2. 宁夏大学 西部特色生物资源保护与利用教育部重点实验室, 银川 750021
2. Key Laboratory of the Protection and Utilization of Biological Resources with Western Characteristics of Ministry of Education, Ningxia University, Yinchuan 750021, China
动物的肌肉发育包括胚胎期和出生后肌纤维的增殖和肥大两个方面,是一个长期发展的生物过程并由众多基因协作调控。部分品种的牛(如比利时蓝牛),其肌肉增大是由于肌肉纤维显著增多,而胰岛素样生长因子(IGFs)则能够促进成肌细胞的增殖;小鼠在缺失肌生成抑制素基因(GDF-8)后,肌肉纤维数量增多,进而增强其骨骼肌质量[1]。骨骼肌是肌肉的重要组成部分,存在于生物体的各种组织中,也是呼吸、代谢等生理过程中不可或缺的组成要素[2]。骨骼肌的生长发育伴随着众多基因的共同调控,骨骼肌卫星细胞是肌源性干细胞,在动物机体受到损伤时,可以增殖、分化为新的肌纤维、形成新的肌肉组织[3-5]。在畜牧生产过程中,动物的产肉性能是决定经济指标的重要因素之一。从不同层面研究肌肉的生长发育调控网络有助于了解和提升动物的产肉性能。
核不均一核糖核蛋白(heterogeneous nuclear ribonucleoproteins, hnRNPs)是一类由RNA聚合酶Ⅱ产生并且与转录物结合的核蛋白[6],是一类参与了多种重要生命活动的RNA结合蛋白。HNRNPs家族成员约有20多个,按分子量大小命名从A到U,主要成员包括hnRNPA、hnRNPA/B、hnRNPC等,hnRNPs的生物学功能较为广泛,它能够利用其自身特有的结构同前体mRNA相结合,以两者复合体的形式参与mRNA的剪接、翻译、mRNA的稳定以及转录等调控过程,还有少部分未命名的成员由于表达较少并且不具备与前体mRNA结合的能力从而负责协助其他hnRNPs完成部分生物学功能[7-8]。hnRNP蛋白能够进行翻译后修饰,从而使生物活性及亚细胞定位发生改变。其在基因表达调控中同样发挥重要作用,研究表明许多hnRNPs参与癌症的发生和转移,例如hnRNP A1在肺癌样本中的表达显著增加,并与肿瘤的增殖有关;hnRNP A2/B1可以显著提升癌细胞的增殖从而促进人乳腺癌细胞的侵袭;hnRNP C能够调节乳腺癌的致癌基因等[9-11]。在肌肉发育方面,hnRNP A1能够调控小鼠肌肉相关基因的表达和选择性剪接,从而影响肌肉收缩[12];降低小鼠骨骼肌中hnRNP A1的表达能够通过抑制糖原合成影响该组织的代谢特性和胰岛素敏感性[13];hnRNP H在胚胎间充质细胞中高表达,并且在胚胎间充质细胞向平滑肌细胞分化时,hnRNP H的表达显著降低,表明hnRNP H在平滑肌的生成调节中具有重要作用[14];hnRNP L对肌源性分化具有重要作用,并能够作为潜在的治疗靶点调节肌强直性营养不良病[15]等;HNRNPAB作为hnRNPs家族的核心蛋白,由两段RNA识别基序、一段RGG盒以及两段富含甘氨酸结构域的辅助区域构成,其中的RNA识别基序约由90~100个氨基酸组成,能够与Pre-mRNA的3′非翻译(3′UTR)区域结合,而富含甘氨酸的区域在核定位功能上具有重要作用。HNRNPAB在生物学功能上主要参与调控mRNA转运、代谢及剪切等过程[16-17],并且与诸多恶性肿瘤的发生发展密切相关[18]。但HNRNPAB参与动物骨骼肌发育的研究鲜有报道。基于此,本研究利用牛骨骼肌卫星细胞体外诱导成肌分化模型,通过干扰牛骨骼肌卫星细胞中HNRNPAB的表达,研究HNRNPAB对牛骨骼肌卫星细胞体外成肌分化的作用,为进一步挖掘影响牛肌肉发育的基因奠定一定基础。
1 材料与方法 1.1 主要材料牛骨骼肌卫星细胞由天津市农业动物繁育与健康养殖重点实验室分离冻存。
1.2 主要仪器荧光显微镜(Leica);CO2培养箱(SANYO);脱色摇床(IKA);酶标仪(Thermo-scientific);垂直电泳槽(Bio-Rad);LightCycle 96实时荧光定量PCR仪(Roche);PowerPac Basic电泳仪;ChemiDocTM Imaging System(Bio-Rad)等。
1.3 主要试剂胎牛血清(FBS)和马血清(HS)(Gibco, USA);PBS缓冲液、0.25%胰蛋白酶(Solarbio, 北京);DMEM高糖培养基(Hyclone, USA);EDU细胞增殖检测试剂盒(锐博,广州);RNA快速提取试剂盒(艾德莱,北京);BCA试剂盒(康为世纪,北京);PAGE凝胶快速制备试剂盒(雅酶生物,上海);鼠抗GAPDH,羊抗鼠二抗和羊抗兔二抗(中杉金桥,北京);兔抗HNRNPAB(Abcam, USA);PrimeScript II 1 st Strand cDNA Synthesis Kit(TaKaRa,大连)等。
