在奶牛饲养中发现,高精料/低粗料日粮或饲粮中添加油脂都会造成牛乳中乳脂肪含量降低,形成低脂乳症现象(milk fat depression,MFD)。对此现象,前人在研究过程中提出了多个理论进行解释,如脂肪动员学说、乙酸不足学说、胰岛素-葡萄糖学说等。反式脂肪酸理论认为,是日粮在瘤胃氢化过程中产生的反式脂肪酸(trans-FA)导致了乳脂肪降低[1],后来Bauman和Griinari[2]通过试验明确提出,油脂在瘤胃的代谢产物共轭亚油酸(conjugated linoleic acid,CLA)可以有效抑制乳腺脂肪合成。共轭亚油酸(CLA)是亚油酸的同分异构体,是一系列在碳9、11或10、12位具有共轭双键的高不饱和脂肪酸[3]。研究发现,给奶牛饲喂共轭亚油酸钙,或者肠道灌注trans-10,cis-12 CLA[4-6],会导致奶牛发生MFD,改变了乳中脂肪含量与脂肪酸组成,也调控了乳腺组织的基因表达,但对此过程的内在机理还存在很多未知之处。
乳脂肪以脂肪球(milk fat globule,MFG)的形式从哺乳动物的乳腺中分泌到乳中。每毫升牛乳中,大约有150亿个脂肪球,以大小不同的小液滴形状分散存在于乳中形成乳浊液。乳脂肪球颗粒直径大小从0.2 μm到15 μm不等[7],主要受到动物种类[8]、遗传倾向[9]、泌乳时期[10]、季节和营养[11]的影响。研究发现,乳脂肪球的粒径大小与乳脂肪含量有显著相关性[12],直接影响了乳品的营养特性和动物的肠道吸收,不同粒径的脂肪球还能够改变奶酪等下游乳产品的加工性质[13]。一些饲料营养因素可以改变脂肪球的直径,Argov-Argaman等[14]采用高精料日粮饲喂奶牛,发现脂肪球的平均粒径从3.51 μm减少到3.3 μm。那么饲喂奶牛CLA造成乳脂肪含量减少的同时,是不是也伴随着脂肪球粒径大小的变化?这个问题目前还不清楚。因此,本研究通过在荷斯坦奶牛日粮中添加CLA,采用激光粒度仪检测其对乳脂肪球粒径大小及分布的影响,为从乳脂肪球角度阐明反式脂肪酸造成奶牛MFD的内在机理奠定基础。
1 材料与方法 1.1 试验动物和饲养管理本试验选择24头泌乳中期荷斯坦奶牛,试验奶牛的日粮参照NRC(2001)中育成奶牛营养标准进行配制,日粮配方及营养成分见表 1。试验所用奶牛在同一双列式牛舍内饲养,试验期间采用TMR日粮,按照自由采食,自由饮水的方式进行饲养。
|
|
表 1 奶牛基础日粮的成分和化学组成 Table 1 Ingredient and chemical composition of the basal diets of cows |
选取24头体型、身体状况、产犊日期相近的泌乳中期荷斯坦奶牛(体重(583±34.6)kg,产奶量(27.2±2.4)kg·d-1)随机分为4组,每组6个重复,每个重复1头牛,分别为对照组(C group)饲喂基础日粮(表 1),低剂量组(L group)每头饲喂基础日粮+150 g·d-1 CLA;中剂量组(M group)饲喂基础日粮+300 g·d-1 CLA;高剂量组(H group)饲喂基础日粮+400 g·d-1 CLA。整个饲养周期为7 d,包括2 d适应期和5 d试验期,每天分别在07:00和16:00饲喂,每天挤奶3次。试验用共轭亚油酸微囊粉购自青岛澳海生物有限公司,其中分别含有cis-9,trans-11 CLA 39.6%和trans-10,cis-12 CLA 39.4%。
1.3 样品采集试验期间每天记录奶牛采食量和产奶量,试验期每天收集一次乳样,每头奶牛在早、中、晚按30∶40∶30取样后制成混合乳样,每天将50 mL混合乳样送至河南省奶牛生产性能测定中心采用乳成分分析仪(Foss 120 Milko-Scan,丹麦)进行乳脂、乳蛋白、乳糖含量的检测;在饲喂试验的第5天取中午的奶样,迅速将其送到实验室,采用激光粒度分析仪(Mastersizer 3000,英国)检测乳脂肪球粒径。
1.4 乳脂肪球染色观察对鲜牛乳的脂肪球进行尼罗红染色。吸取1 mL牛奶样品,加入4 mL超纯水进行稀释,加入1 mL尼罗红染料(100 μg·mL-1),混匀后避光反应20 min;取适量染色后的样品与琼脂糖(5 g·L-1) 等量混合后滴到玻底皿中,用倒置荧光显微镜(OLYMPUS IX73,日本)观察。
