2. 农业部种养结合重点实验室, 南京 210014;
3. 北京美联众合动物医院, 北京 100101
2. Key Laboratory of Crop and Livestock Integrated Farming, Ministry of Agriculture, Nanjing 210014, China;
3. Beijing Meilianzhonghe Animal Hospital, Beijing 100101, China
光照作为环境因子,显著影响动物的生理活动,如季节性繁殖、毛皮生长、春季脱毛等,在动物生产中起着重要作用。人工光照已经在畜禽养殖中得到应用。家兔是对光高度敏感的畜种,光照可调控褪黑激素分泌,通过MT1受体途径促进家兔发情[1]。有研究报道,光照时长、强度及光照制度会影响母兔的产奶量、产仔窝重,从而影响母兔的生产性能,光照的强度和制度甚至会影响母兔的采食量[2]。在自然光周期缩短的情况下,使用30 lx光照强度(白炽灯泡),每天补充光照14 h可提高母兔生产性能[3]。Mousa-Balabel[4]研究发现,每天使用20 lx的荧光灯照明能改善母兔的生产性能。EFSA规定,为满足家兔生理活动、视觉及环境需要,光照强度必须达到50 lx[5]。光照也是影响毛皮动物皮毛生长发育的关键因子。Fushimi等[6]研究发现,LED光照可以促进小鼠毛发的生长,但光照对长毛兔影响的研究报道很少,特别是LED光。本研究拟探讨不同LED光对3月龄苏系长毛兔生产性能、毛品质及皮肤毛囊发育的影响,为LED光照在毛兔生产中的应用提供数据支撑。
1 材料与方法 1.1 试验动物3月龄苏系长毛兔来自江苏省农业科学院六合动物科学基地实验兔场。
1.2 试验设计试验地点为江苏省农业科学院六合实验兔场。将50只3月龄、健康、体重相近((2.245 ± 0.296) kg)的苏系长毛兔随机分为5组,每组5个重复,每个重复2只,采用不同LED光处理,光照强度为50 lx,处理组分别为LED红光组、LED绿光组、LED蓝光组、黑暗组和对照组(自然光组),试验期73 d。
1.3 饲养管理试验前对兔舍兔笼进行彻底清扫、消毒,长毛兔统一剪毛。一兔一笼,饲喂同一基础日粮,由江苏康迪富尔饲料股份有限公司生产。每天饲喂两次,早上8:30和下午15:30,自由采食、饮水。光照采用16L∶8D光照制度,采用时间控制器,每天早上4:00开始启动灯带,晚上20:00关闭灯带,光照强度一致,为50 lx。
1.4 样本采集试验初始及试验末,称量每只试验兔体重,计算平均日增重(ADG)。试验期记录每周每组试验兔总采食量,计算平均采食量。试验末统一剪毛,称量产毛重量。
试验末禁食24 h,于早晨9:00,每组10只苏系长毛兔,耳缘静脉采集血样,分离血清,-20 ℃保存用于褪黑激素(MT)、催乳素(PRL)、三碘甲状原氨酸(T3)、四碘甲状原氨酸(T4)、生长激素(GH)的测定,按照ELISA试剂盒(购自南京建成生物工程研究所)说明书的方法进行检测。取肩胛部、背部、腹部相同部位的1 cm2毛样,用于毛纤维长度、细度、粗毛率和回潮率测定。
取肩胛皮肤,铺平并固定在带孔的木板上,然后固定于Bouin氏液中。皮肤在Bouin氏液中固定2 h之后进行修块,修成1 cm×1 cm,用于皮肤毛囊观察。
1.5 指标测定毛纤维长度测定采用手排法,即在黑绒板上将兔毛纤维整理成一端平齐的毛束,将纤维由长到短、自左向右均匀排列成底线平齐的纤维长度分布图,把长度分布图描绘在坐标纸上,根据作图计算纤维平均长度指标。粗毛率采用重量法测定,称取1 g毛样进行测定,先将毛样中的粗毛检出,剩余的毛为细毛和两型毛,干燥器中平衡2 h,天平称其重量,计算其比例。毛纤维细度采用投射显微镜法[7],应用Q-capture Pro 6.0软件获得纤维切面照片,Image-pro plus 6.0图像分析系统进行分析,测量200毛样直径。回潮率采用国家标准(GB/T13835.4-92)规定的方法测定[8]。
皮肤组织固定完成后,脱水制成石蜡切片,苏木精-伊红(HE) 染色,毛囊群密度采用光学显微镜检测,在目镜为10倍、物镜为10倍条件下观察,读取原生毛囊数。
1.6 数据处理运用Excel 2016整理数据,采用SPSS17.0进行单因素方差分析(one-way ANOVA),Duncan氏法多重比较,P < 0.05表示差异显著;P < 0.01表示差异极显著。
2 结果 2.1 LED光照对苏系长毛兔生产性能的影响由表 1可知,LED光照对苏系长毛兔终末体重、平均日增重、采食量无显著的影响(P>0.05),但红光组的产毛量要高于其余各组(P=0.050)。
|
|
表 1 LED光照对苏系长毛兔生产性能的影响 Table 1 Effects of LED lights on the growth performance of Su line Angora rabbits |
