J]. Artif Cells Nanomed Biotechnol, 2018, 46(S3): S1004-S1010. |
| [20] |
LI Y M, ZHANG J Z, PAN S, et al. CircRNA CDR1as knockdown inhibits progression of non-small-cell lung cancer by regulating miR-219a-5p/SOX5 axis[J]. Thorac Cancer, 2020, 11(3): 537-548. DOI:10.1111/1759-7714.13274 |
| [21] |
LIU T L, FAN X C, LI Y H, et al. Expression profiles of mRNA and lncRNA in HCT-8 cells infected with Cryptosporidium parvum Ⅱd subtype[J]. Front Microbiol, 2018, 9: 1409. DOI:10.3389/fmicb.2018.01409 |
| [22] |
LIU T L, FAN X C, WANG Y, et al. Micro-RNA expression profile of chicken small intestines during Eimeria necatrix infection[J]. Poult Sci, 2020, 99(5): 2444-2451. DOI:10.1016/j.psj.2019.12.065 |
| [23] |
曹大龙. 双荧光素酶报告基因检测miR-185与AKT1靶标关系[D]. 蚌埠: 蚌埠医学院, 2015. CAO D L. Research on target relationship between miR-185 and AKT1 by dual luciferase report gene assay[D]. Bengbu: Bengbu Medical College, 2015. (in Chinese) |
| [24] |
SUBAUSTE C S. Recent advances in the roles of autophagy and autophagy proteins in host cells during Toxoplasma gondii infection and potential therapeutic implications[J]. Front Cell Dev Biol, 2021, 9: 673813. DOI:10.3389/fcell.2021.673813 |
| [25] |
SCHMUCKLI-MAURER J, REBER V, WACKER R, et al. Inverted recruitment of autophagy proteins to the Plasmodium berghei parasitophorous vacuole membrane[J]. PLoS One, 2017, 12(8): e0183797. DOI:10.1371/journal.pone.0183797 |
| [26] |
MUNIZ-FELICIANO L, VAN GROL J, PORTILLO J A C, et al. Toxoplasma gondii-induced activation of EGFR prevents autophagy protein-mediated killing of the parasite[J]. PLoS Pathog, 2013, 9(12): e1003809. DOI:10.1371/journal.ppat.1003809 |
| [27] |
REAL E, RODRIGUES L, CABAL G G, et al. Plasmodium UIS3 sequesters host LC3 to avoid elimination by autophagy in hepatocytes[J]. Nat Microbiol, 2018, 3(1): 17-25. DOI:10.1038/s41564-017-0054-x |
| [28] |
ONIZUKA Y, TAKAHASHI C, UEMATSU A, et al. Inhibition of autolysosome formation in host autophagy by Trypanosoma cruzi infection[J]. Acta Trop, 2017, 170: 57-62. DOI:10.1016/j.actatropica.2017.02.021 |
| [29] |
THOMAS S A, NANDAN D, KASS J, et al. Countervailing, time-dependent effects on host autophagy promotes intracellular survival of Leishmania[J]. J Biol Chem, 2018, 293(7): 2617-2630. DOI:10.1074/jbc.M117.808675 |
| [30] |
蒋衡. 隐孢子虫通过microRNA-MAPK信号通路调控细胞自噬的机制[D]. 长春: 吉林大学, 2020. JIANG H. Mechanism of cell autophagy regulated by Cryptosporidium parvum via microRNA-MAPK signaling pathway[D]. Changchun: Jilin University, 2020. (in Chinese) |
| [31] |
PRIYAMVADA S, JAYAWARDENA D, BHALALA J, et al. Cryptosporidium parvum infection induces autophagy in intestinal epithelial cells[J]. Cell Microbiol, 2021, 23(4): e13298. |
| [32] |
ZHOU Z X, ZHANG Y F, GAO J N, et al. Circular RNAs act as regulators of autophagy in cancer[J]. Mol Ther Oncolytics, 2021, 21: 242-254. DOI:10.1016/j.omto.2021.04.007 |
| [33] |
MENG L J, LIU S H, DING P A, et al. Circular RNA ciRS-7 inhibits autophagy of ESCC cells by functioning as miR-1299 sponge to target EGFR signaling[J]. J Cell Biochem, 2020, 121(2): 1039-1049. DOI:10.1002/jcb.29339 |
| [34] |
ZHOU X D, LI J, ZHOU Y S, et al. Down-regulated ciRS-7/up-regulated miR-7 axis aggravated cartilage degradation and autophagy defection by PI3K/AKT/mTOR activation mediated by IL-17A in osteoarthritis[J]. Aging (Albany NY), 2020, 12(20): 20163-20183. |
| [35] |
CHEN X M, SPLINTER P L, O'HARA S P, et al. A cellular micro-RNA, let-7i, regulates Toll-like receptor 4 expression and contributes to cholangiocyte immune responses against Cryptosporidium parvum infection[J]. J Biol Chem, 2007, 282(39): 28929-28938. DOI:10.1074/jbc.M702633200 |
| [36] |
LI J, JIN K H, LI M, et al. A host cell long noncoding RNA NR_033736 regulates type Ⅰ interferon-mediated gene transcription and modulates intestinal epithelial anti-Cryptosporidium defense[J]. PLoS Pathog, 2021, 17(1): e1009241. DOI:10.1371/journal.ppat.1009241 |
| [37] |
HE W, LI J, GONG A Y, et al. Cryptosporidial infection suppresses intestinal epithelial cell MAPK signaling impairing host anti-parasitic defense[J]. Microorganisms, 2021, 9(1): 151. DOI:10.3390/microorganisms9010151 |
(编辑 白永平)



