microRNAs(miRNAs)是一类由约22个核苷酸组成的短链非编码RNA分子,其通过自身种子序列与靶基因特定位点进行碱基配对结合[1-2],广泛参与动物卵巢类固醇激素合成与分泌的调控过程[3-5]。
近年来,大量研究已报道,miRNAs能调节卵巢颗粒细胞类固醇激素的生物合成[6-8]。研究发现,miR-15a参与了人卵巢颗粒细胞中黄体酮、雄激素和雌激素的释放,并且在过表达miR-15a后可以促进孕酮和睾酮生成[9]。Zhang等[10]发现,在FSH处理后的牛颗粒细胞中,miR-31和miR-143通过靶向FSHR基因抑制孕酮的分泌。在猪颗粒细胞中,miR-378通过靶向芳香化酶的3′UTR来调节卵巢中雌二醇的分泌[4]。而在小鼠颗粒细胞中,miR-383可以靶向RBMS1促进雌二醇的代谢[5]。这些结果表明,miRNAs在哺乳动物卵巢颗粒细胞的类固醇激素合成与分泌方面有重要调控作用。相比miRNAs在哺乳动物中的研究,其在禽类卵巢中的研究大多集中在对卵巢细胞增殖和凋亡的调控。比如,Kang等[11]研究表明,miR-26a-5p通过靶向TNRC6A基因促进鸡卵巢卵泡膜细胞的增殖。miR-26b-5p受到转录因子SMAD4的调控,靶向INHBB介导TGF-β/SMADs信号通路的表达,从而促进鹅卵泡颗粒细胞的增殖[12]。此外,鹅颗粒细胞的增殖和凋亡也受miR-181a-5p的调控,其通过靶向SIRT1参与FOXO信号通路[13]。然而,miRNAs对禽类卵泡颗粒细胞的类固醇激素的合成与分泌的调控机制还未见报道。
过氧化物酶体增殖物激活型受体(peroxisome proliferator activated receptors,PPARs)是核受体超家族的配体依赖型转录因子,主要包括α、β、γ三种亚型[14-15]。其中,PPARγ参与调节卵巢的卵泡发育、排卵及卵子的质量和类固醇激素(雌激素和孕激素)的分泌[16-17]。Kim等[16]研究表明,PPARγ通过调控孕激素受体参与小鼠颗粒细胞类固醇激素的合成。然而,PPARγ基因是否影响鹅卵巢颗粒细胞的生物学功能目前尚不清楚。
本课题组前期转录组测序发现,miR-148a-3p在鹅4~6 mm、8~10 mm和F5卵泡颗粒层中的表达逐渐升高。因此,本研究首先检测miR-148a-3p在鹅不同阶段卵泡颗粒层中的表达,然后在体外培养的鹅卵泡颗粒细胞中通过转染mimics和inhibitor模拟miR-148a-3p的过表达和抑制,利用qPCR法检测与类固醇激素合成分泌相关基因的表达变化,接着通过双荧光素酶报告系统验证靶标关系,最后干扰靶基因检测miR-148a-3p对孕酮合成变化的影响,为进一步阐明miR-148a-3p在禽类颗粒细胞中的功能提供依据。
1 材料与方法 1.1 试验动物和样品采集本试验共选用12只天府肉鹅母系母鹅,其中,6只进行组织样品采集,6只进行颗粒细胞的分离培养。所选用的试验鹅均由四川农业大学家禽育种场提供,试验鹅在相同的条件下饲养,开产时间和体重基本一致。鹅麻醉致死后,迅速取出整个卵巢,用游标卡尺测量不同直径大小的卵泡(2~4 mm、4~6 mm、6~8 mm、8~10 mm、F5、F4、F3、F2、F1),根据甘翔等[18]的方法进一步分离不同阶段卵泡颗粒层,PBS漂洗3~4次,将颗粒层附着的卵黄清洗干净后,剪碎并置于-80 ℃冻存,用于RNA提取。
1.2 miR-148a-3p在不同物种中的保守性分析在miRBase(http://www.Mirbase.org/)数据库中在线检索人(Homo sapiens, GenBank_No.:NC_000003.12)、小鼠(Mus musculus, GenBank_No.:NC_000072.6)、鸡(Gallus gallus, GenBank_No.:NC_006099.5)、猪(Sus scrofa, GenBank_No.:NC_010455.5)、牛(Bos Taurus, GenBank_No.:NC_037349.1)、恒河猴(Macaca mulatta, GenBank_No.:NC_041755.1)、绿蜥蜴(Anolis carolinensis, GenBank_No.:NC_014777.1)、大西洋鲑鱼(Salmo salar, GenBank_No.:NC_027314.1)8个物种的miR-148a-3p成熟体序列,鹅的miR-148a-3p成熟体序列由课题组前期miRNA测序所得,采用MEGA 7进行保守性分析。