1.4 方法1.4.1 牛骨骼肌卫星细胞的复苏、传代及诱导分化 牛骨骼肌卫星细胞体外诱导成肌分化模型的建立参考天津市农业动物繁育与健康养殖重点实验室建立的方法[19],液氮中取出细胞,37 ℃水浴快速摇晃使细胞复苏,用等体积的增殖培养基(20%FBS+ 80%DMEM)中和,立刻转移到离心管中离心10 min(1 000 r·min-1),离心后弃上清,加入3 mL增殖培养基重悬,均匀接种于60 mm细胞培养板,当细胞融合度达到80%,细胞量约为1×106时传代,用胰酶进行消化,增殖培养基中和以终止消化,差速离心收集沉淀,加入增殖培养基重悬后接种于6孔和24孔板中培养,24 h后收取增殖期细胞(GM期细胞)。细胞融合度达到80%~90%后,换分化培养基(2%HS+98%DMEM)进行体外诱导分化,培养24、48、72 h后收取分化期第1(DM1)、2(DM2)、3(DM3)天的细胞。
1.4.2 HNRNPAB干扰效果检测 根据HNRNPAB的CDS区序列设计干扰RNA(序列为:CCAAAGAGGTTTATCAACA),由广东锐博公司合成。将细胞培养于24孔板和6孔板中,设置试验组和对照组,每组3个重复,细胞融合度达80%,细胞量约为1×106时参考Lipofectamine3000说明书转染si-HNRNPAB及对照。转染24 h后收取增殖期(GM)细胞,同时更换增殖培养基为分化培养基,换分化培养基24、72 h后分别收取分化第1天(DM1)和第3天(DM3)的细胞。通过qRT-PCR技术和Western blot技术检测GM期、DM1期和DM3期的干扰效果。
1.4.3 EdU染色法检测细胞增殖 按Cell-LightTM EdU Apollo 567 In Vitro Kit说明操作EdU染色试验,于96孔板中培养细胞,转染24 h后对细胞进行处理,利用荧光显微镜检测阳性细胞数,每组设置3个生物学重复,荧光显微镜拍照存图,每孔至少采集5个不同视野。
1.4.4 荧光定量PCR检测基因表达 24孔板中培养细胞,收取细胞后按照试剂盒说明书提取总RNA,RNA浓度检测合格后进行反转录,利用PrimeScript II 1 st Strand cDNA Synthesis Kit进行cDNA第一链的合成,稀释cDNA 5倍后进行qRT-PCR检测HNRNPAB的mRNA表达水平、Pax7、Cyclin D1(增殖标志因子)和MyoG、MyHC(分化标志因子)的mRNA表达水平。荧光定量PCR体系(总20 μL):cDNA 2 μL,上、下游引物各0.5 μL,Mix 10 μL,RNase free water 7 μL。荧光定量PCR程序:95 ℃预变性60 s;95 ℃变性10 s;61 ℃退火20 s;72 ℃延伸15 s,40个循环;熔解曲线:95 ℃ 10 s;65 ℃ 60 s;97 ℃ 1 s;冷却:37 ℃ 30 s。qRT-PCR反应引物详情见表 1。
|
|
表 1 qRT-PCR引物信息 Table 1 qRT-PCR primers information |
1.4.5 Western blot检测蛋白表达 6孔板中培养细胞,待转染处理48、96 h后,加入含1% PMSF的RIPA裂解液收集蛋白,4 ℃超速离心10 min后取上清液保存于-80 ℃。根据BCA试剂盒测定蛋白浓度后对蛋白进行变性,根据蛋白浓度利用Western blot检测HNRNPAB、Pax7(增殖标志因子)、MyoG、MyHC(分化标志因子)的蛋白表达量。
1.4.6 统计分析 qRT-PCR及Western blot结果用t检验分析(SPSS和Image Lab), EDU细胞增殖试验采用t检验分析(ImageJ软件进行细胞计数),“*”表示差异显著(P < 0.05),“**”表示差异极显著(P < 0.01),“N.S.”表示差异不显著(P>0.05)。
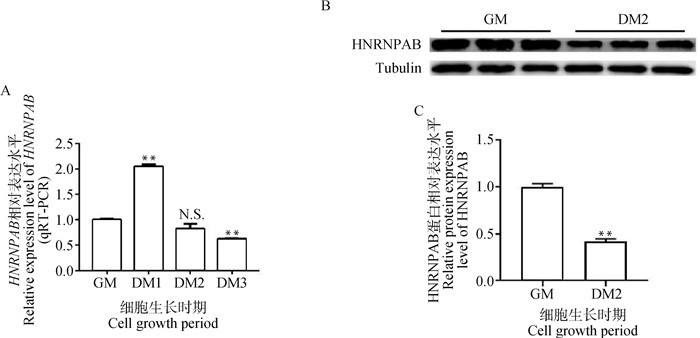
2 结果 2.1 牛骨骼肌卫星细胞成肌分化前后HNRNPAB表达谱分析为鉴定HNRNPAB是否在成肌分化前后具有关键作用,通过qRT-PCR对HNRNPAB进行时间序列表达分析,结果显示,随着牛骨骼肌卫星细胞分化时间的延长,HNRNPAB的mRNA表达水平呈现先上升后下降的趋势,且在诱导分化第1天HNRNPAB的mRNA表达量最高(图 1A),分化期HNRNPAB的蛋白水平表达量较增殖期(分化前)相比极显著降低(P < 0.01),表明HNRNPAB可能具有调控牛骨骼肌卫星细胞成肌分化的作用(图 1B、C)。
|
A.HNRNPAB 时间序列 mRNA 表达谱;B.HNRNPAB 成肌分化前后蛋白表达水平;C.Western blot 量化结果 A.HNRNPAB time-series mRNA expression profile; B.Protein expression levels of HNRNPAB before and after myogenic differentiation; C.Western blot quantification results 图 1 牛骨骼肌卫星细胞成肌分化前后HNRNPAB表达量 Fig. 1 Expression of HNRNPAB in bovine skeletal muscle satellite cells before and after myogenic differentiation |
