2. 西南大学医学研究院免疫学研究中心,重庆 402460
2. Immunology Research Center, Medical Research Institute, Southwest University, Chongqing 402460, China
鸭疫里氏杆菌(Riemerella anatipestifer)是一种不运动、无芽胞、革兰阴性短小杆菌,是鸭传染性浆膜炎的主要病原,鸭疫里氏杆菌已成为严重危害养鸭业的主要病原之一[1-4]。该病表现为急性、败血性传染病,发病率和死亡率均很高,一旦鸭疫里氏杆菌感染鸭群,就会迅速在鸭群中传播,并有发展为地方流行性疾病的风险[5]。由于鸭疫里氏杆菌血清型众多,且各血清型菌株间缺少交叉免疫保护,耐药性严重[6-8],目前疫苗免疫及药物防治鸭传染性浆膜炎均遇到困难,鸭疫里氏杆菌的致病机制及新的防治措施是当前的研究热点[9]。鸭疫里氏杆菌感染可以引起雏鸭严重的组织损伤和炎症反应,但其机制尚不清楚。
补体系统是天然免疫系统的重要组成部分,在维护机体免疫平衡及抵抗病原微生物入侵等方面发挥了重要作用[10-13]。病原微生物进入机体后,补体系统可以通过3种途径激活:经典途径(classical pathway,CP)、凝集素途径(lectin pathway,LP)和旁路途径(alternate pathway,AP)。3种途径最终均会级联激活C5分子,C5分子进一步裂解为C5a和C5b,C5b通过级联反应与C6-C9形成攻膜复合物(membrane attack complex,MAC),MAC可通过裂解方式清除病原[14-16]。C5a可以诱导大量的生物学效应,包括增加血管通透性,细胞因子和趋化因子的释放,炎症细胞的趋化作用以及吞噬作用[17]。而C5a发挥这些生物学效应主要是通过其受体来介导和完成,它的受体有两种,一种是C5aR,另一种是C5L2。C5aR是目前研究得最为彻底的一种受体,C5aR在许多细胞类型中均有表达,尤其在嗜中性粒细胞、嗜酸性粒细胞、嗜碱性粒细胞、单核巨噬细胞、肥大细胞以及树突状细胞中表达更为丰富[18-20]。C5a与C5aR的N端结合后可诱导C5aR构象改变并产生一系列反应以激活丝裂原活化蛋白激酶(mitogen-activated protein kinase,MAPK)和cAMP信号通路[21-22]。C5a-C5aR轴与许多细菌性疾病的发生密切相关,C5a-C5aR轴在某些革兰阴性菌感染模型中可以过度激活,以诱发强烈的炎症反应。应用C5a-C5aR轴拮抗剂(C5a抗体或C5aR抗体)能够减缓疾病发展过程,减轻组织病变[23].
鸭疫里氏杆菌感染能够引起雏鸭严重的纤维素性渗出性炎症,推测在鸭疫里氏杆菌感染中C5a-C5aR轴发挥了重要作用。因此本研究通过全血试验和动物试验,从体外和体内水平上验证C5a-C5aR轴激活对鸭疫里氏杆菌感染雏鸭组织损伤及炎症反应的影响,阻断C5a-C5aR轴能显著降低雏鸭发病率和死亡率及炎性因子的表达水平,减轻组织损伤。研究结果对于进一步阐明鸭疫里氏杆菌致病机制具有重要意义,同时也将为后续开发针对鸭疫里氏杆菌新的疫苗靶点提供理论依据。
1 材料与方法 1.1 材料1.1.1 菌株和试验动物 鸭疫里氏杆菌AF株由西南大学动物医学院动物疫病防控与兽医公共卫生研究室分离并保存。10日龄健康雏鸭(樱桃谷鸭)购自重庆永健生物技术有限公司,所有试验用鸭经本实验室检测鸭疫里氏杆菌阴性。
1.1.2 主要试剂 鸭C5a、TNF-α、IL-6和IL-1β ELISA检测试剂盒购自上海泛柯实业有限公司。PrimeScriptTMRT reagent Kit with gDNA Eraser (Perfect Real Time)、DL2000 DNA Marker、DEPC、Premix TaqTM、TB GreenTM Premix Ex TaqTM Ⅱ购自宝生物工程(大连)有限公司;荧光定量PCR八联管购自生工生物工程(上海)股份有限公司。
1.2 方法1.2.1 兔抗C5a抗体和兔抗C5aR抗体制备 为制备兔抗C5a抗体和兔抗C5aR抗体,根据鸭C5(GenBank No. XP_027326464.1)和C5aR(GenBank No. XP_027303283.1)序列进行结构分析,选取C5a和C5aR功能区序列,依据大肠杆菌最适密码子进行合成,将合成的C5a片段及C5aR片段克隆至原核表达载体pET-32a(+),分别命名为pET-32a-C5a和pET-32a-C5aR。将pET-32a-C5a和pET-32a-C5aR转化至大肠杆菌BL21(DE3),用1.0 mmol·L-1IPTG诱导表达蛋白,重组蛋白经SDS-PAGE检测,镍柱亲和层析纯化的重组蛋白免疫新西兰大白兔,制备多克隆抗体,间接ELISA测定多抗效价,Western blot鉴定多抗特异性。
1.2.2 全血试验 新鲜鸭外周血采自10日龄雏鸭,以肝素钠为抗凝剂制备抗凝血。将-80 ℃保存的鸭疫里氏杆菌AF菌种划线接种于巧克力营养琼脂平板,37 ℃培养至单菌落形成;挑取单菌落接种于LB液体培养基中,37 ℃振荡培养至饱和;细菌悬液于4 ℃条件下5 000 r·min-1离心15 min,菌泥用0.01 mol·L-1 PBS(pH7.4) 重悬洗涤2次,制成终浓度为1×109 CFU·mL-1菌液。试验分为4组,第1~3组的5 mL抗凝血中分别加入15 μL鸭疫里氏杆菌悬液,第4组加入15 μL PBS,混匀;第1~2组分别加入15 μL的兔抗C5a抗体和兔抗C5aR抗体,第3~4组分别加入15 μL灭活兔健康血清,轻轻混匀;于37 ℃恒温摇床中孵育1、2和4 h后取样,加入20 mmol·L-1乙二胺四乙酸二钠,2 500 r·min-1离心10 min,分离血浆,于-80 ℃保存备用。
1.2.3 动物试验 依据“1.2.2”中的方法制备鸭疫里氏杆菌菌液,调整浓度为1×109CFU·mL-1。在进行动物试验之前筛选兔抗C5a抗体和兔抗C5aR抗体最佳注射剂量和注射时间,经测定,兔抗C5a抗体和兔抗C5aR抗体最佳注射剂量为0.5 mL·只-1,最佳注射时间为鸭疫里氏杆菌感染同时皮下注射抗体。
44只10日龄健康雏鸭随机分为4组,每组11只,隔离饲养。第1~3组动物颈部皮下注射5×108 CFU·mL-1鸭疫里氏杆菌后,分别腿部皮下注射0.5 mL兔抗C5a抗体、C5aR抗体和灭活健康兔血清;第4组为健康对照组,颈部皮下注射0.5 mL PBS和腿部皮下注射0.5 mL灭活健康兔血清。每组动物随机分成2个小组,第1小组5只,用于每日观察记录发病率、死亡率和临床症状;第2小组6只,于感染后12、24、48和72 h经颈静脉采血,制备抗凝血和分离血清。鸭疫里氏杆菌感染后72 h捕杀第1小组的全部雏鸭,采集心、肝和脾进行组织病理学检查,采集肝用于载菌量测定和基因表达检测。