1.3 鹅等级卵泡颗粒细胞分离培养与转染参考Deng等[19]的方法进行鹅等级卵泡颗粒细胞的体外培养,待细胞汇合度为70%~80%时,根据LipofectamineⓇ 3000(Invitrogen,USA)试剂使用说明,先将lipofectamine 3000分别和miR-148a-3p mimics、mimics NC、inhibitor、inhibitor NC、Control在DMEM/F12(HyClone,USA)培养基内形成脂质体复合物,然后共转染到颗粒细胞中,在含5% CO2的37 ℃培养箱中培养。转染后6 h对细胞状态进行一次观察,转染24 h后收取上清液和细胞用于后续的检测。miR-148a-3p mimics、mimics NC、inhibitor、inhibitor NC由上海吉玛制药技术有限公司合成(表 1)。
|
|
表 1 miR-148a-3p mimics和inhibitor合成序列 Table 1 The sequences of miR-148a-3p mimics and inhibitor |
利用RNAiso Plus(TaKaRa, China)提取鹅卵泡颗粒层组织和细胞的总RNA,利用Nano Drop 2000紫外分光光度计检测总RNA的浓度和纯度,使用Prime ScripTM RT reagent Kit(TaKaRa, China) 进行mRNA反转录,按照上海吉玛制药技术有限公司的miRNA反转录试剂盒反转录miRNA,反应结束后置于-20 ℃保存备用。
1.5 qPCR检测miR-148a-3p及关键基因的表达利用课题组前期对鹅颗粒细胞miRNA测序得到的miR-148a-3p序列,由上海吉玛制药技术有限公司合成miR-148a-3p定量检测试剂盒,其他mRNAs定量检测引物由华大基因科技有限公司合成(表 2)。使用SYBRⓇ Premix Ex TaqTM II(TaKaRa, China) 检测mRNAs表达量,mRNAs反应体系为:SYBRⓇ Premix Ex TaqTM II 6.25 μL,上、下游引物各0.5 μL,模板1 μL,无菌水4.25 μL,总反应体系为12.5 μL。反应条件:95 ℃ 10 min;95 ℃ 30 s,退火温度30 s,72 ℃延伸30 s,72 ℃ 10 min, 总共40个循环;设置熔解曲线为65~95 ℃,每5 s增加0.5 ℃,以检测引物特异性。使用miR-148a-3p定量检测试剂盒检测miR-148a-3p的表达量,miR-148a-3p的反应体系为:2×Real-time PCR Master Mix (SYBR) 10 μL,上、下游引物各0.4 μL,Taq DNA polymerase 0.2 μL,模板2 μL,无菌水7 μL,总反应体系为20 μL。反应条件为:95 ℃ 3 min;95 ℃ 12 s,退火温度40 s,共40个循环。每个样本设3个技术重复,miRNA表达量计算以U6为内参,mRNAs表达量计算以GAPDH和β-actin为内参。
|
|
表 2 用于qPCR的引物 Table 2 Primers used for qPCR |
通过miRecords_version4、miRTarBase、Tarbase 7.0等数据库中收录的经试验验证的miR-148a-3p靶基因,综合TragetScan 7.1、miRanda、miRWalk 2.0、DIANA-microT等在线软件预测的结果发现,PPARγ-3′UTR与miR-148a-3p种子序列存在结合位点(图 1)。根据鹅PPARγ-3′UTR序列设计含结合位点在内的扩增引物(表 3),将所得到的PPARγ-3′UTR目的片段和pmir GLO空载质粒(Promega,USA)分别用Sac Ⅰ和Xba Ⅰ进行双酶切,酶切反应完毕后进行纯化回收。将经过切胶纯化后的目的片段和已切开的pmir GLO(Promega,USA)空载质粒进行连接,构建野生型靶标验证载体PGL/PPARγ-WT。将野生型载体的潜在结合位点突变为其互补序列(图 1),从而得到突变型重组载体即PGL/PPARγ-Mut,突变载体由上海吉玛制药技术有限公司完成。

|
黑色加粗字体为种子序列和突变序列 Seed sequences and mutation sequences are showed in black bold 图 1 PPARγ 基因3′UTR与miR-148a-3p的结合位点 Fig. 1 The target site of miR-148a-3p with the 3′UTR of the PPARγ mRNA transcript |
|
|
表 3 PPARγ-3′UTR序列扩增引物 Table 3 Primer sequence used for PPARγ-3′UTR amplification |
使用中国仓鼠卵巢细胞系(CHO,中国科学院昆明动物研究所)用于双荧光素酶报告系统检测。CHO细胞系复苏后接种于培养皿中,待细胞长满后用胰酶消化,然后用含10% FBS(Gibco, USA)的DMEM/F12(HyClone, USA)培养基重悬细胞,以4×106个·mL-1的密度接种于96孔板。待细胞密度达到约60%~70%时,按照LipofectamineⓇ 3000(Invitrogen,USA)说明书将miR-148a-3p mimics分别与PGL/PPARγ-WT或PGL/PPARγ-Mut两两进行共转染,每个处理设置3孔重复。转染后24 h使用Dual-LuciferaseⓇ Reporter试剂盒(Promega, China)检测细胞荧光素酶活性。以萤火虫荧光素酶作为试验报告基因,海肾荧光素酶作为对照报告基因,二者的比值即为相对荧光素活性。