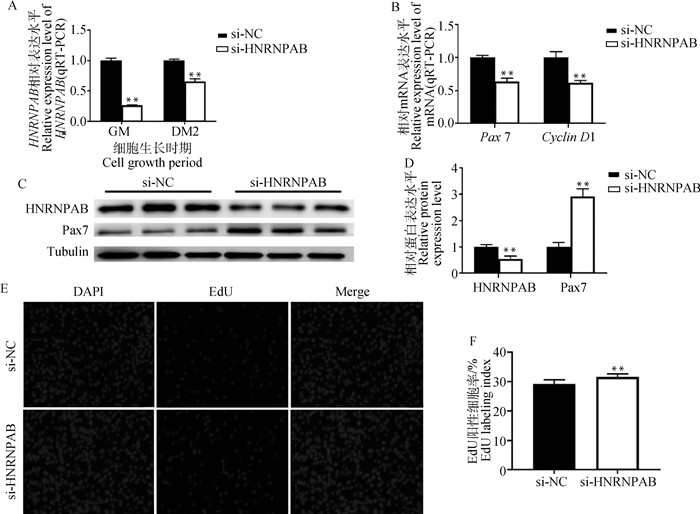
构建牛骨骼肌卫星细胞抑制表达模型,对牛骨骼肌细胞进行转染si-HNRNPAB处理24 h后,EdU细胞增殖试验检测细胞的增殖情况,qRT-PCR检测增殖期细胞中HNRNPAB和增殖标志因子Pax7、Cyclin D1的mRNA水平变化,Western blot检测细胞中HNRNPAB与Pax7蛋白表达水平变化。转染si-HNRNPAB后,HNRNPAB的mRNA水平与蛋白表达水平均极显著降低(P < 0.01),增殖标志因子Pax7和Cyclin D1的mRNA表达水平极显著降低(P < 0.01,图 2B),Pax7的蛋白表达水平极显著上升(P < 0.01,图 2C、2D),EdU阳性细胞率极显著上升(P < 0.01, 图 2E、2F)。结果显示,干扰HNRNPAB后降低了牛骨骼肌卫星细胞增殖标志因子的转录水平,升高了Pax7的蛋白表达水平。
|
A.HNRNPAB 干扰效果分析;B.增殖标志因子 mRNA 表达水平;C.增殖期 HNRNPAB 与 Pax7 蛋白表达水平;D.Western blot 量化结果;E.EdU 染色(200×);F. EdU 标记指数 A. Analysis of the interference effect of HNRNPAB; B. mRNA expression levels of proliferation marker factors; C. Protein expression levels of HNRNPAB and Pax7 in the proliferation phase; D. Western blot quantification results; E. EdU staining (200×); F. EdU labeling index 图 2 干扰HNRNPAB对牛骨骼肌卫星细胞增殖的影响 Fig. 2 The effect of interfering with HNRNPAB on the proliferation of bovine skeletal muscle satellite cells |
转染si-HNRNPAB处理牛骨骼肌卫星细胞48、96 h后,qRT-PCR和Western blot检测细胞中HNRNPAB以及分化标志因子MyHC、MyoG的mRNA表达水平与蛋白表达水平的变化,显微镜观察细胞成肌分化后的肌管形态。转染si-HNRNPAB 48及96 h后,HNRNPAB的mRNA表达水平极显著降低(P < 0.01),分化标志因子MyoG、MyHC的mRNA表达水平均极显著上升(P < 0.01,图 3A、3B),转染si-HNRNPAB 96 h后,HNRNPAB的蛋白表达水平极显著降低(P < 0.01),分化标志因子MyoG、MyHC的蛋白表达水平显著上升(P < 0.05,图 3C、3D),倒置显微镜下观察到干扰HNRNPAB组与对照组相比,肌管明显变粗且分化状态明显(图 3E)。结果显示,干扰HNRNPAB对牛骨骼肌卫星细胞分化有促进作用。

|
A.分化第1天 HNRNPAB 与分化标志因子 mRNA 表达水平;B.分化第3天 HNRNPAB 与分化标志因子 mRNA 表达水平;C.分化第3天 HNRNPAB 与标志因子蛋白表达水平;D.Western blot 量化结果;E.诱导分化第3天,倒置显微镜下细胞生长状态(200×) A.The mRNA expression levels of HNRNPAB and differentiation marker factors on the first day of differentiation; B.The mRNA expression levels of HNRNPAB and differentiation marker factors on the third day of differentiation; C.The protein expression levels of HNRNPAB and marker factors on the third day of differentiation; D.Western blot quantification results; E.On the third day of induction of differentiation, the growth state of cells under an inverted microscope (200×) 图 3 干扰HNRNPAB对牛骨骼肌卫星细胞分化的影响 Fig. 3 Effects of interfering with HNRNPAB on the differentiation of bovine skeletal muscle satellite cells |