1.2.4 组织病理学检查 鸭疫里氏杆菌感染72 h处死全部雏鸭,尸体剖检观察主要组织器官病理变化,并用10%福尔马林固定心、肝和脾制备病理组织切片,进行苏木精-伊红(hematoxylin-eosin,HE)染色,光学显微镜观察。
1.2.5 血清酶活性测定 鸭疫里氏杆菌感染后12、24、48和72 h,采集各组雏鸭颈静脉血,分离血清,利用迈瑞Mindray生化分析自动仪检测血清中谷丙转氨酶(alanine aminotransferase,ALT)和谷草转氨酶(aspartate aminotransferase,AST)的含量。
1.2.6 外周血及肝细菌数量测定 在无菌条件下采集捕杀鸭肝组织,称量后玻璃研磨器中研磨,加入灭菌生理盐水混匀,室温静置10 min,上层匀浆液用于细菌计数,计数方法参照文献[24],并进行适当改进。利用巧克力营养琼脂平板以涂布的方式进行抗凝血和肝组织匀浆液的细菌计数,样品10倍递进稀释,每个样品选择3个合适的稀释度,每个稀释度的样品涂布3个营养琼脂平板,0.1 mL·板-1。在37 ℃恒温培养箱培养18~24 h至菌落形成后计数。
1.2.7 C5a及炎性因子测定 利用商品化ELISA检测全血试验和动物试验中采集的血清样品C5a、TNF-α、IL-6和IL-1β水平。具体步骤:标准品孔各加不同浓度的标准品50 μL;加样孔分为空白孔和待测样品孔,待检样品孔中加样品稀释液40 μL和待测样品10 μL;加入酶标试剂100 μL·孔-1,空白孔除外;37 ℃恒温培养箱中孵育1 h;弃掉液体,加入洗涤液300 μL·孔-1,重复5次;每孔加入底物A、B各50 μL,37 ℃避光孵育15 min;加入终止液50 μL·孔-1,15 min内用酶标仪测定OD450 nm。
1.2.8 荧光定量PCR检测肝各基因的表达 利用Trizol抽提法提取肝总RNA,然后根据反转录试剂盒说明书进行反转录扩增cDNA,荧光定量PCR检测C5aR、鞘氨醇激酶-1(sphingosine kinase 1,SphK1)、纤维蛋白原样蛋白2(fibrinogen-like protein 2,FGL2)、TNF-α和IL-1β mRNA水平,β-actin为内参基因。各基因引物:C5aR-F:CAGCTCCTTGTTCAAGAGCG;C5aR-R:CATCACCACGTAGATGATGGG;SphK1-F:GAAGAAGCTGC-TGACGAACT;SphK1-R:GCCCAGGAAGGAGA-AGAAAC;FGL2-F:GTACCTGAAGTACCGTC-TAAGCG;FGL2-R:ATGCCCCACAGTTTCCTGAG;TNF-α-F:CAGATGGGAAGGGAATGAA-C;TNF-α-R:GAACTGGGCGGTCATAAAAT;IL-1β-F:GCTTCATCTTCTACCGCCTGGAC;IL-1β-R:TTAGCTTGTAGGTGGCGATGTTGAC;β-actin-F:ACCGCAAATGCTTCTAAACC:β-actin-R:ATCCTGAGTCAAGCGCCAAA。反应体系:SYBR Green Mix 12.5 μL,模板2 μL,上游引物1 μL,下游引物1 μL,DEPC水8.5 μL。反应条件:95 ℃ 3 min;95 ℃ 10 s,60 ℃ 1 min,40个循环。采用相对比较Ct值法对数据进行分析,先计算各组Ct差值(ΔCt=目的基因Ct-内参基因Ct);再计算ΔΔCt(即ΔCt试验组—ΔCt对照组),最后将结果转化相对差异倍数(QR=2-ΔΔCt)。
1.2.9 统计学分析 数据采集自3次独立重复试验,不同组别的数据统计为mean±S.E.M.,用单因素方差分析和Bonferroni检验分析各组之间的差异显著性,分析软件是SPSS 20.0(SPSS Inc., Chicago, IL, USA),P<0.05为差异显著。
2 结果 2.1 重组蛋白的表达、鉴定和纯化依据大肠杆菌最适密码子进行序列合成,将合成的C5a片段及C5aR片段克隆至原核表达载体pET-32a(+)。将重组质粒转化至大肠杆菌BL21(DE3),IPTG诱导表达,经SDS-PAGE检测,分别获得约为29和25 ku的重组目的蛋白,与预期条带大小一致,表明成功表达重组蛋白C5a和C5aR(图 1)。经镍柱亲和层析纯化和SDS-PAGE检测,在洗脱液中可见1条清晰的条带,杂蛋白较少(图 2)。将纯化的重组蛋白免疫新西兰大白兔制备多克隆抗体,经间接ELISA测定,兔抗C5a抗体和兔抗C5aR抗体效价均超过1∶12 800(表 1),且特异性良好。
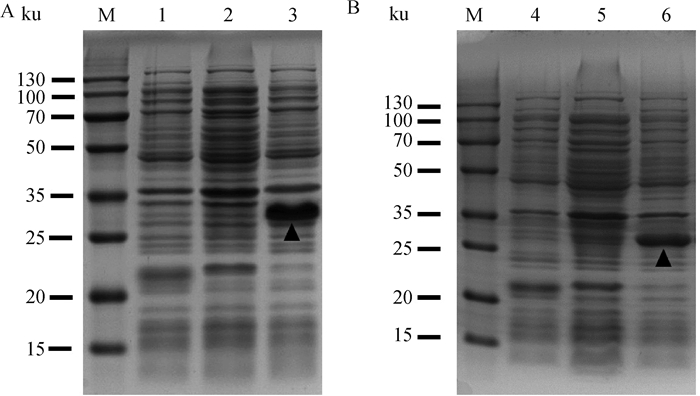
|
M. 双色预染蛋白相对分子质量标准;1. pET-32a/BL21诱导;2. pET-32a-C5a/BL21未诱导;3. pET-32a-C5a/BL21诱导;4. pET-32a/BL21诱导;5. pET-32a-C5aR/BL21未诱导;6. pET-32a-C5aR/BL21诱导;▲.代表各蛋白目的条带 M. Two-color pre-stained protein marker; 1. Induced pET-32a/BL21; 2. Non-induced pET-32a-C5a/BL21; 3. Induced pET-32a-C5a/BL21; 4. Induced pET-32a/BL21; 5. Non-induced pET-32a-C5aR/BL21; 6. Induced pET-32a-C5aR/BL21; ▲. The targeted bands of the proteins 图 1 pET-32a-C5a及pET-32a-C5aR重组表达SDS-PAGE分析 Fig. 1 Identification of pET-32a-C5a and pET-32a-C5aR by SDS-PAGE |
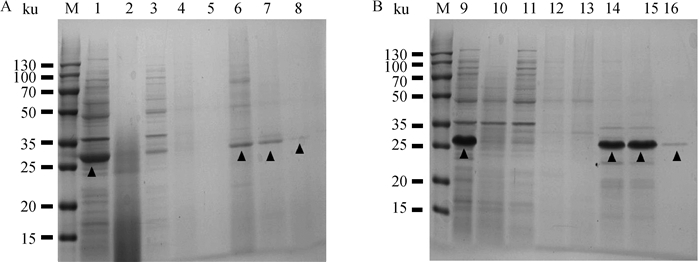
|
