2. 瑞普(保定)生物药业有限公司, 保定 071001;
3. 行唐县动物卫生监督所, 行唐 050600
2. Ringpu(Baoding) Biological Pharmaceutical Co. Ltd., Baoding 071001, China;
3. Xingtang Animal Health Supervision Institution, Xingtang 050600, China
猪流行性腹泻病毒(porcine epidemic diarrhea virus, PEDV)主要引起新生仔猪水样腹泻,致死率高达80%~100%[1]。自1973年,猪流行性腹泻在我国多有发生[2],2010年,在我国大规模暴发,给我国生猪产业带来了严重的损失[3]。目前,市面上商品化的灭活疫苗和弱毒活疫苗不够安全[4]。所以,研制一种安全且有效的新型PED疫苗就显得尤为重要。
PEDV主要编码E、S、M和N蛋白这4种结构蛋白[5]。将PEDV的S蛋白分为S1、S2两个区域,S1集中了多个中和表位,其中,包含两个短的保守的B细胞中和表位,分别为744YSNIGVCK752和756SQYGQVKI771,它们主要决定病毒的抗原性[6-8]。所以,PED亚单位疫苗多基于S1蛋白来构建。
乙肝核心抗原(hepatitis B core antigen, HBcAg)能够在酵母表达系统、大肠杆菌表达系统及杆状病毒-昆虫表达系统等多种表达系统中表达[9]。本研究选择的大肠杆菌表达系统和其他系统相比具有表达周期短、经济、易于操作和表达蛋白量高等优点[10]。当外源基因表位插入到HBcAg的主要免疫优势区(MIR)时,可在原核系统中进行表达,重组蛋白自发装配成病毒样颗粒(virus like particle, VLP),且可增强外源基因表位的免疫原性[11-12]。VLP是具备病毒的一些抗原性,但不具备感染性的能够自我组装的类病毒粒子。
因此,本研究以HBcAg作为呈现PEDV S1抗原表位的载体,构建了重组质粒,表达获得重组蛋白HBcAg-PEDV S1,通过透射电镜检测发现其可以形成VLP,这为今后研制PED的新型疫苗提供研究方法及理论依据。
1 材料与方法 1.1 材料和试剂限制性内切酶为宝生物工程(大连)有限公司产品;PEDV HB/HS株(JQ862708.1) 及大肠杆菌DH5α和BL21(DE3)感受态菌细胞由河北农业大学动物医学院动物传染病实验室保存;pcDNA3.1(+)-HBcAg(编码1—144 aa)质粒由河北农业大学王家鑫教授惠赠。
1.2 方法1.2.1 引物设计 根据GenBank中乙肝核心抗原基因序列(KC774394)及PEDV的S基因(JQ862708.1,与CV777相似性为96.9%),分别设计引物P1、P2及P5并添加相应酶切位点及终止密码子;设计引物P3-R重叠S1的18个碱基,设计引物P4-F重叠HBcAg的17个碱基(表 1)。
|
|
表 1 构建重组质粒的引物 Table 1 Primers for constructing recombinant plasmid |
1.2.2 重组质粒的构建 用引物P3、P4通过融合PCR扩增HBcAg(编码1—74 aa)-S1片段;用引物P5进行PCR扩增HBcAg(编码83—144 aa)片段;回收目的片段进行连接转化并且测序正确后,提取质粒。对上述两个质粒用HindⅢ和XhoⅠ进行双酶切并连接。取连接完成的重组质粒pET-32a(+)-HBcAg-PEDV S1,用EcoR Ⅰ和XhoⅠ进行双酶切鉴定, 再送生工生物工程(上海)股份有限公司测序。
1.3 目的蛋白的表达及纯化将测序正确的重组质粒pET-32a(+)-HBcAg- PEDV S1转化至大肠杆菌BL21(DE3)中,表达并纯化重组蛋白,SDS-PAGE检测。
1.4 重组蛋白的复性用尿素浓度梯度法透析法复性重组蛋白,检测复性后的蛋白浓度,SDS-PAGE检测。
1.5 透射电镜检测HBcAg-PEDV S1病毒样颗粒结构将复性蛋白浓度调整至200 μL·mL-1,取蛋白样品滴于铜网上,滴加2%的磷钨酸负染液,自然干燥后,将样品置于透射电子显微镜下观察样品形态。
2 结果 2.1 重组质粒pET-32a(+)-HBcAg-PEDV S1的鉴定用EcoR Ⅰ和Xho Ⅰ对pET-32a(+)-HBcAg- PEDV S1重组质粒进行双酶切鉴定,经琼脂糖凝胶电泳检测酶切产物,可见702 bp的目的条带,对重组质粒的测序结果与目的序列进行比对,结果显示, 与预期完全相符,表明成功构建重组质粒。