骨骼肌是哺乳动物机体内的重要组成部分,在动物机体的运动、产热等方面发挥着不可小觑的作用[20]。骨骼肌卫星细胞对骨骼肌的生长、修复、再生过程都具有调控作用。当骨骼肌受损后,骨骼肌卫星细胞被调控因子激活,随后进入增殖、分化进程,最后形成新的肌纤维[21]。骨骼肌的生长发育不仅包括肌源性调节因子,许多转录因子也参与到调控进程中。
Pre-mRNA在成为成熟的mRNA的过程中需要多种蛋白质的参与,大多蛋白质会同RNA形成核糖核蛋白复合物[22]。其中的核内不均一核糖核蛋白复合物(hnRNP)家族成员约有20多个,除少数成员外,hnRNPs家族成员都含有RBD(RNA-binding domain)结构域,除了基本的RBD结构域还有其他辅助的结构域(如富含甘氨酸、脯氨酸或酸性的结构域)[23]。有研究表明,hnRNP E1可以下调HPV16 E6癌基因的表达,从而抑制宫颈癌变[24]。敲除小鼠中hnRNP-U的表达能够导致糖酵解肌肉的选择性萎缩,发展为肌病[25]。RNA结合基序蛋白X-连锁蛋白(RBMX)通过竞争性抑制hnRNP A1介导的PKM可变剪接机制抑制转移性膀胱癌(BCa)的发展和致瘤性[26]。hnRNP C可以促进新的异质核RNA(hnRNAs,也称为pre-mRNAs)发展成为成熟mRNAs,并且可以稳定mRNAs,控制其翻译。它与一些癌症基因和其他生物分子相互作用,其主要功能是调节癌基因的稳定性和翻译水平[27]。hnRNP K能够在转录水平上抑制肌细胞生成素的表达[28]。综上研究可知,hnRNP这个蛋白超家族具有广泛的生物学功能,但大多表现为参与肿瘤的发生、癌症的发展等。
HNRNPAB作为hnRNPs的主要家族成员之一,主要参与调控mRNA转运、代谢、剪切及表达等过程[29-30]。近年来,HNRNPAB也被发现与肿瘤发生发展密切相关,同时,研究发现HNRNPAB影响了众多细胞的增殖和分化,但和肌肉发育相关的研究还未见报道,所以本研究探索了HNRNPAB在牛骨骼肌卫星细胞分化前后的表达量是否存在显著差异,结果显示随牛骨骼肌卫星细胞分化时间的不断延长,HNRNPAB的mRNA表达水平呈现先上升后下降的趋势,且在诱导分化第1天表达量最高,蛋白表达水平在分化前后也具有显著差异。暗示HNRNPAB可能具有调控牛骨骼肌细胞增殖、分化的作用;随后通过干扰HNRNPAB的表达分析其对牛骨骼肌卫星细胞增殖分化的影响,结果显示,干扰HNRNPAB后细胞增殖标志因子Pax7和Cyclin D1的mRNA水平同对照组相比极显著降低,Pax7的蛋白表达水平极显著升高,EdU阳性细胞率与对照组相比极显著上升;干扰HNRNPAB后分化标志因子MyoG和MyHC的mRNA水平及蛋白表达水平同对照组相比显著升高,肌管数量和肌管直径明显大于对照组。由此可知,干扰HNRNPAB对牛骨骼肌卫星细胞的增殖、分化具有相应的影响,抑制HNRNPAB表达后会抑制牛骨骼肌卫星细胞增殖标志因子的转录水平,提升Pax7的蛋白表达水平,同时促进牛骨骼肌卫星细胞的分化。类似的,HNRNPAB对其他细胞的增殖、分化也有着一定的作用,Dang等[31]发现,通过调控miR-M4-5p降低了HNRNPAB的表达量后,促进了原代鸡胚成纤维细胞的增殖,说明HNRNPAB具有抑制原代鸡胚成纤维细胞增殖活性的作用。Taga等[32]发现,在293T细胞或乳腺癌MCF-7细胞中转染过表达HNRNPAB的载体后,两种细胞的增殖受到抑制作用,表明HNRNPAB可以抑制细胞增殖进程。这可能是由于HNRNPAB在不同类型的细胞中会发挥不同的作用,有待进一步验证。而对于本试验中干扰HNRNPAB后增殖标志因子的mRNA表达水平降低,Pax7的蛋白表达水平却升高,可能是蛋白质稳定性、翻译效率或蛋白降解所导致,具体原因还需要通过进一步试验加以验证。
4 结论本研究利用牛骨骼肌卫星细胞体外成肌诱导分化模型,探究hnRNPs家族核心蛋白HNRNPAB对牛骨骼肌卫星细胞增殖分化的调控作用,结果显示,干扰HNRNPAB后抑制牛骨骼肌卫星细胞增殖标志因子的转录水平,促进Pax7的蛋白表达水平,并促进了牛骨骼肌卫星细胞的分化。本研究可为进一步深入探索HNRNPAB及其在动物骨骼肌的发育分化调控机制等方面提供参考。
| [1] |
BASS J, OLDHAM J, SHARMA M, et al. Growth factors controlling muscle development[J]. Domest Anim Endocrinol, 1999, 17(2-3): 191-197. DOI:10.1016/S0739-7240(99)00036-3 |
| [2] |
KRAUSS R S, JOSEPH G A, GOEL A J. Keep your friends close: Cell-cell contact and skeletal myogenesis[J]. Cold Spring Harb Perspect Biol, 2017, 9(2): a029298. DOI:10.1101/cshperspect.a029298 |