M. 双色预染蛋白相对分子质量标准;1. pET-32a-C5a;2. pET-32a-C5a过柱后;3. pET-32a-C5a第1次洗涤;4. pET-32a-C5a第2次洗涤;5. pET-32a-C5a第3次洗涤;6. pET-32a-C5a第1次洗脱;7. pET-32a-C5a第2次洗脱;8. pET-32a-C5a第3次洗脱;9. pET-32a-C5aR;10. pET-32a-C5aR过柱后;11. pET-32a-C5aR第1次洗涤;12. pET-32a-C5aR第2次洗涤;13. pET-32a-C5aR第3次洗涤;14. pET-32a-C5aR第1次洗脱;15. pET-32a-C5aR第2次洗脱;16. pET-32a-C5aR第3次洗脱;▲.代表各蛋白目的条带 M. Two-color pre-stained protein marker; 1. pET32a-C5a; 2. pET-32a-C5a after column; 3. pET-32a-C5a first washing; 4. pET-32a-C5a second washing; 5. pET-32a-C5a third washing; 6. pET-32a-C5a first elution; 7. pET-32a-C5a second elution; 8. pET-32a-C5a third elution; 9. pET32a-C5aR; 10. pET-32a-C5aR after column; 11. pET-32a-C5aR first washing; 12. pET-32a-C5aR second washing; 13. pET-32a-C5aR third washing; 14. pET-32a-C5aR first elution; 15. pET-32a-C5aR second elution; 16. pET-32a-C5aR third elution; ▲. The targeted bands of the proteins 图 2 pET-32a-C5a及pET-32a-C5aR的纯化SDS-PAGE分析 Fig. 2 Purification of pET-32a-C5a and pET-32a-C5aR by SDS-PAGE |
|
|
表 1 C5a及C5aR多克隆抗体ELISA结果(OD450 nm) Table 1 ELISA results of polyclonal antibodies of C5a and C5aR (OD450 nm) |
为检测C5a-C5aR轴在鸭疫里氏杆菌感染影响补体系统和炎症反应中的作用,ELISA检测全血和雏鸭外周血血清中C5a、TNF-α、IL-6和IL-1β水平。在全血试验中,鸭外周抗凝血受到鸭疫里氏杆菌刺激后,C5a、TNF-α和IL-6表达呈上调趋势,抗C5a抗体和抗C5aR抗体均能抑制C5a、TNF-α、IL-6和IL-1β的表达(图 3)。动物试验中,鸭疫里氏杆菌感染雏鸭后,各组雏鸭血清C5a、TNF-α、IL-6和IL-1β表达也呈上调趋势,抗C5a抗体和抗C5aR抗体均能抑制雏鸭血清C5a、TNF-α、IL-6和IL-1β的表达(图 4)。结果表明,阻断C5a-C5aR轴能够降低鸭疫里氏杆菌感染的补体反应和炎症反应。
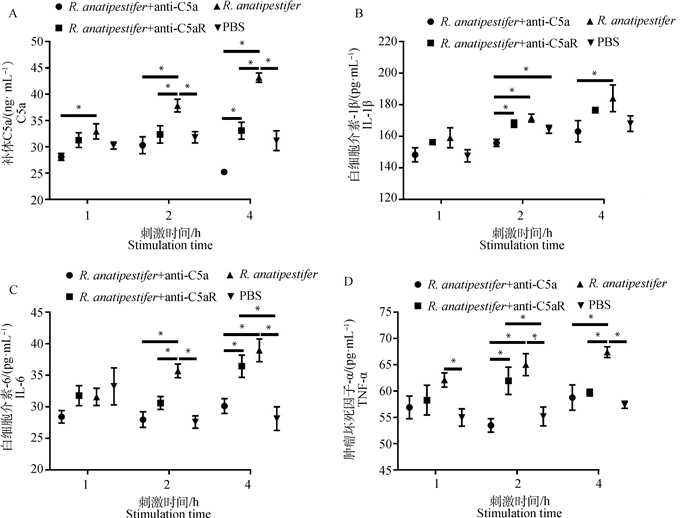
|
A. 全血中C5a表达量检测;B. 全血中IL-1β表达量检测;C. 全血中IL-6表达量检测;D. 全血中TNF-α表达量检测。ELISA检测全血中C5a、IL-1β、IL-6和TNF-α表达量。数据采集自3次重复试验,结果以平均值±标准误表示,*表示差异显著(P<0.05) A. Detection of C5a in the whole blood; B. Detection of IL-1β in the whole blood; C. Detection of IL-6 in the whole blood; D. Detection of TNF-α in the whole blood. ELISA was used to detect the expression of C5a, TNF-α, IL-6, IL-1β in the whole blood. Data were collected from three repeated experiments and the results were represented as mean ± SEM. An asterisk (*) shows a statistically significant difference (P < 0.05) 图 3 ELISA检测全血中C5a及炎性因子表达水平 Fig. 3 The expression of C5a and inflammatory factors in the whole blood detected by ELISA |
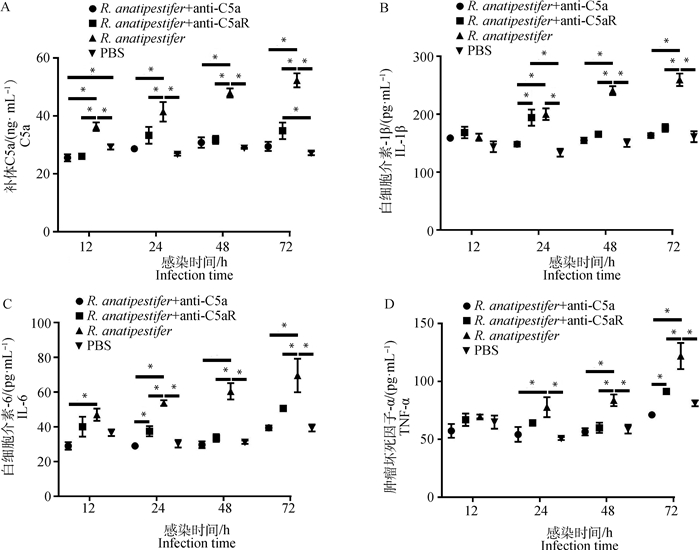
|
A. 雏鸭血清C5a表达量检测;B. 雏鸭血清IL-1β表达量检测;C. 雏鸭血清IL-6表达量检测;D. 雏鸭血清TNF-α表达量检测。ELISA检测雏鸭血清样品C5a、IL-1β、IL-6和TNF-α表达量,数据采集自3次重复试验,结果以“平均值±标准误”表示,*.P<0.05 A. Detection of C5a in the serum of ducklings; B. Detection of IL-1β in the serum of ducklings; C. Detection of IL-6 in the serum of ducklings; D. Detection of TNF-α in the serum of ducklings. ELISA was used to detect the expression of C5a, TNF-α, IL-6, IL-1β in the serum of ducklings. Data were collected from three repeated experiments and the results were represented as mean±SEM. *.P < 0.05 图 4 ELISA检测雏鸭血清样品C5a及炎性因子表达水平 Fig. 4 The expression of C5a and inflammatory factors in the serum of ducklings detected by ELISA |
鸭疫里氏杆菌感染雏鸭后,每日观察记录各组雏鸭临床症状,并且在感染后72 h处死雏鸭采集心、肝和脾进行病理组织学检查。结果显示,鸭疫里氏杆菌感染后,雏鸭出现神经症状,嗜睡,缩颈蹲伏,排黄绿色稀粪,气喘,呼吸困难等症状;而抗C5a抗体和抗C5aR抗体处理后雏鸭仅表现为蹲地不起,神经症状,临床症状明显减轻。病理组织学检查结果显示,R. anatipestifer组雏鸭可见明显的黄白色纤维素性渗出物,尤其是心、肝和气囊,主要表现为纤维素性心包炎、肝周炎和气囊炎。两个抗体处理组雏鸭仅在心和肝可见少量的纤维素性渗出物。R. anatipestifer组镜下病变表现为心肌纤维肿胀、颗粒变性,部分心肌纤维断裂;肝表现为中央静脉和肝窦充满红细胞,肝细胞发生水泡变性和/或脂肪变性;脾表现为脾窦高度扩张、淤血及出血,白髓淋巴滤泡数量减少,淋巴细胞稀疏分布,红髓和白髓的界限不清晰。两个抗体处理组镜下病变表现为心肌纤维轻度颗粒变性,肝细胞脂肪变性,脾结构完整,仅见少量淋巴细胞减少及炎性细胞浸润(图 5)。