2.2 重组蛋白HBcAg-PEDV S1的纯化和复性SDS-PAGE结果(图 1)显示,重组蛋白HBcAg-PEDV S1通过纯化和复性后, 可见43.1 ku目的蛋白;复性蛋白浓度约为1.5 mg·mL-1。

|
M.蛋白相对分子质量标准;1.pET-32a(+)空载体对照;2.纯化蛋白;3.复性蛋白 M. Protein marker; 1. pET-32a(+) null vector; 2. Purified protein; 3. Renatured protein 图 1 重组蛋白HBcAg-PEDV S1纯化和复性的SDS-PAGE检测 Fig. 1 Purification and renaturation of recombinant HBcAg-PEDV S1 by SDS-PAGE |
透射电镜结果显示,可看到大小均匀的粒子形成,并且直径在30 nm左右,与预期相符(图 2)。
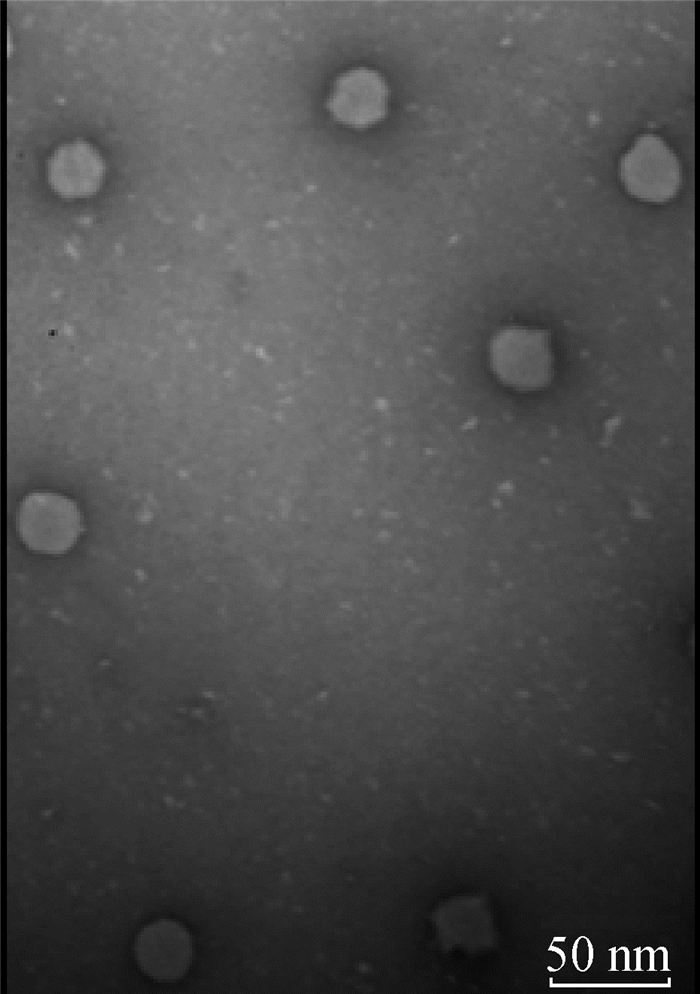
|
图 2 HBcAg-PEDV S1 VLP的透射电镜观察 Fig. 2 Transmission electron microsecope of HBcAg-PEDV S1 VLPs |
目前, 国内控制PEDV的主要手段是为猪群接种传统灭活疫苗和弱毒活疫苗。而传统灭活疫苗多选用经典毒株,这造成疫苗对各地的猪群免疫保护能力参差不齐;而弱毒活疫苗虽然免疫效果远优于传统灭活疫苗,但其研发成本较高、周期较长,并且基于目前的研究和临床观察发现,有弱毒活疫苗与流行毒株基因重组的现象发生[13-14],这就要求对其进行进一步研究,研发有效且安全的PED新型疫苗。
以HBcAg为载体可用于新型疫苗的研发,在美国等国家允许应用于疫苗中[15]。近期在研发PED的亚单位疫苗中,以基因重组HBcAg与PEDV S蛋白上的B细胞表位构建了VLP,并且可在小鼠体内诱导产生特异性抗体,证明成功构建PED的基因工程疫苗[16-17]。与其相比,本研究优势在于利用融合PCR将其B细胞表位插入到HBcAg中,而不是直接由生物公司合成,降低成本的同时,可以达到相同的免疫效果。
VLP能够自发装配成类病毒粒子,具备病毒的一些抗原性,但不具备感染性[18]。1986年,乙肝表面抗原(HBsAg)作为第一种基于病毒样颗粒的商业疫苗被批准上市[19]。大肠杆菌表达系统是第一个用于生产VLPs的系统,已经生产了几种商业VLPs疫苗,例如,抗戊型肝炎病毒VLPs疫苗[20]。
PEDV S1区包含多个主要B细胞表位和受体结合域,与病毒抗原性和吸附入侵密切相关。其主要的两个B细胞表位为744YSNIGVCK752、756SQYGQVKI771[21]。据此,本研究通过设计融合PCR[22]将PEDV S1的B细胞表位插入到HBcAg中,成功构建了pET-32a(+)-HBcAg-PEDV S1重组质粒。将其诱导表达、纯化并复性,通过透射电镜检测到形成VLPs,表明利用大肠杆菌表达系统成功表达了PEDV S1 VLPs。本研究为后续PED疫苗的研制提供了进一步的参考。
4 结论作者将PEDV S1中含B细胞表位的270 bp片段插入到HBcAg的MIR区域,利用大肠杆菌表达系统表达其重组蛋白,经过透射电镜检测到成功形成HBcAg-PEDV S1 VLPs,为今后PED疫苗的研究提供了进一步的理论依据。
| [1] | STEVENSON G W, HOANG H, SCHWARTZ K J, et al. Emergence of porcine epidemic diarrhea virus in the United States: clinical signs, lesions, and viral genomic sequences[J]. J Vet Diagn Invest, 2013, 25(5): 649–654. DOI: 10.1177/1040638713501675 |
| [2] | LIN C M, SAIF L J, MARTHALER D, et al. Evolution, antigenicity and pathogenicity of global porcine epidemic diarrhea virus strains[J]. Virus Res, 2016, 226: 20–39. DOI: 10.1016/j.virusres.2016.05.023 |