1.8 miR-148a-3p靶基因的干扰从NCBI获取鹅PPARγ CDS区预测序列,由上海吉玛制药技术有限公司构建si-PPARγ,序列见表 4。结合LipofectamineⓇ 3000(Invitrogen,USA)试剂使用说明,使用12孔板转染siRNA及其NC,转染24 h后收集样品进行关键基因表达量的检测及激素含量的检测。
|
|
表 4 PPARγ干扰序列 Table 4 The sequences of si-PPARγ |
细胞转染24 h后收取细胞上清液,根据ELISA试剂盒(上海恒远科技有限公司,中国)说明书在板条标准品孔内加50 μL不同浓度标准品,样本孔加10 μL待测样本和40 μL稀释液,空白孔不加,其余孔中加入100 μL辣根过氧化物酶(HRP) 标记的抗体,封板膜密封,37 ℃恒温箱温育60 min;弃去液体,纸上拍干,每孔加满洗涤液,静置1 min,甩去洗涤液,纸上拍干,重复洗板5次;每孔各加入50 μL底物A、B,37 ℃避光孵育15 min;每孔加入50 μL终止液混匀,15 min内在450 nm波长处检测各孔OD值。在Excel中,以标准品浓度为横坐标,OD值为纵坐标,绘制出标准品线性回归曲线,按曲线方程计算各样本浓度值,从而得到不同处理组中孕酮含量。
1.10 数据的统计与分析采用SPSS 19.0软件对试验数据进行单因素方差分析,组间差异显著性检验采用独立样本t检验进行分析,利用公式2-△△Ct计算所选目标基因的相对表达量[20]。P < 0.05表示具有统计学意义,数据用“平均值±标准误”表示。
2 结果 2.1 miR-148a-3p在不同物种中的保守性分析通过miRBase数据库下载不同物种的miR-148a-3p的成熟体序列,比对不同物种的成熟体序列,结果发现,miR-148a-3p的成熟体序列在各个物种中高度保守,其核心的种子序列基本一致(表 5)。
|
|
表 5 miR-148a-3p序列保守性分析 Table 5 Sequence conservation analysis of miR-148a-3p |
结果如图 2所示,随着卵泡直径的增加,miR-148a-3p的表达量呈现先升高后下降的趋势,且在等级卵泡F4颗粒层中表达最高。此外,miR-148a-3p在等级阶段卵泡颗粒层的表达也高于等级前卵泡(图 2),因此,选择等级卵泡颗粒细胞用于后续的细胞试验。
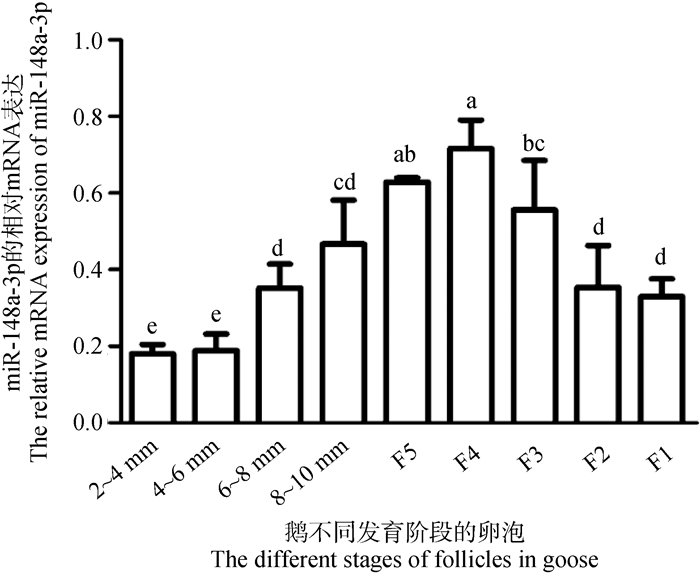
|
不同小写字母表示差异显著(P < 0.05),下同 The different lowercase letters represent the significant differences(P < 0.05), the same as below 图 2 miR-148a-3p在鹅不同阶段卵泡颗粒层中的表达 Fig. 2 The expression of miR-148a-3p in granulosa layer at different stages of goose |
为了探究miR-148a-3p对鹅卵泡颗粒细胞功能的影响,在鹅等级卵泡颗粒细胞中转染miR-148a-3p mimics和inhibitor。定量结果显示,与mimics NC组相比,miR-148a-3p mimics能显著下调3β-HSD表达(图 3A, P < 0.05),而与inhibitor NC组相比,miR-148a-3p inhibitor能显著上调3β-HSD表达(图 3C, P < 0.05)。同时,ELISA结果显示,与mimics NC组相比,miR-148a-3p mimics能显著抑制孕酮的合成(图 3B, P < 0.05),而与inhibitor NC组相比,miR-148a-3p inhibitor能显著促进孕酮的合成(图 3D, P < 0.05)。由此可知,miR-148a-3p能抑制鹅等级卵泡颗粒细胞中孕酮的合成。
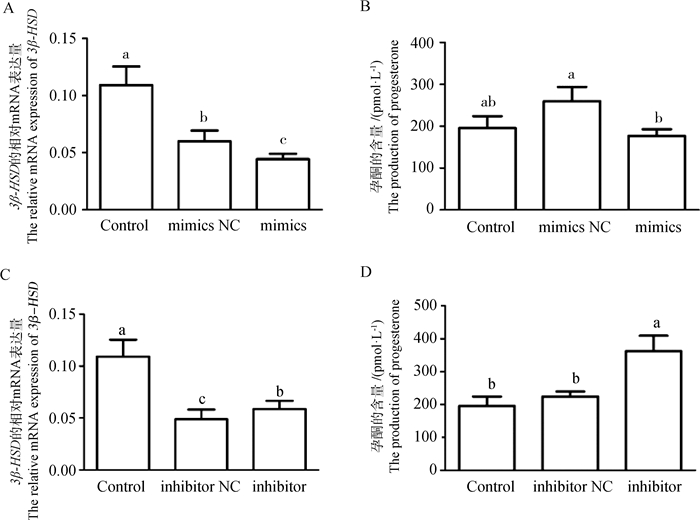
|