由表 2可知,LED光照对长毛兔肩胛部毛纤维长度有显著的影响,红光组肩胛部毛纤维长度显著长于对照组和绿光组,比对照组和绿光组长35.36%(P < 0.05)。背部和腹部毛纤维长度组间无显著差异(P>0.05),但红光组的背部毛纤维最长。
|
|
表 2 LED光照对苏系长毛兔毛纤维长度的影响 Table 2 Effects of LED lights on the fiber lengths of Su line Angora rabbits |
由表 3可知,LED光照对苏系长毛兔粗毛细度和粗毛率有显著影响,与对照组相比,绿光组和黑暗组显著提高了粗毛的细度(P < 0.05),红光组和蓝光组显著降低了粗毛率(P < 0.05)。此外,红光组的细毛细度度最低细毛细度,但无统计学差异(P>0.05)。LED光照对回潮率没有显著影响(P>0.05)。
|
|
表 3 LED光照对长毛兔毛纤维细度、回潮率、粗毛率的影响 Table 3 Effects of of LED lights on the fiber diameters, moisture contents and coarse fiber ratios of Su line Angora rabbits |
由表 4可知,LED光照对长毛兔血清MT、T3有显著影响。与对照组相比,红光组显著提高了血清MT和T3的浓度(P < 0.05),黑暗组显著降低了血清PRL的浓度(P < 0.05)。血清T4和GH浓度在组间无显著差异(P>0.05)。
|
|
表 4 LED光照对苏系长毛兔血清激素的影响 Table 4 Effects of LED lights on serum hormones of Su line Angora rabbits |
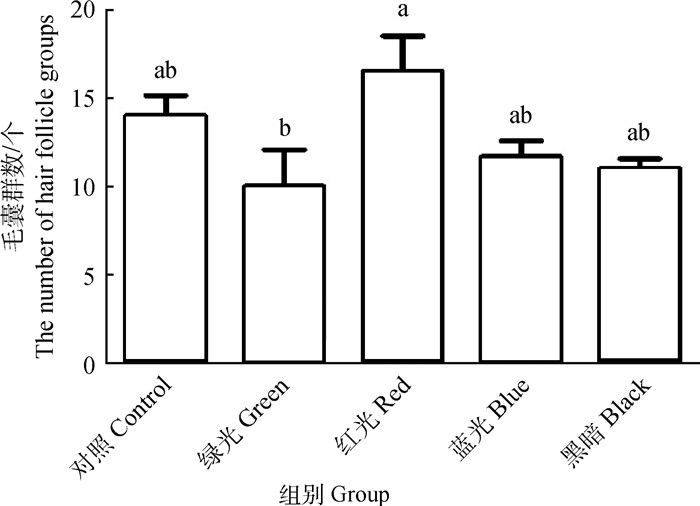
由图 1可知,相同放大倍数视野中,红光组毛囊群个数最多(n=16.50),依次为对照组(n=14.00)、蓝光组(n=11.67)、黑光组(n=11.00),绿光组毛囊群个数最少(n=10.00),红光组与绿光组之间毛囊群个数具有显著性差异(P < 0.05),但其余各组间比较差异不显著(P >0.05)。
|
不同小写字母表示差异显著(P < 0.05) The different small letters show significant difference (P < 0.05) 图 1 LED光照对苏系长毛兔皮肤毛囊群个数的影响 Fig. 1 Effect of LED lights on hair follicle groups of Su line Angora rabbits |
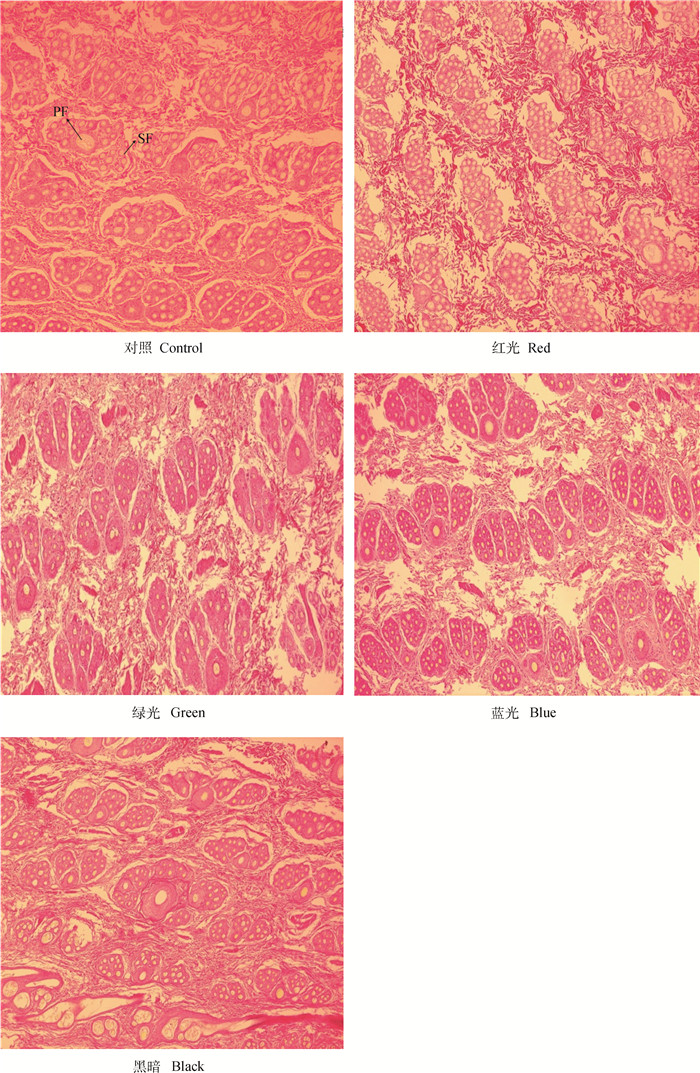
图 2为HE染色结果,LED光照对长毛兔皮肤毛囊结构有明显影响。对照组毛囊群结构排列整齐,一个初级毛囊被3~4个次级毛囊群环绕,次级毛囊群的扇叶结构明显,每个扇叶结构中有5~8个次级毛囊。红光组毛囊群结构排列整齐,但每个毛囊群几乎全是次级毛囊,且次级毛囊个数较多,而初级毛囊极少,同时,次级毛囊群的扇叶结构不明显。绿光组毛囊群个数较少,囊群结构明显,一个初级毛囊被3~4个次级毛囊群环绕,次级毛囊群的扇叶结构明显。蓝光组毛囊群结构排列整齐,次级毛囊的囊群个数较多,最多一个初级毛囊被8个次级毛囊包围,每个扇叶结构中有最少5个,最多10多个次级毛囊。黑暗组毛囊群个数多于绿光组的但少于蓝光组的,每个初级毛囊被3~5个次级毛囊群包围,次级毛囊扇叶结构明显,次级毛囊群中毛囊个数最少3个,最多10多个,部分为初级毛囊。
|
PF为初级毛囊,SF为次级毛囊 PF and SF represent primary follicle and secondary follicle, respectively 图 2 长毛兔皮肤毛囊结构组织切片(100×) Fig. 2 Skin tissue sections with hair follicles of Angora rabbits (100×) |