| [3] | WANG D, FANG L R, XIAO S B. Porcine epidemic diarrhea in China[J]. Virus Res, 2016, 226: 7–13. DOI: 10.1016/j.virusres.2016.05.026 |
| [4] | SEKHON S S, NGUYEN P L, AHN J Y, et al. Porcine epidemic diarrhea (PED) infection, diagnosis and vaccination: A mini review[J]. Toxicol Environ Health Sci, 2016, 8(5): 277–289. DOI: 10.1007/s13530-016-0287-8 |
| [5] | SONG D, PARK B. Porcine epidemic diarrhoea virus: a comprehensive review of molecular epidemiology, diagnosis, and vaccines[J]. Virus Genes, 2012, 44(2): 167–175. DOI: 10.1007/s11262-012-0713-1 |
| [6] | LEE D K, PARK C K, KIM S H, et al. Heterogeneity in spike protein genes of porcine epidemic diarrhea viruses isolated in Korea[J]. Virus Res, 2010, 149(2): 175–182. DOI: 10.1016/j.virusres.2010.01.015 |
| [7] | WICHT O, LI W, WILLEMS L, et al. Proteolytic activation of the porcine epidemic diarrhea coronavirus spike fusion protein by trypsin in cell culture[J]. J Virol, 2014, 88(14): 7952–7961. DOI: 10.1128/JVI.00297-14 |
| [8] | SUN D B, FENG L, SHI H Y, et al. Spike protein region (aa 636789) of porcine epidemic diarrhea virus is essential for induction of neutralizing antibodies[J]. Acta Virol, 2007, 51(3): 149–156. |
| [9] | SCHÖDEL F, PETERSON D, MILICH D. Hepatitis B virus core and e antigen: immune recognition and use as a vaccine carrier moiety[J]. Intervirology, 1996, 39(1-2): 104–110. DOI: 10.1159/000150481 |
| [10] | LEE P S, LEE K H. Escherichia coli-a model system that benefits from and contributes to the evolution of proteomics[J]. Biotechnol Bioeng, 2003, 84(7): 801–814. DOI: 10.1002/bit.10848 |
| [11] | LIN L C, LEE T H, CHANG C H, et al. Predictors of clinical deterioration during hospitalization following acute ischemic stroke[J]. Eur Neurol, 2012, 67(3): 186–192. DOI: 10.1159/000334723 |
| [12] | SCHÖDEL F, PETERSON D, HUGHES J, et al. Hybrid hepatitis B virus core antigen as a vaccine carrier moiety: I. presentation of foreign epitopes[J]. J Biotechnol, 1996, 44(1-3): 91–96. DOI: 10.1016/0168-1656(95)00118-2 |