A. miR-148a-3p mimics对3β-HSD的mRNA表达的影响;B. miR-148a-3p mimics对孕酮合成的影响;C. miR-148a-3p inhibitor对3β-HSD的mRNA表达的影响;D. miR-148a-3p inhibitor对孕酮合成的影响。NC. 阴性对照组;mimics. miR-148a-3p mimics;inhibitor. miR-148a-3p inhibitor;Control. 空白对照组 A. The effect of miR-148a-3p mimics on the mRNA expression of 3β-HSD; B. The effect of miR-148a-3p mimics on the production of progesterone; C. The effect of miR-148a-3p inhibitor on the mRNA expression of 3β-HSD; D. The effect of miR-148a-3p inhibitor on the production of progesterone. NC. Negative control; mimics. miR-148a-3p mimics; inhibitor. miR-148a-3p inhibitor; Control. Blank control group 图 3 miR-148a-3p对3β-HSD的表达及孕酮合成的影响 Fig. 3 The effect of miR-148a-3p on the expression of 3β-HSD and the progesterone production |
为了进一步探究miR-148a-3p抑制鹅卵泡颗粒细胞孕酮合成的分子机制,在CHO细胞系中共转染miR-148a-3p mimics与野生型载体PGL/PPARγ-WT或突变型载体PGL/PPARγ-Mut,采用双荧光素酶报告系统检测双荧光素酶的活性。结果显示,当miR-148a-3p与PPARγ的结合位点突变后,双荧光素酶活性显著升高(图 4,P < 0.05),证实PPARγ是miR-148a-3p的靶基因。
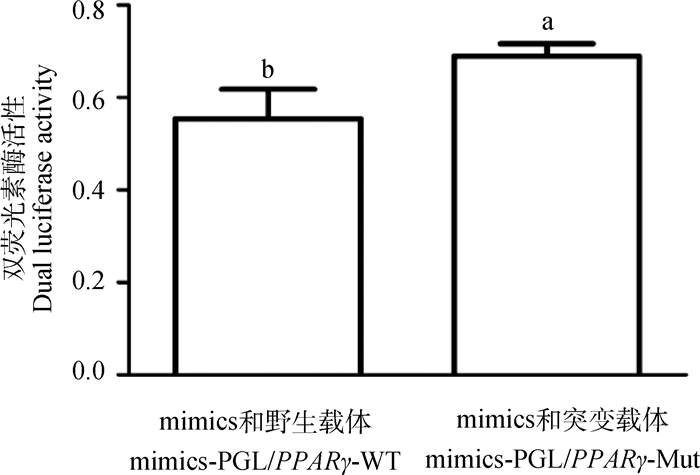
|
图 4 miR-148a-3p与PPARγ靶标关系的双荧光素检测 Fig. 4 Double luciferase reporter assay of targeting relationship between miR-148a-3p and PPARγ |
为了探究miR-148a-3p对靶基因的转录调控的影响,在鹅卵泡颗粒细胞中转染miR-148a-3p mimics和miR-148-3p inhibitor后检测靶基因PPARγ的mRNA表达变化。结果如图 5所示,与mimics NC组相比,miR-148a-3p mimics能显著降低鹅颗粒细胞中PPARγ的表达(图 5A, P < 0.05)。与inhibitor NC组相比,miR-148a-3p inhibitor能显著上调PPARγ的表达(图 5B, P < 0.05)。由此可知,miR-148a-3p能调控靶基因PPARγ的转录水平。
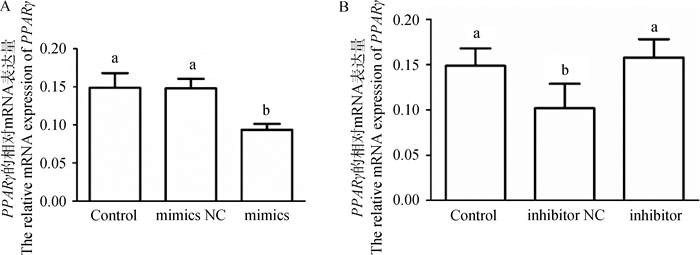
|
A. miR-148a-3p mimics对PPARγ的mRNA表达的影响;B. miR-148a-3p inhibitor对PPARγ的mRNA表达的影响 A. The effect of miR-148a-3p mimics on the mRNA expression of PPARγ; B. The effect of miR-148a-3p inhibitor on the mRNA expression of PPARγ 图 5 miR-148a-3p mimics和inhibitor对鹅卵泡颗粒细胞中PPARγ表达的影响 Fig. 5 Effects of miR-148a-3p mimics and inhibitor on the expression of PPARγ in goose granulosa cells |
为了探究miR-148a-3p是否会通过其靶基因的转录调控影响鹅卵泡颗粒细胞的激素合成,在颗粒细胞中干扰PPARγ后检测关键基因的表达变化及孕酮含量。结果如图 6所示,与对照组和si-PPARγ NC组相比,干扰PPARγ基因后,3β-HSD的表达量显著降低(图 6A, P < 0.05),孕酮的含量也降低,但差异不显著(图 6B, P>0.05)。由此可知,鹅颗粒细胞中孕酮的含量受PPARγ的转录调控。

|
A.干扰PPARγ对3β-HSD的mRNA表达的影响;B.干扰PPARγ对孕酮合成的影响 A. The effect of PPARγ interference on the mRNA expression of 3β-HSD; B. The effect of PPARγ interference on the progesterone production 图 6 干扰PPARγ对颗粒细胞孕酮分泌的影响 Fig. 6 The effect of PPARγ interference on the progesterone production in granulosa cells |