毛产量和纤维品质是毛用动物生产的重要指标,受众多因素影响,包括年龄、遗传、环境和营养。遗传和营养方面,毛用动物生产中已做了大量的研究。Wnt10b可以通过经典Wnt信号通路促进毛囊的生长[9],其基因可激活Wnt/β-catenin途径调节毛囊的发育[10]。Yang等[11]研究报道,CSDC2基因可能是促进山羊绒生长的重要转录因子。不同蛋白质水平或蛋白补充也能够影响安哥拉山羊的产毛性能和毛纤维特性[7, 12]。然而,关于环境因素——光照对毛用动物生产的影响研究较少,主要集中于绒山羊。缩短光照周期可以诱导7月份绒山羊皮肤次级毛囊发育,提高10月份产绒量及绒纤维长度[13]。这与Liu等[14]研究结果基本一致,短光照周期可促进毛(绒)生长,调节光照有效提高绒产量,可以广泛应用于畜牧生产。在非产绒期,也可通过光照调控促进羊绒生长,显著提高个体产绒量,增加绒纤维长度[15]。其它毛用动物光照研究也有相似的结果。高志光[16]研究发现,缩短光照能诱导貉冬毛提前生长并成熟,光照能改变换毛及冬毛生长的进程,不改变其生长规律。本试验选择不同颜色LED光,研究其对苏系长毛兔毛产量和毛纤维品质的影响,结果表明,在16L∶8D的光照制度下,不同颜色LED光照对毛纤维品质有不同的影响,LED红光可以提高兔毛产量,显著提高毛纤维长度,降低细度和粗毛率。毛纤维长度和细度直接影响纺织加工工艺,细度是影响毛纤维价值的主要性状[17],LED红光提高毛纤维长度,降低细度在生产中很有意义。Sheen等[18]研究发现,红光组小鼠的毛进入生长期速度比绿光组和蓝光组的快。Han等[19]报道,在体外培养模型中,655 nm红光+LED能促进人的头发生长,减少毛囊向退化期转变。由此可见,LED红光可以促进毛纤维的生长。
在本试验中,还检测了血清MT、PRL、T3等激素的浓度,发现,LED红光组可以提高血清MT和T3的浓度,这与王兴涛[20]研究的发现基本相似,调控光照可以提高绒山羊血清MT浓度。毛纤维生长受褪黑激素的调节,而褪黑激素分泌受光照周期调控。在动物体内,一定强度的光向松果体发出信号,启动或终止褪黑激素的合成和分泌,褪黑激素以周期性的节律传递信号,调节季节性繁殖和其他生物生理过程,如冬眠、迁徙和皮毛变化等[6]。Coelho等[21]报道,在南半球低纬度地区,自然光周期下毛用母羊的血浆褪黑激素浓度表现出相同的周年规律,春季外源性褪黑激素处理对Rasa Aragonesa母羊羊毛品质有积极影响。外源褪黑激素还能提高毛产量和纤维质量[22]。长毛兔皮下埋植40和55 mg褪黑激素能够提高公兔夏季产毛量且抑制母兔夏季产毛量的降低,同时增加公、母兔粗毛长度[23]。Duan等[24-25]研究发现,两次(4月下旬和6月)植入褪黑激素(2 mg·kg-1体重),可诱导羊绒生长和降低纤维细度,提高羊绒产量,且不改变母羊生长速度和繁殖性能,但在生长慢速期(12月和2月)埋植褪黑激素对产绒量没有显著影响。Cong等[26]指出,冬至期间植入褪黑激素可有效延长辽宁绒山羊羊绒生长周期,处理后羊绒纤维的数量和质量都有所提高。因此,光照处理提高毛纤维品质可能与褪黑激素有关。在本研究中,LED红光效果优于自然光或其他LED光照组,LED红光对长毛兔的毛纤维品质起着关键作用。
Yang等[27]揭示,褪黑激素能够促进早产绒山羊皮肤次级毛囊的发育,进一步拓展了褪黑激素在毛纤维生产中应用的认识。本研究为验证上述结果,进一步测定和观察了毛囊的发育和结构,结果显示,相同视野下LED红光组的毛囊群数为16.5,且由众多次级毛囊和少量初级毛囊组成。这一结果与LED红光提高毛纤维长度、降低细度的结果一致。有关光照处理下毛囊特性变化的报道较少。王兴涛[20]研究发现,调控光照可以提高绒山羊次级毛囊的密度。此外,Lanszki等[22]发现,褪黑激素处理可使每组毛囊的活性毛囊(初级和次级)数量增加32%。光照影响褪黑激素的合成和分泌,而褪黑激素影响毛囊活动,LED红光通过改变褪黑激素分泌来增加长毛兔或其他毛皮动物(如绵羊、羊绒山羊)的次级毛囊数量。但其刺激褪黑激素分泌的作用机制需进一步研究。
4 结论 4.1本试验条件下,LED红光显著提高了苏系长毛兔毛纤维长度,降低了细度,提高了毛产量。
4.2LED红光显著提高苏系长毛兔血清MT的浓度,增加了苏系长毛兔皮肤组织毛囊群数和次级毛囊数。LED红光可能通过提高机体MT浓度影响苏系长毛兔毛产量及毛纤维品质。
| [1] |
王文利, 张玉仙, 原展航, 等. 光照周期对褪黑激素受体在母兔下丘脑-垂体-卵巢轴中分布与表达的影响[J]. 畜牧兽医学报, 2019, 50(12): 2518-2528. WANG W L, ZHANG Y X, YUAN Z H, et al. Effect of photoperiods on distribution and expression of melatonin receptors in hypothalamus-pituitary-ovary axis in female rabbits[J]. Acta Veterinaria et Zootechnica Sinica, 2019, 50(12): 2518-2528. DOI:10.11843/j.issn.0366-6964.2019.12.016 (in Chinese) |
| [2] |