| [3] |
SHAHINI A, VYDIAM K, CHOUDHURY D, et al. Efficient and high yield isolation of myoblasts from skeletal muscle[J]. Stem Cell Res, 2018, 30: 122-129. DOI:10.1016/j.scr.2018.05.017 |
| [4] |
CRAMERI R M, HENNING L, MAGNUSSON P, at el. Changes in satellite cells in human skeletal muscle after a single bout of high intensity exercise[J]. J Physiol, 2004, 558(Pt 1): 333-340. |
| [5] |
MEGENEY L A, KABLAR B, GARRETT K, et al. MyoD is required for myogenic stem cell function in adult skeletal muscle[J]. Genes Dev, 1996, 10(10): 1173-1183. DOI:10.1101/gad.10.10.1173 |
| [6] |
HAN S P, TANG Y H, SMITH R. Functional diversity of the hnRNPs: Past, present and perspectives[J]. Biochem J, 2010, 430(3): 379-392. DOI:10.1042/BJ20100396 |
| [7] |
DAS U, NGUYEN H, XIE J Y. Transcriptome protection by the expanded family of hnRNPs[J]. RNA Biol, 2019, 16(2): 155-159. DOI:10.1080/15476286.2018.1564617 |
| [8] |
刘玉娟. hnRNPs的结构、功能及其相关疾病的研究进展[J]. 江西医药, 2020, 55(10): 1537-1541. LIU Y J. Research progress on the structure, function and related diseases of hnRNPs[J]. Jiangxi Medical Journal, 2020, 55(10): 1537-1541. DOI:10.3969/j.issn.1006-2238.2020.10.063 (in Chinese) |
| [9] |
LIU X X, ZHOU Y, LOU Y Q, et al. Knockdown of HNRNPA1 inhibits lung adenocarcinoma cell proliferation through cell cycle arrest at G0/G1 phase[J]. Gene, 2016, 576(2 Pt 2): 791-797. |
| [10] |
LI H, GUO L, HUANG A X, et al. Nanoparticle-conjugated aptamer targeting hnRNP A2/B1 can recognize multiple tumor cells and inhibit their proliferation[J]. Biomaterials, 2015, 63: 168-176. DOI:10.1016/j.biomaterials.2015.06.013 |
| [11] |
GEUENS T, BOUHY D, TIMMERMAN V. The hnRNP family: Insights into their role in health and disease[J]. Hum Genet, 2016, 135(8): 851-867. DOI:10.1007/s00439-016-1683-5 |
| [12] |
LIU T Y, CHEN Y C, JONG Y J, et al. Muscle developmental defects in heterogeneous nuclear Ribonucleoprotein A1 knockout mice[J]. Open Biol, 2017, 7(1): 160303. DOI:10.1098/rsob.160303 |
| [13] |