miR-148a-3p的序列已经在多个物种中被鉴定[21],而在鹅上,miR-148a-3p的序列是通过前期转录组测序获得。本研究通过对多个物种的序列进行保守性比对分析发现,miR-148a-3p在不同物种中高度保守,推测miR-148a-3p在不同的物种中可能具有类似的功能,但关于miR-148a-3p调控鹅卵泡颗粒细胞类固醇激素分泌的相关研究未见报道。
目前的研究发现,miR-148a-3p在许多生理过程中均发挥重要作用,比如免疫、肿瘤和癌症[22-23],骨骼肌细胞的分化、增殖和凋亡以及脂肪细胞的增殖分化[24-26]。本研究中,miR-148a-3p在鹅不同发育阶段的卵泡颗粒层中均有表达,且随着卵泡直径的增加,其表达量呈现先升高后下降的趋势,在F4、F5阶段卵泡达到顶峰。F4、F5阶段卵泡为等级前卵泡发育到等级卵泡的过渡时期[27],推测miR-148a-3p可能在卵泡发育或卵泡选择过程中有重要作用,这也表明,miR-148a-3p也参与禽类的繁殖生理过程。进一步体外培养鹅颗粒细胞,转染miR-148a-3p mimics能显著下调3β-HSD表达,抑制孕酮的合成;而转染miR-148a-3p inhibitor能显著上调3β-HSD表达,促进孕酮的合成。3β-HSD是孕酮合成的关键基因[28],禽类卵泡颗粒层中3β-HSD的表达量随着卵泡的发育逐渐升高[29-30]。这些结果表明,miR-148a-3p可以抑制3β-HSD的表达降低孕酮的合成,进而影响鹅卵泡的选择和发育。miRNAs主要通过与靶基因的3′-UTR结合调控靶基因表达继而影响靶细胞的生物学功能[1]。研究表明,在食管癌中,miR-148a-3p通过靶向DNA甲基转移酶1(DNA (cytosine-5)-methyltransferase 1, DNMT1)调控癌细胞的增殖和入侵[31]。在人卵巢癌细胞中,miR-148a-3p通过靶向络氨酸蛋白激酶Met(tyrosine-protein kinase Met, c-Met)抑制癌细胞的增殖[32]。而在正常的细胞中,如C2C12肌细胞系和原代老鼠细胞中,miR-148a能直接通过靶向含蛋白激酶1的Rho相关螺旋线圈(Rho-associated coiled-coil containing protein kinase 1, ROCK1)的3′UTR来促进成肌细胞分化[33]。在牛的骨骼肌细胞中,miR-148a-3p通过转录后水平下调类Krüppel因子6(Krüppel-like factor 6, KLF6)的表达抑制成肌细胞的增殖,促进其凋亡[34]。而在鸡的骨骼肌卫星细胞中,miR-148a-3p通过直接靶向间质同源框2(mesenchyme homeobox 2, Meox2)来促进细胞的分化,抑制凋亡,但不影响增殖,同时能增加PI3K/AKT信号通路[35]。本课题组前期通过靶基因在线预测软件发现,miR-148a-3p与PPARγ 3′-UTR存在潜在结合位点,双荧光素酶报告载体试验结果显示,miR-148a-3p可以直接靶向结合PPARγ调控鹅颗粒细胞的生理功能。进一步检测miR-148a-3p对PPARγ表达量的影响时发现,转染miR-148a-3p mimics能显著降低鹅颗粒细胞中PPARγ表达,而转染miR-148a-3p inhibitor后,PPARγ的表达显著升高。研究发现,PPARγ可以影响大鼠[36]、猪[37]、绵羊[38]、牛[39]卵巢颗粒细胞产生类固醇激素。Komar[40]研究表明,PPARγ可以引起人孕酮分泌量的改变。本研究中,干扰PPARγ能显著降低3β-HSD表达水平和孕酮的含量。这些结果就表明,在鹅卵泡颗粒细胞中,miR-148a-3p通过靶向PPARγ来抑制3β-HSD的表达,进而降低孕酮的合成。
4 结论本研究发现,miR-148a-3p在鹅等级卵泡颗粒细胞中通过靶向结合PPARγ来抑制3β-HSD的表达,从而降低孕酮的合成,初步探讨了miR-148a-3p对禽类卵泡颗粒细胞孕酮合成的分子机制,为深入探究miRNA在禽类卵巢颗粒细胞中的功能提供理论依据。
| [1] | BARTEL D P. MicroRNAs: Target recognition and regulatory functions[J]. Cell, 2009, 136(2): 215–233. DOI: 10.1016/j.cell.2009.01.002 |
| [2] | KLINGE C M. miRNAs regulated by estrogens, tamoxifen, and endocrine disruptors and their downstream gene targets[J]. Mol Cell Endocrinol, 2015, 418(03): 273–297. |