VIRÁG G, PAPP Z, RAFAI P, et al. Effects of an intermittent lighting schedule on doe and suckling rabbit's performance[J]. World Rabbit Sci, 2000, 8(suppl.1): S477-S481. |
| [3] |
MATTARAIA V G M, BIANOSPINO E, FERNANDES S, et al. Reproductive responses of rabbit does to a supplemental lighting program[J]. Livest Prod Sci, 2005, 94(3): 179-187. DOI:10.1016/j.livprodsci.2004.10.012 |
| [4] |
MOUSA-BALABEL T M. Using light and melatonin in the management of New Zealand white rabbits[J]. Open Vet J, 2011, 1(1): 1-6. DOI:10.4236/ojvm.2011.11001 |
| [5] |
MARINA V, BLASCO A, CAVANI C, et al. The impact of the current housing and husbandry systems on the health and welfare of farmed domestic rabbits[J]. Sci Rep, 2005, 267: 1-31. |
| [6] |
FUSHIMI T, INUI S, OGASAWARA M, et al. Narrow-band red LED light promotes mouse hair growth through paracrine growth factors from dermal papilla[J]. J Dermatol Sci, 2011, 64(3): 246-248. DOI:10.1016/j.jdermsci.2011.09.004 |
| [7] |
GRÉGOIRE R J, FAHMY M H, BOUCHER J M, et al. Effect of four protein supplements on growth, feed conversion, mohair production, fibre characteristics and blood parameters of Angora goats[J]. Small Rumin Res, 1996, 19(2): 121-130. DOI:10.1016/0921-4488(95)00723-7 |
| [8] |
国家技术监督局. GB/T13835.4-92免毛回潮率试验方法烘箱法[S]. 北京: 中国标准出版社, 1993. The State Bureau of Quality and Technical Supervision. GB/T13835.4-92 Test method for moisture regain of angora rabbit hair—Drying oven method[S]. Beijing: Standards Press of China, 1993. (in Chinese) |
| [9] |
LI Y H, ZHANG K, YE J X, et al. Wnt10b promotes growth of hair follicles via a canonical Wnt signalling pathway[J]. Clin Exp Dermatol, 2011, 36(5): 534-540. DOI:10.1111/j.1365-2230.2011.04019.x |
| [10] |
OUJI Y, YOSHIKAWA M, SHIROI A, et al. Promotion of hair follicle development and trichogenesis by Wnt-10b in cultured embryonic skin and in reconstituted skin[J]. Biochem Biophys Res Commun, 2006, 345(2): 581-587. DOI:10.1016/j.bbrc.2006.04.142 |
| [11] |
YANG M, SONG S, DONG K Z, et al. Skin transcriptome reveals the intrinsic molecular mechanisms underlying hair follicle cycling in Cashmere goats under natural and shortened photoperiod conditions[J]. Sci Rep, 2017, 7(1): 13502. DOI:10.1038/s41598-017-13986-w |