| [13] | LI W, LI H, LIU Y, et al. New variants of porcine epidemic diarrhea virus, China, 2011[J]. Emerg Infect Dis, 2012, 18(8): 1350–1353. DOI: 10.3201/eid1803.120002 |
| [14] | LI R F, QIAO S L, YANG Y Y, et al. Genome sequencing and analysis of a novel recombinant porcine epidemic diarrhea virus strain from Henan, China[J]. Virus Genes, 2016, 52(1): 91–98. DOI: 10.1007/s11262-015-1254-1 |
| [15] | SCHÖDEL F, KELLY S M, PETERSON D, et al. Development of recombinant Salmonellae expressing hybrid hepatitis B virus core particles as candidate oral vaccines[J]. Dev Biol Stand, 1994, 82: 151–158. |
| [16] | GILLAM F, ZHANG J, ZHANG C. Hepatitis B core antigen based novel vaccine against porcine epidemic diarrhea virus[J]. J Virol Methods, 2018, 253: 61–69. DOI: 10.1016/j.jviromet.2017.11.003 |
| [17] | GILLAM F, ZHANG C. Epitope selection and their placement for increased virus neutralization in a novel vaccination strategy for porcine epidemic diarrhea virus utilizing the Hepatitis B virus core antigen[J]. Vaccine, 2018, 36(30): 4507–4516. DOI: 10.1016/j.vaccine.2018.06.015 |
| [18] | ROLDÃO A, MELLADO M C M, CASTILHO L R, et al. Virus-like particles in vaccine development[J]. Expert Rev Vaccines, 2010, 9(10): 1149–1176. DOI: 10.1586/erv.10.115 |
| [19] | MICHEL M L, TIOLLAIS P. Hepatitis B vaccines: protective efficacy and therapeutic potential[J]. Pathol Biol (Paris), 2010, 58(4): 288–295. DOI: 10.1016/j.patbio.2010.01.006 |
| [20] | DENNER J. Hepatitis E virus (HEV)-The future[J]. Viruses, 2019, 11(3): 251. DOI: 10.3390/v11030251 |
| [21] | WANG K, LU W, CHEN J, et al. PEDV ORF3 encodes an ion channel protein and regulates virus production[J]. FEBS Lett, 2012, 586(4): 384–391. DOI: 10.1016/j.febslet.2012.01.005 |
| [22] | LIAO L B, YANG L, XU Y X, et al. Fusion-PCR generates attL recombination site adaptors and allows rapid one-step gateway (ROG) cloning[J]. Biochimie, 2020, 174: 69–73. DOI: 10.1016/j.biochi.2020.04.002 |