| [3] | ROBINSON C L, ZHANG L, SCHVTZ L F, et al. MicroRNA 221 expression in theca and granulosa cells: hormonal regulation and function 1[J]. J Anim Sci, 2018, 96(2): 641–652. DOI: 10.1093/jas/skx069 |
| [4] | XU S, KATJA L M, YANG B B, et al. Micro-RNA378 (miR-378) regulates ovarian estradiol production by targeting aromatase[J]. Endocrinology, 2011, 152(10): 3941–3951. DOI: 10.1210/en.2011-1147 |
| [5] | YIN M, LV M, YAO G, et al. Transactivation of microRNA-383 by steroidogenic factor-1 promotes estradiol release from mouse ovarian granulosa cells by targeting RBMS1[J]. Mol Endocrinol, 2012, 26(7): 1129–1143. DOI: 10.1210/me.2011-1341 |
| [6] | NAN Y, YANG B Q, YU L, et al. Follicle-stimulating hormone regulation of microRNA expression on progesterone production in cultured rat granulosa cells[J]. Endocrine, 2010, 38(2): 158–166. DOI: 10.1007/s12020-010-9345-1 |
| [7] | DONADEU F X, SCHAUER S N, SONTAKKE S D. Involvement of miRNAs in ovarian follicular and luteal development[J]. J Endocrinol, 2012, 215(3): 323–334. DOI: 10.1530/JOE-12-0252 |
| [8] | HU Z, SHEN W J, YUAN C, et al. Hormonal regulation of microRNA expression in steroid producing cells of the ovary, testis and adrenal gland[J]. PLoS One, 2013, 8(10): e78040. DOI: 10.1371/journal.pone.0078040 |
| [9] | SIROTKIN A V, KISOVÁ G, BRENAUT P, et al. Involvement of microRNA Mir15a in control of human ovarian granulosa cell proliferation, apoptosis, steroidogenesis, and response to FSH[J]. Microrna, 2014, 3(1): 29–36. DOI: 10.2174/2211536603666140227232824 |
| [10] | ZHANG Z, CHEN C Z, XU M Q, et al. MiR-31 and miR-143 affect steroid hormone synthesis and inhibit cell apoptosis in bovine granulosa cells through FSHR[J]. Theriogenology, 2019, 123: 45–53. DOI: 10.1016/j.theriogenology.2018.09.020 |
| [11] | KANG L, YANG C, WU H, et al. MiR-26a-5p regulates TNRC6A expression and facilitates theca cell proliferation in chicken ovarian follicles[J]. DNA Cell Biol, 2017, 36(11): 922–929. DOI: 10.1089/dna.2017.3863 |
| [12] |
路璐. miR-26b-5p调控鹅卵泡颗粒细胞增殖作用机制研究[D]. 扬州: 扬州大学, 2019.