| [12] |
DEAVILLE E R, GALBRAITH H. Effect of dietary protein level and yeast culture on growth, blood prolactin and mohair fibre characteristics of British Angora goats[J]. Anim Feed Sci Technol, 1992, 38(2-3): 123-133. DOI:10.1016/0377-8401(92)90097-P |
| [13] |
ZHANG C Z, SUN H Z, LI S L, et al. Effects of photoperiod on nutrient digestibility, hair follicle activity and cashmere quality in Inner Mongolia white cashmere goats[J]. Asian-Australas J Anim Sci, 2019, 32(4): 541-547. |
| [14] |
LIU B, GAO F Q, GUO J, et al. A microarray-based analysis reveals that a short photoperiod promotes hair growth in the Arbas Cashmere goat[J]. PLoS One, 2016, 11(1): e0147124. DOI:10.1371/journal.pone.0147124 |
| [15] |
吴丽媛, 刘斌, 辛雷勇, 等. 内蒙古阿尔巴斯型绒山羊光控增绒效果分析[J]. 中国畜牧兽医, 2018, 45(7): 1972-1977. WU L Y, LIU B, XIN L Y, et al. Effect of light control on cashmere growth of Inner Mongolia Arbas Cashmere goats[J]. China Animal Husbandry & Veterinary Medicine, 2018, 45(7): 1972-1977. (in Chinese) |
| [16] |
高志光. 光照对貉冬毛生长的影响[J]. 北华大学学报: 自然科学版, 2005, 6(3): 272-274. GAO Z G. Effect of light on the fur growth of nyctereutes procyonoides in winter[J]. Journal of Beihua University: Natural Science, 2005, 6(3): 272-274. DOI:10.3969/j.issn.1009-4822.2005.03.025 (in Chinese) |
| [17] |
TSEVEENJAV B, GARRICK D J, BATJARGAL E, et al. Economic selection index to improve fiber quality in Mongolian Cashmere goats[J]. Livest Sci, 2020, 232: 103898. DOI:10.1016/j.livsci.2019.103898 |
| [18] |
SHEEN Y S, FAN S M Y, CHAN C C, et al. Visible red light enhances physiological anagen entry in vivo and has direct and indirect stimulative effects in vitro[J]. Lasers Surg Med, 2015, 47(1): 50-59. DOI:10.1002/lsm.22316 |
| [19] |
HAN L, LIU B, CHEN X Y, et al. Activation of Wnt/β-catenin signaling is involved in hair growth-promoting effect of 655-nm red light and LED in in vitro culture model[J]. Lasers Med Sci, 2018, 33(3): 637-645. DOI:10.1007/s10103-018-2455-3 |