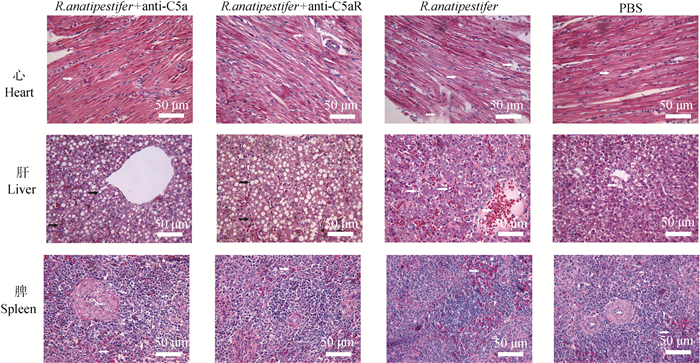
|
图 5 阻断C5a-C5aR轴对鸭疫里氏杆菌感染雏鸭主要组织病理变化的影响(标尺:50 μm,箭头所指为病变位置) Fig. 5 The influence of blocking C5a-C5aR axis on pathological changes of main tissues in R. anatipestifer infected ducklings (Bar: 50 μm, the arrow refers to the location of the lesions) |
鸭疫里氏杆菌感染雏鸭后,每日观察记录各组雏鸭发病率及死亡率。结果显示,R. anatipestifer组雏鸭在感染后48 h发病率平均值为53.3%,而两个抗体处理组雏鸭在感染后72 h内发病率均低于50%;R. anatipestifer组雏鸭在感染后72 h存活率平均值为33.3%,而两个抗体处理组雏鸭在感染后72 h存活率均超过70%(图 6)。
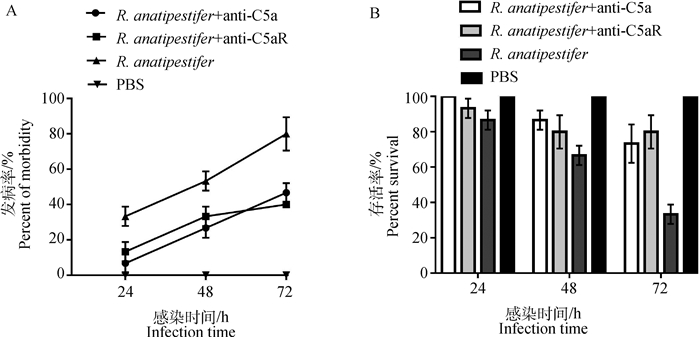
|
A. 鸭疫里氏杆菌感染72 h内各组雏鸭发病率检测;B. 鸭疫里氏杆菌感染72 h内各组雏鸭存活率检测 A. The morbidity of ducklings in different groups was detected within 72 h after R. anatipestifer infection; B. The survival rates of ducklings in different groups were detected within 72 h after R. anatipestifer infection 图 6 鸭疫里氏杆菌感染雏鸭发病率和存活率测定 Fig. 6 Detection of the morbidity and mortality of ducklings infected by R. anatipestifer |
各组雏鸭颈静脉无菌采血制备抗凝血进行细菌计数,鸭疫里氏杆菌感染雏鸭后,抗C5a抗体和抗C5aR抗体均能降低外周血细菌数量(图 7A)。并且在鸭疫里氏杆菌感染后72 h,各组无菌采集肝进行肝组织载菌量测定。结果显示,与R. anatipestifer组相比,两个抗体处理组肝组织细菌载量也显著降低(P<0.05)(图 7B)。
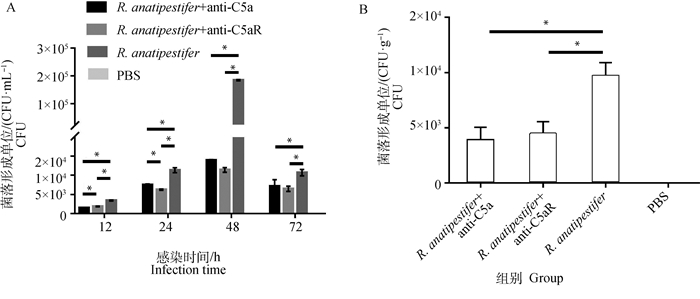
|
A. 12~72 h外周血细菌数量测定;B. 72 h肝载菌量测定。*.P<0.05 A. Detection of the number of bacteria in the peripheral blood on 12-72 h; B. Detection of the number of bacteria in the livers on 72 h. *.P < 0.05 图 7 阻断C5a-C5aR轴对鸭疫里氏杆菌感染鸭外周血和肝细菌数量的测定 Fig. 7 Detection of the number of bacteria in the peripheral blood and livers of R. anatipestifer infected ducklings after C5a-C5aR blockade |
外周血ALT和AST水平可以反映肝功能。结果显示,鸭疫里氏杆菌感染后,雏鸭外周血ALT和AST水平呈上调趋势,但是与R. anatipestifer组相比,R. anatipestifer + anti-C5a组和R. anatipestifer + anti-C5aR组ALT和AST水平显著降低(P<0.05)(图 8)。
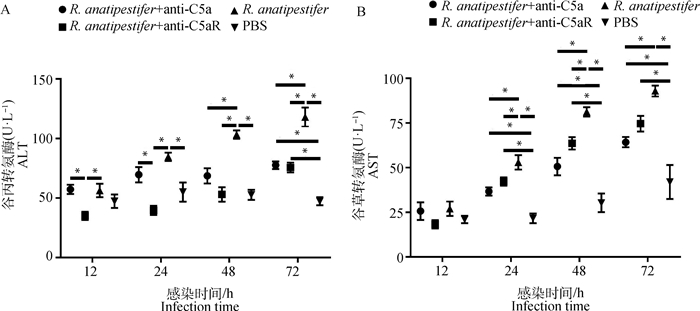
|
A. 血清ALT水平检测;B. 血清AST水平检测。数据采集自3次重复试验,结果以“平均值±标准误”表示,*.P<0.05 A. Detection of serum ALT levels; B. Serum AST levels were determined at the indicated times after different treatments. Data were collected from three repeated experiments and the results were represented as mean±SEM. *.P < 0.05 图 8 阻断C5a-C5aR轴对鸭疫里氏杆菌感染鸭肝生化指标的影响 Fig. 8 The influence of blocking C5a-C5aR axis on biochemical criterion of liver tissues in R. anatipestifer infected ducklings |
荧光定量PCR检测鸭疫里氏杆菌感染鸭肝主要基因C5aR、SphK1、FGL2、TNF-α和IL-1β mRNA转录水平。结果显示,R. anatipestifer组C5aR、SphK1、FGL2、TNF-α和IL-1β相对mRNA转录水平显著高于R. anatipestifer + anti-C5a组和R. anatipestifer+anti-C5aR组(P<0.05)(图 9)。

|
A. C5aR mRNA相对转录量;B. SphK1 mRNA相对转录量;C. FGL2 mRNA相对转录量;D. TNF-α mRNA相对转录量;E. IL-1β mRNA相对转录量。数据采集自3次重复试验,结果以“平均值±标准误”表示,*P<0.05 A. Relative C5aR mRNA level; B. Relative SphK1 mRNA level; C. Relative FGL2 mRNA level; D. Relative TNF-α mRNA level; E. Relative IL-1β mRNA level. Data were collected from three repeated experiments and the results were represented as mean±SEM. *. P < 0.05 图 9 荧光定量PCR检测鸭疫里氏杆菌感染鸭肝脏主要基因mRNA转录水平 Fig. 9 The fluorescence quantitative PCR detection of mRNA transcriptional levels of major genes in the livers of ducklings infected by R. anatipestifer |