LU L. Mechanism of miR-26b-5p regulating the proliferation of follicular granulosa cells in goose[D]. Yangzhou: Yangzhou University, 2019. (in Chinese) |
| [13] |
莫远亮. miR-181a-5p靶基因预测及其在鹅颗粒细胞增殖凋亡中的作用研究[D]. 成都: 四川农业大学, 2018.
MO Y L. Target gene prediction of miR-181a-5p and its role in proliferation and apoptosis of goose granulosa cells[D]. Chengdu: Sichuan Agricultural University, 2018. (in Chinese) |
| [14] | ISSEMANN I, GREEN S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators[J]. Nature, 1990, 347(6294): 645–650. DOI: 10.1038/347645a0 |
| [15] | MIRZA A Z, ALTHAGAFI I I, SHAMSHAD H. Role of PPAR receptor in different diseases and their ligands: Physiological importance and clinical implications[J]. Eur J Med Chem, 2019, 166: 502–513. DOI: 10.1016/j.ejmech.2019.01.067 |
| [16] | KIM J, SATO M, LI Q, et al. Peroxisome proliferator-activated receptor gamma is a target of progesterone regulation in the preovulatory follicles and controls ovulation in mice[J]. Mol Cell Biol, 2008, 28(5): 1770–1782. DOI: 10.1128/MCB.01556-07 |
| [17] | BANERJEE J, KOMAR C M. Effects of luteinizing hormone on peroxisome proliferator-activated receptor gamma in the rat ovary before and after the gonadotropin surge[J]. Reproduction, 2006, 131(1): 93–101. DOI: 10.1530/rep.1.00730 |
| [18] |
甘翔, 王继文, 李琴, 等. 鹅成熟卵泡壁层组织动态发育特点—卵泡发育与分级的新视角[J]. 畜牧兽医学报, 2019, 50(8): 1607–1613.
GAN X, WANG J W, LI Q, et al. Dynamic development characteristics of mature follicular wall in goose: A new perspective of follicle development and grading[J]. Acta Veterinaria et Zootechnica Sinica, 2019, 50(8): 1607–1613. (in Chinese) |
| [19] | DENG Y, GAN X, CHEN D, et al. Comparison of growth characteristics of in vitro cultured granulosa cells from geese follicles at different developmental stages[J]. Biosci Rep, 2018, 38(2): BSR20171361. DOI: 10.1042/BSR20171361 |
| [20] | LIVAK K J, SCHMITTGEN T D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔ Ct method[J]. Methods, 2001, 25(4): 402–408. DOI: 10.1006/meth.2001.1262 |
| [21] | KOZOMARA A, GRIFFITHS-JONES S. miRBase: annotating high confidence microRNAs using deep sequencing data[J]. Nucleic Acids Res, 2014, 42(Database issue): D68–D73. |
| [22] | TAKAHASHI M, CUATRECASAS M, BALAGUER F, et al. The clinical significance of MiR-148a as a predictive biomarker in patients with advanced colorectal cancer[J]. PLoS One, 2012, 7(10): e46684. DOI: 10.1371/journal.pone.0046684 |
| [23] | YUAN K, LIAN Z, SUN B, et al. Role of miR-148a in hepatitis B associated hepatocellular carcinoma[J]. PLoS One, 2012, 7(4): e35331. DOI: 10.1371/journal.pone.0035331 |
| [24] | LI T, WU R, ZHANG Y, et al. A systematic analysis of the skeletal muscle miRNA transcriptome of chicken varieties with divergent skeletal muscle growth identifies novel miRNAs and differentially expressed miRNAs[J]. BMC Genomics, 2011, 12: 186. DOI: 10.1186/1471-2164-12-186 |
| [25] | HOU X, TANG Z, LIU H, et al. Discovery of microRNAs associated with myogenesis by deep sequencing of serial developmental skeletal muscles in pigs[J]. PLoS One, 2012, 7(12): e52123. DOI: 10.1371/journal.pone.0052123 |
| [26] | HE H, CAI M, ZHU J, et al. miR-148a-3p promotes rabbit preadipocyte differentiation by targeting PTEN[J]. In Vitro Cell Dev Biol Anim, 2018, 54(3): 241–249. DOI: 10.1007/s11626-018-0232-z |