ZHAO M X, SHEN L H, OUYANG Z J, et al. Loss of hnRNP A1 in murine skeletal muscle exacerbates high-fat diet-induced onset of insulin resistance and hepatic steatosis[J]. J Mol Cell Biol, 2020, 12(4): 277-290. DOI:10.1093/jmcb/mjz050 |
| [14] |
LIU J H, BEQAJ S, YANG Y, et al. Heterogeneous nuclear ribonucleoprotein-H plays a suppressive role in visceral myogenesis[J]. Mech Dev, 2001, 104(1-2): 79-87. DOI:10.1016/S0925-4773(01)00377-X |
| [15] |
ALEXANDER M S, HIGHTOWER R M, REID A L, et al. hnRNP L is essential for myogenic differentiation and modulates myotonic dystrophy pathologies[J]. Muscle Nerve, 2021, 63(6): 928-940. DOI:10.1002/mus.27216 |
| [16] |
BOUKAKIS G, PATRINOU-GEORGOULA M, LEKARAKOU M, et al. Deregulated expression of hnRNP A/B proteins in human non-small cell lung cancer: parallel assessment of protein and mRNA levels in Paired tumour/non-tumour tissues[J]. BMC Cancer, 2010, 10: 434. DOI:10.1186/1471-2407-10-434 |
| [17] |
BRONSTEIN N B, KISHORE R, ISMAIL Z, et al. cDNA cloning and spatiotemporal expression during avian embryogenesis of hnRNP A1, a regulatory factor in alternative splicing[J]. Gene Expr Patterns, 2003, 3(3): 285-295. DOI:10.1016/S1567-133X(03)00048-6 |
| [18] |
HUA J T, AHMED M, GUO H Y, et al. Risk SNP-mediated promoter-enhancer switching drives prostate cancer through lncRNA PCAT19[J]. Cell, 2018, 174(3): 564-575.e18. DOI:10.1016/j.cell.2018.06.014 |
| [19] |
王轶敏, 代阳, 刘新峰, 等. 牛骨骼肌卫星细胞的分离鉴定和诱导分化[J]. 中国畜牧兽医, 2014, 41(7): 142-147. WANG Y M, DAI Y, LIU X F, et al. Isolation, identification and induced differentiation of bovine skeletal muscle satellite cells[J]. China Animal Husbandry & Veterinary Medicine, 2014, 41(7): 142-147. (in Chinese) |
| [20] |
FORTIN M, VIDEMAN T, GIBBONS L E, et al. Paraspinal muscle morphology and composition: A 15-yr longitudinal magnetic resonance imaging study[J]. Med Sci Sports Exerc, 2014, 46(5): 893-901. DOI:10.1249/MSS.0000000000000179 |
| [21] |
MAVALLI M D, DIGIROLAMO D J, FAN Y, et al. Distinct growth hormone receptor signaling modes regulate skeletal muscle development and insulin sensitivity in mice[J]. J Clin Invest, 2010, 120(11): 4007-4020. DOI:10.1172/JCI42447 |
| [22] |