补体系统作为天然免疫系统的重要组成部分,在很多疾病发生发展过程中发挥了重要作用,例如败血症、创伤、自身免疫性疾病和癌症等[25-29],但是补体系统在鸭疫里氏杆菌感染过程中的作用尚不清楚。一旦识别病原微生物,宿主补体系统可通过3种途径立即被激活:CP、LP和AP。3种途径最终均会级联激活C5分子,随后C5裂解为活性片段C5a和C5b以执行相关生物学功能[14]。C5a是否参与了鸭疫里氏杆菌感染过程以及C5a在鸭疫里氏杆菌感染过程中发挥的作用尚未见相关报道。本研究结果表明,鸭疫里氏杆菌感染能够激活宿主补体系统,强烈刺激C5a和炎性细胞因子(TNF-α、IL-6和IL-1β)表达量上调(图 1~2)。R. anatipestifer + anti-C5a组和R. anatipestifer + anti-C5aR组全血和动物试验证实阻断C5a-C5aR轴能够降低鸭疫里氏杆菌感染对雏鸭造成的组织损伤和炎症反应(图 3~6)。荧光定量PCR结果表明,C5a-C5aR轴在鸭疫里氏杆菌感染过程中对SphK1和FGL2激活也起到重要作用(图 7)。
补体激活产物C5a与其受体C5aR结合后能够介导促炎反应和趋化作用[30]。本研究采集雏鸭外周血制备抗凝血,血液与鸭疫里氏杆菌混匀后与分别兔抗C5a抗体和兔抗C5aR抗体进行孵育,结果显示,C5a抗体和C5aR抗体均能显著降低鸭疫里氏杆菌孵育后血液中C5a水平。动物试验结果也表明,R. anatipestifer + anti-C5a组和R. anatipestifer + anti-C5aR组雏鸭C5a水平也显著降低,阻断C5a-C5aR轴可有效降低鸭疫里氏杆菌感染雏鸭的发病率和死亡率;另外C5a或C5aR抗体可减轻心、肝和脾等组织的病理损伤,显著降低雏鸭外周血细菌数量。全血试验和动物试验结果表明,C5a-C5aR轴有助于鸭疫里氏杆菌感染雏鸭,并发挥了重要作用。
鸭疫里氏杆菌感染可诱发宿主强烈的炎症反应[31]。为验证C5a-C5aR轴是否参与调控鸭疫里氏杆菌感染的炎症反应过程,在体内水平和体外水平检测主要的炎性因子(IL-1β、IL-6和TNF-α)。在全血试验和动物试验中,C5a抗体或C5aR抗体处理组3种细胞因子表达水平均显著降低,表明在鸭疫里氏杆菌感染过程中C5a-C5aR轴可以调控这3种细胞因子的表达。以往研究显示,脂多糖(lipopolysaccharide,LPS)能够刺激枯否细胞表达释放IL-1β、TNF-α和其他炎症因子,进而诱导肝细胞焦亡。此外,随着肝星状细胞的激活,产生大量炎症介质,使胶原蛋白、蛋白多糖和黏连蛋白在肝中过度沉积和异常分布,造成严重的肝损伤[32]。LPS已被证实是鸭疫里氏杆菌最重要的毒力因子之一[33],同时鸭疫里氏杆菌也可引起严重的肝损伤[33-34]。因此,减轻炎症反应可以提高雏鸭存活率。在鸭疫里氏杆菌感染72 h,C5a抗体和C5aR抗体处理的雏鸭存活率均为100%,也证明了这一点。同时,随着IL-1β、IL-6和TNF-α的下降,雏鸭组织损伤和主要肝功能指标(ALT和AST)也降低,结果表明阻断C5a-C5aR轴能够减轻鸭疫里氏杆菌感染引起的炎症反应。
SphK1是一种细胞内信号酶,可产生脂质介质1-磷酸鞘氨醇(S1P)[35]。包括C5a在内的几种促炎刺激物均能激活巨噬细胞上的SphK1,并且阻断SphK1会减弱炎症反应[36]。有研究表明,SphK1在脓毒症诱导的炎症反应中起着关键作用[37],C5a-C5aR轴的激活也在急性肝衰竭中SphK1的活化起重要作用[38]。本研究应用荧光定量PCR检测肝中SphK1 mRNA水平,经C5a抗体或C5aR抗体处理后,鸭疫里氏杆菌感染的雏鸭SphK1的转录量显著降低,表明C5a-C5aR轴在鸭疫里氏杆菌感染中对SphK1激活中也起重要作用。已有研究表明,C5a能够激活包括巨噬细胞在内的大多数炎症细胞中的3条主要MAPK通路[39],C5a-C5aR轴对于巨噬细胞p38-MAPK磷酸化所必需的[40],MAPK激活在C5a诱导的炎性因子表达中起着重要作用[39]。因此,鸭疫里氏杆菌感染的机制可能是C5a抗体或C5aR抗体阻断C5a-C5aR轴,进而导致p38-MAPK未被激活,巨噬细胞中SphK1的表达水平下降。除了SphK1,作者还检测了FGL2在肝中的转录。FGL2是纤维蛋白沉积和微血管血栓形成的关键因子[41-42]。已有研究表明,在MHV-3诱导的暴发性肝炎模型的肝细胞坏死区域(特别是在肝窦内皮细胞中)检测到大量表达的FGL2[43]。研究表明,C5aR、TNF-α和FGL2可以形成一个整合网络,有助于病毒性肝炎过程中补体系统的激活[44]。荧光定量PCR结果显示,在鸭疫里氏杆菌感染后,各C5a-C5aR阻断组雏鸭肝FGL2 mRNA水平显著降低,表明C5a-C5aR轴的激活在鸭疫里氏杆菌感染过程中FGL2活化也发挥了重要作用。
4 结论本研究成功证实C5a-C5aR轴激活有助于鸭疫里氏杆菌感染雏鸭,阻断C5a-C5aR轴可显著减轻雏鸭组织损伤和炎症反应;SphK1和FGL2的表达和活化可能有助于C5a-C5aR轴参与鸭疫里氏杆菌感染引起的炎症反应过程。本研究为下一步开发抗鸭疫里氏杆菌感染药物提供了新思路。
| [1] |
覃宗华, 蔡建平, 吕敏娜, 等. 鸭疫里默氏菌病和大肠杆菌病鉴别诊断双重PCR方法的建立和应用[J]. 畜牧兽医学报, 2008, 39(4): 517-521. QIN Z H, CAI J P, LÜ M N, et al. Establishment and application of dulex PCR assay for differentiating Riemerella anatipestifer and Escherichia coli infection in ducks[J]. Acta Veterinaria et Zootechnica Sinica, 2008, 39(4): 517-521. DOI:10.3321/j.issn:0366-6964.2008.04.024 (in Chinese) |
| [2] |
FLORES R A, CAMMAYO P L T, NGUYEN B T, et al. Duck interleukin-22:identification and expression analysis in Riemerella anatipestifer infection[J]. J Immunol Res, 2021, 2021: 3862492. |