| [27] | HU S, GAO S, ZHU J, et al. Differential actions of diacylglycerol acyltransferase (DGAT) 1 and 2 in regulating lipid metabolism and progesterone secretion of goose granulosa cells[J]. J Steroid Biochem Mol Biol, 2020, 202: 105721. DOI: 10.1016/j.jsbmb.2020.105721 |
| [28] | PAPACLEOVOULOU G, EDMONDSON R J, CRITCHLEY H O D, et al. 3β-Hydroxysteroid dehydrogenases and pre-receptor steroid metabolism in the human ovarian surface epithelium[J]. Mol Cell Endocrinol, 2009, 301(1-2): 65–73. DOI: 10.1016/j.mce.2008.08.010 |
| [29] | MARRONE B L, SEBRING R J. Quantitative cytochemistry of 3 beta-hydroxysteroid dehydro-genase activity in avian granulosa cells during follicular maturation[J]. Biol Reprod, 1989, 40(5): 1007–1011. DOI: 10.1095/biolreprod40.5.1007 |
| [30] | NITTA H, MASON J I, BAHR J M. Localization of 3 beta-hydroxysteroid dehydrogenase in the chicken ovarian follicle shifts from the theca layer to granulosa layer with follicular maturation[J]. Biol Reprod, 1993, 48(1): 110–116. DOI: 10.1095/biolreprod48.1.110 |
| [31] | WANG Y, HU Y, GUO J, et al. miR-148a-3p suppresses the proliferation and invasion of esophageal cancer by targeting DNMT1[J]. Genet Test Mol Biomarkers, 2019, 23(2): 98–104. DOI: 10.1089/gtmb.2018.0285 |
| [32] | WANG W, DONG J, WANG M, et al. miR-148a-3p suppresses epithelial ovarian cancer progression primarily by targeting c-Met[J]. Oncol Lett, 2018, 15(5): 6131–6136. |
| [33] | ZHANG J, YING Z Z, TANG Z L, et al. MicroRNA-148a promotes myogenic differentiation by targeting the ROCK1 gene[J]. J Biol Chem, 2012, 287(25): 21093–21101. DOI: 10.1074/jbc.M111.330381 |
| [34] | SONG C C, YANG J M, JIANG R, et al. miR-148a-3p regulates proliferation and apoptosis of bovine muscle cells by targeting KLF6[J]. J Cell Physiol, 2019, 234(9 Pt.2): 15742–15750. |
| [35] | YIN H, HE H, CAO X, et al. MiR-148a-3p regulates skeletal muscle satellite cell differentiation and apoptosis via the PI3K/AKT signaling pathway by targeting Meox2[J]. Front Genet, 2020, 11: 512. DOI: 10.3389/fgene.2020.00512 |
| [36] | KOMAR C M, BRAISSANT O, WAHLI W, et al. Expression and localization of PPARs in the rat ovary during follicular development and the periovulatory period[J]. Endocrinology, 2001, 142(11): 4831–4838. DOI: 10.1210/endo.142.11.8429 |
| [37] | SCHOPPEE P D, GARMEY J C, VELDHUIS J D. Putative activation of the peroxisome proliferator-activated receptor γ impairs androgen and enhances progesterone biosynthesis in primary cultures of porcine theca cells[J]. Biol Reprod, 2002, 66(1): 190–198. DOI: 10.1095/biolreprod66.1.190 |
| [38] | FROMENT P, FABRE S, DUPONT J, et al. Expression and functional role of peroxisome proliferator-activated receptor-gamma in ovarian folliculogenesis in the sheep[J]. Biol Reprod, 2003, 69(5): 1665–1674. DOI: 10.1095/biolreprod.103.017244 |
| [39] | SUNDVOLD H, BRZOZOWSKA A, LIEN S. Characterisation of bovine peroxisome proliferator-activated receptors gamma 1 and gamma 2: genetic mapping and differential expression of the two isoforms[J]. Biochem Biophys Res Commun, 1997, 239(3): 857–861. DOI: 10.1006/bbrc.1997.7564 |
| [40] | KOMAR C M. Peroxisome proliferator-activated receptors (PPARs) and ovarian function - implications for regulating steroidogenesis, differentiation, and tissue remodeling[J]. Reprod Biol Endocrinol, 2005, 3: 41. DOI: 10.1186/1477-7827-3-41 |