| [20] |
王兴涛. 陕北白绒山羊光控增绒及其分子机制探究[D]. 杨凌: 西北农林科技大学, 2017. WANG X T. Study on molecular mechanism of Increasing Cashmere by light controlling[D]. Yang-ling: Northwest A & F University, 2017. (in Chinese) |
| [21] |
COELHO L A, RODRIGUES P A, NONAKA K O, et al. Annual pattern of plasma melatonin and progesterone concentrations in hair and wool ewe lambs kept under natural photoperiod at lower latitudes in the southern hemisphere[J]. J Pineal Res, 2006, 41(2): 101-107. DOI:10.1111/j.1600-079X.2006.00333.x |
| [22] |
LANSZKI J, THÉBAULT R G, ALLAIN D, et al. The effects of melatonin treatment on wool production and hair follicle cycle in Angora rabbits[J]. Anim Res, 2001, 50(1): 79-89. DOI:10.1051/animres:2001118 |
| [23] |
黄冬维, 丁海生, 赵辉玲, 等. 褪黑激素对长毛兔产毛性能及兔毛品质的影响[J]. 中国草食动物科学, 2019, 39(2): 9-12. HUANG D W, DING H S, ZHAO H L, et al. The effect of melatonin on wool production performance and wool quality in Angora rabbit[J]. China Herbivore Science, 2019, 39(2): 9-12. DOI:10.3969/j.issn.2095-3887.2019.02.003 (in Chinese) |
| [24] |
DUAN C H, XU J H, SUN C M, et al. Effects of melatonin implantation on cashmere yield, fibre characteristics, duration of cashmere growth as well as growth and reproductive performance of Inner Mongolian cashmere goats[J]. J Anim Sci Biotechnol, 2015, 6(1): 22. DOI:10.1186/s40104-015-0023-2 |
| [25] |
DUAN C H, XU J H, ZHANG Y, et al. Effects of melatonin implantation during the slow period of cashmere growth on fibre production[J]. South Afr J Anim Sci, 2016, 46(2): 214-219. DOI:10.4314/sajas.v46i2.13 |
| [26] |
CONG Y Y, DENG H W, FENG Y L, et al. Melatonin implantation from winter solstice could extend the cashmere growth phase effectively[J]. Small Rumin Res, 2011, 99(1): 48-53. DOI:10.1016/j.smallrumres.2011.03.055 |
| [27] |
YANG C H, XU J H, REN Q C, et al. Melatonin promotes secondary hair follicle development of early postnatal cashmere goat and improves cashmere quantity and quality by enhancing antioxidant capacity and suppressing apoptosis[J]. J Pineal Res, 2019, 67(1). |
(编辑 范子娟)