| [3] |
HUANG L, LIU M F, AMMANATH A V, et al. Identification of the natural transformation genes in Riemerella anatipestifer by random transposon mutagenesis[J]. Front Microbiol, 2021, 12: 712198. DOI:10.3389/fmicb.2021.712198 |
| [4] |
SHOUSHA A, AWAD A, YOUNIS G. Molecular characterization, virulence and antimicrobial susceptibility testing of Riemerella anatipestifer isolated from ducklings[J]. Biocontrol Sci, 2021, 26(3): 181-186. DOI:10.4265/bio.26.181 |
| [5] |
LI J X, TANG Y, GAO J Y, et al. Riemerella anatipestifer infection in chickens[J]. Pak Vet J, 2011, 31(1): 65-69. |
| [6] |
PATHANASOPHON P, PHUEKTES P, TANTICHAROENYOS T, et al. A potential new serotype of Riemerella anatipestifer isolated from ducks in Thailand[J]. Avian Pathol, 2002, 31(3): 267-270. DOI:10.1080/03079450220136576 |
| [7] |
张先福, 韦强, 鲍国连, 等. 黄连汤对鸭疫里默氏菌的抑菌作用及对菌体形态的影响[J]. 畜牧兽医学报, 2010, 41(4): 489-494. ZHANG X F, WEI Q, BAO G L, et al. Inhibition activity of Coptidis rhizoma dicoction against Riemerella anatipestifer and its effects on morphology of bacterial body[J]. Acta Veterinaria et Zootechnica Sinica, 2010, 41(4): 489-494. (in Chinese) |
| [8] |
TSAI H J, LIU Y T, TSENG C S, et al. Genetic variation of the ompA and 16S rRNA genes of Riemerella anatipestifer[J]. Avian Pathol, 2005, 34(1): 55-64. DOI:10.1080/03079450400025471 |
| [9] |
李德龙, 谷九龙, 徐兴胜, 等. 与鸭C4结合蛋白互作的鸭疫里默菌外膜蛋白的筛选及鉴定[J]. 畜牧兽医学报, 2020, 51(11): 2825-2835. LI D L, GU J L, XU X S, et al. Screening and identification of outer membrane proteins of Riemerella anatipestifer recruited duck C4b-binding protein[J]. Acta Veterinaria et Zootechnica Sinica, 2020, 51(11): 2825-2835. DOI:10.11843/j.issn.0366-6964.2020.11.021 (in Chinese) |
| [10] |
WALPORT M J. Complement. First of two parts[J]. N Engl J Med, 2001, 344(14): 1058-1066. DOI:10.1056/NEJM200104053441406 |
| [11] |
KING B C, BLOM A M. Complement in metabolic disease: metaflammation and a two-edged sword[J]. Semin Immunopathol, 2021, 43(6): 829-841. DOI:10.1007/s00281-021-00873-w |
| [12] |
TILLE A, LEHNERT T, ZIPFEL P F, et al. Quantification of factor H mediated self vs. Non-self discrimination by mathematical modeling[J]. Front Immunol, 2020, 11: 1911. DOI:10.3389/fimmu.2020.01911 |
| [13] |
TOP O, PARSONS J, BOHLENDER L L, et al. Recombinant production of MFHR1, a novel synthetic multitarget complement inhibitor, in moss bioreactors[J]. Front Plant Sci, 2019, 10: 260. DOI:10.3389/fpls.2019.00260 |
| [14] |
MERLE N S, CHURCH S E, FREMEAUX-BACCHI V, et al. Complement system part Ⅰ-molecular mechanisms of activation and regulation[J]. Front Immunol, 2015, 6: 262. |
| [15] |
DOORDUIJN D J, HEESTERBEEK D A C, RUYKEN M, et al. Polymerization of C9 enhances bacterial cell envelope damage and killing by membrane attack complex pores[J]. PLoS Pathog, 2021, 17(11): e1010051. DOI:10.1371/journal.ppat.1010051 |