FAKAN S. Perichromatin fibrils are in situ forms of nascent transcripts[J]. Trends Cell Biol, 1994, 4(3): 86-90. DOI:10.1016/0962-8924(94)90180-5 |
| [23] |
KRECIC A M, SWANSON M S. hnRNP complexes: Composition, structure, and function[J]. Curr Opin Cell Biol, 1999, 11(3): 363-371. DOI:10.1016/S0955-0674(99)80051-9 |
| [24] |
SONG L, MAO R, DING L, et al. hnRNP E1 regulates HPV16 oncogene expression and inhibits cervical cancerization[J]. Front Oncol, 2022, 12: 905900. DOI:10.3389/fonc.2022.905900 |
| [25] |
BAGCHI D, MASON B D, BALDINO K, et al. Adult-onset myopathy with constitutive activation of akt following the loss of hnRNP-U[J]. iScience, 2020, 23(7): 101319. DOI:10.1016/j.isci.2020.101319 |
| [26] |
YAN Q X, ZENG P, ZHOU X Q, et al. RBMX suppresses tumorigenicity and progression of bladder cancer by interacting with the hnRNP A1 protein to regulate PKM alternative splicing[J]. Oncogene, 2021, 40(15): 2635-2650. DOI:10.1038/s41388-021-01666-z |
| [27] |
MO L Y, MENG L J, HUANG Z C, et al. An analysis of the role of HnRNP C dysregulation in cancers[J]. Biomark Res, 2022, 10(1): 19. DOI:10.1186/s40364-022-00366-4 |
| [28] |
HITACHI K, KIYOFUJI Y, NAKATANI M, et al. Myoparr-associated and -independent multiple roles of heterogeneous nuclear ribonucleoprotein K during skeletal muscle cell differentiation[J]. Int J Mol Sci, 2022, 23(1): 108. |
| [29] |
WEIGHARDT F, BIAMONTI G, RIVA S. The roles of heterogeneous nuclear ribonucleoproteins (hnRNP) in RNA metabolism[J]. Bioessays, 1996, 18(9): 747-756. DOI:10.1002/bies.950180910 |
| [30] |
PIÑOL-ROMA S. HnRNP proteins and the nuclear export of mRNA[J]. Semin Cell Dev Biol, 1997, 8(1): 57-63. DOI:10.1006/scdb.1996.0122 |
| [31] |
DANG L, TENG M, LI H Z, et al. Marek's disease virus type 1 encoded analog of miR-155 promotes proliferation of chicken embryo fibroblast and DF-1 cells by targeting hnRNPAB[J]. Vet Microbiol, 2017, 207: 210-218. DOI:10.1016/j.vetmic.2017.06.015 |
| [32] |
TAGA Y, MIYOSHI M, OKAJIMA T, et al. Identification of heterogeneous nuclear ribonucleoprotein A/B as a cytoplasmic mRNA-binding protein in early involution of the mouse mammary gland[J]. Cell Biochem Funct, 2010, 28(4): 321-328. DOI:10.1002/cbf.1662 |
(编辑 郭云雁)