| [16] |
CUGNO M, MACOR P, GIORDANO M, et al. Consumption of complement in a 26-year-old woman with severe thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination[J]. J Autoimmun, 2021, 124: 102728. DOI:10.1016/j.jaut.2021.102728 |
| [17] |
LI L S, YANG W N, SHEN Y B, et al. The evolutionary analysis of complement component C5 and the gene co-expression network and putative interaction between C5a and C5a anaphylatoxin receptor (C5AR/CD88) in human and two Cyprinid fish[J]. Dev Comp Immunol, 2021, 116: 103958. DOI:10.1016/j.dci.2020.103958 |
| [18] |
DUMITRU A C, DEEPAK R N V K, LIU H, et al. Submolecular probing of the complement C5a receptor-ligand binding reveals a cooperative two-site binding mechanism[J]. Commun Biol, 2020, 3(1): 786. DOI:10.1038/s42003-020-01518-8 |
| [19] |
PERONATO A, FRANCHI N, BALLARIN L. Insights into the complement system of tunicates: C3a/C5aR of the colonial ascidian Botryllus schlosseri[J]. Biology (Basel), 2020, 9(9): 263. |
| [20] |
GORMAN D M, LI X X, LEE J D, et al. Development of potent and selective agonists for complement C5a receptor 1 with in vivo activity[J]. J Med Chem, 2021, 64(22): 16598-16608. DOI:10.1021/acs.jmedchem.1c01174 |
| [21] |
YANG X S, LIU M Y, ZHANG H M, et al. Protein kinase C-δ mediates sepsis-induced activation of complement 5a and urokinase-type plasminogen activator signaling in macrophages[J]. Inflamm Res, 2014, 63(7): 581-589. DOI:10.1007/s00011-014-0729-1 |
| [22] |
MA H J, LIU C, SHI B Y, et al. Mesenchymal stem cells control complement C5 activation by factor H in lupus nephritis[J]. eBioMedicine, 2018, 32: 21-30. DOI:10.1016/j.ebiom.2018.05.034 |
| [23] |
JIANG Y T, ZHAO G Y, SONG N P, et al. Blockade of the C5a-C5aR axis alleviates lung damage in hDPP4-transgenic mice infected with MERS-CoV[J]. Emerg Microbes Infect, 2018, 7: 77. |
| [24] |
谢小伟, 孙志良, 李元义, 等. 猪链球菌2型生长曲线与半数致死量的测定[J]. 中兽医医药杂志, 2016, 35(4): 8-11. XIE X W, SUN Z L, LI Y Y, et al. Determination of growth curve and median lethal dose of Streptococcus suis type 2[J]. Journal of Traditional Chinese Veterinary Medicine, 2016, 35(4): 8-11. (in Chinese) |
| [25] |
RICKLIN D, HAJISHENGALLIS G, YANG K, et al. Complement: a key system for immune surveillance and homeostasis[J]. Nat Immunol, 2010, 11(9): 785-797. |
| [26] |
RITTIRSCH D, FLIERL M A, WARD P A. Harmful molecular mechanisms in sepsis[J]. Nat Rev Immunol, 2008, 8(10): 776-787. |
| [27] |
HUBER-LANG M, KOVTUN A, IGNATIUS A. The role of complement in trauma and fracture healing[J]. Semin Immunol, 2013, 25(1): 73-78. DOI:10.1016/j.smim.2013.05.006 |
| [28] |
OKROJ M, HEINEGÅRD D, HOLMDAHL R, et al. Rheumatoid arthritis and the complement system[J]. Ann Med, 2007, 39(7): 517-530. DOI:10.1080/07853890701477546 |
| [29] |
MARKIEWSKI M M, DEANGELIS R A, BENENCIA F, et al. Modulation of the antitumor immune response by complement[J]. Nat Immunol, 2008, 9(11): 1225-1235. DOI:10.1038/ni.1655 |
| [30] |
GERARD N P, GERARD C. The chemotactic receptor for human C5a anaphylatoxin[J]. Nature, 1991, 349(6310): 614-617. DOI:10.1038/349614a0 |
| [31] |
FERNANDEZ C P, AFRIN F, FLORES R A, et al. Downregulation of inflammatory cytokines by berberine attenuates Riemerella anatipestifer infection in ducks[J]. Dev Comp Immunol, 2017, 77: 121-127. |
| [32] |
PALACIOS-MACAPAGAL D, CONNOR J, MUSTELIN T, et al. Cutting edge: eosinophils undergo caspase-1-mediated pyroptosis in response to necrotic liver cells[J]. J Immunol, 2017, 199(3): 847-853. |
| [33] |
WANG X L, DING C, WANG S H, et al. The AS87_04050 gene is involved in bacterial lipopolysaccharide biosynthesis and pathogenicity of Riemerella anatipestifer[J]. PLoS One, 2014, 9(10): e109962. |
| [34] |
FERNANDEZ-COLORADO C P, CAMMAYO P L T, FLORES R A, et al. Anti-inflammatory activity of diindolylmethane alleviates Riemerella anatipestifer infection in ducks[J]. PLoS One, 2020, 15(11): e0242198. |
| [35] |
MELENDEZ A J. Sphingosine kinase signalling in immune cells: potential as novel therapeutic targets[J]. Biochim Biophys Acta, 2008, 1784(1): 66-75. |
| [36] |
ZHANG W L, MOTTILLO E P, ZHAO J W, et al. Adipocyte lipolysis-stimulated interleukin-6 production requires sphingosine kinase 1 activity[J]. J Biol Chem, 2014, 289(46): 32178-32185. |
| [37] |
LUFRANO M, JACOB A, ZHOU M, et al. Sphingosine kinase-1 mediates endotoxemia-induced hyperinflammation in aged animals[J]. Mol Med Rep, 2013, 8(2): 645-649. |
| [38] |
LEI Y C, LU C L, CHEN L, et al. C5a/C5aR pathway is essential for up-regulating SphK1 expression through p38-MAPK activation in acute liver failure[J]. World J Gastroenterol, 2016, 22(46): 10148-10157. |
| [39] |
SCHAEFFER V, CUSCHIERI J, GARCIA I, et al. The priming effect of C5a on monocytes is predominantly mediated by the p38 MAPK pathway[J]. Shock, 2007, 27(6): 623-630. |
| [40] |
ISSUREE P D A, MARETZKY T, MCILWAIN D R, et al. iRHOM2 is a critical pathogenic mediator of inflammatory arthritis[J]. J Clin Invest, 2013, 123(2): 928-932. |
| [41] |
GHANEKAR A, MENDICINO M, LIU H, et al. Endothelial induction of fgl2 contributes to thrombosis during acute vascular xenograft rejection[J]. J Immunol, 2004, 172(9): 5693-5701. |
| [42] |
LIU M F, MENDICINO M, NING Q, et al. Cytokine-induced hepatic apoptosis is dependent on FGL2/fibroleukin: the role of Sp1/Sp3 and STAT1/PU. 1 composite cis elements[J]. J Immunol, 2006, 176(11): 7028-7038. |
| [43] |
DING J W, NING Q, LIU M F, et al. Fulminant hepatic failure in murine hepatitis virus strain 3 infection: tissue-specific expression of a novel fgl2 prothrombinase[J]. J Virol, 1997, 71(12): 9223-9230. |
| [44] |
LIU J J, TAN Y L, ZHANG J Y, et al. C5aR, TNF-α, and FGL2 contribute to coagulation and complement activation in virus-induced fulminant hepatitis[J]. J Hepatol, 2015, 62(2): 354-362. |
(编辑 白永平)