1.5 乳脂肪球粒径分析采用激光粒度分析仪检测新鲜牛乳样品的脂肪球粒径。根据Mastersizer 3000激光粒度分析仪的湿法指南进行操作,设置激光折射率(纯水=1.330,牛乳=1.560),搅拌器速度2 000 r·min-1。向烧杯中加入400 mL水,然后将搅拌器放入烧杯中。单击开始按钮以初始化仪器和测量背景,然后将1 mL的牛乳样品添加到烧杯中,直到激光度指示为10%~20%。系统将自动测量3次并计算平均值。使用Mastersizer软件对每个样品的获取值进行分析,生成数据包括体积相关直径D[4, 3],表面积相关直径D[3, 2],粒度分布图和粒度分布百分比。
1.6 统计分析使用SPSS 25(SPSS / IBM Corp.,Chicago,IL)的通用线性模型(GLM)中重复测量方差分析CLA处理主效应对产奶量和采食量、乳成分的影响。采用单因素方差分析(one way ANOVA)比较CLA处理对脂肪球粒径及分布比例的影响。以P>0.05表示差异不显著,P<0.05表示差异显著,P<0.01表示差异极显著。
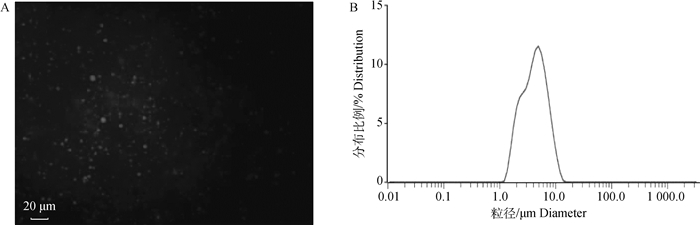
2 结果 2.1 乳脂肪球染色及粒径分布图如图 1A所示,乳脂肪球用尼罗红染色后显示红色荧光(图 1A中发亮之处),表明在牛乳中存在直径大小不一的脂肪球。牛乳的粒径分布如图 1B所示,可以看出,乳脂肪球的粒径主要分布在1~10 μm之间。
|
图 1 牛乳脂肪球染色(A)和粒径分布图(B) Fig. 1 Milk fat globules staining(A) and particle size distribution diagram(B) |
由表 2可知,各组间的产奶量和采食量没有显著差异(P>0.05);从乳成分的变化可以看出,CLA处理对乳糖和乳蛋白的含量没有显著影响,但随着CLA添加水平的增加,处理组牛乳中脂肪的含量极显著降低(P<0.01)。M组和H组的乳脂肪分别为2.34 g·dL-1和2.23 g·dL-1,比对照组分别降低了34.82%和37.88%。
|
|
表 2 CLA对奶牛生产性能及乳成分的影响 Table 2 Effects of CLA on the production performance and milk composition of dairy cows |
由表 3可知,随着CLA添加剂量的加大,显著降低了处理组的乳脂肪球粒径(P < 0.05),其中,M组和H组的脂肪球粒径D[3, 2]和D[4, 3]均显著低于C组。进一步分析不同大小脂肪球占牛乳总脂肪球的比例发现,随着CLA添加剂量的增大,小脂肪球所占总百分比逐渐增加,而大脂肪球所占百分比逐渐减少(表 4)。分布在1.54~2.58 μm的小脂肪球,H组和M组的百分比显著高于C组和L组(P < 0.05),呈现出H组、M组>L组>C组的变化;分布在4.88~6.30 μm的大脂肪球,H组和M组的比例显著低于C组和L组(P < 0.05),呈现出M组、H组 < L组 < C组的变化。
|
|
表 3 CLA对牛乳脂肪球粒径的影响 Table 3 Effects of CLA on the milk fat globule size |
|
|
表 4 CLA对不同粒径牛乳脂肪球比例的影响 Table 4 Effects of CLA on the proportion of milk fat globules with different particle sizes |
研究发现,相较于小粒径MFG,粒径大的牛MFG含有的宿主防御蛋白含量更高,说明可以通过改变牛乳中MFG粒径来增强机体的保护功能[15]。MFG粒径也直接影响了下游食品加工中干酪成熟与乳制品保质期[16-17]。用小粒径MFG生产出的干酪具有较高熔点、较强弹性和较好的流动性等特点[18]。工业上均质化技术可以减小MFG的粒径差异,均质化后形成的脂肪球膜在物理和化学特性上也有较大差异[19-20]。本研究首次发现,泌乳期奶牛进行CLA处理,能够显著降低乳中脂肪含量和脂肪球粒径,并且改变了不同粒径乳脂肪球的比例,这对于生产富含小脂肪球的牛乳制品提供了应用基础。
对于CLA引起的低脂乳症已经进行过很多研究。Perfield等[21]连续140 d给奶牛饲喂CLA,Viswanadha等[22]在5 d内通过颈静脉将不同剂量CLA直接注射给奶牛,结果均发现,CLA只是特异性抑制乳脂肪合成,而对乳蛋白、乳糖和产奶量等奶牛生产指标没有显著影响,这与本试验的结果一致。这些结论支持了一个普遍的观点,即对于奶牛来说,有效剂量的CLA只会显著抑制乳脂肪合成而不影响其他乳成分。
脂肪在乳中以脂肪球(MFG)的形式存在,而脂肪球来源于乳腺上皮细胞内合成的胞浆脂滴(LD)[23]。虽然对整个脂滴分泌过程还不是很了解,但目前研究认为,脂滴的分泌遵循这样的细胞机制:三酰甘油在乳腺上皮细胞的内质网合成后,体积逐渐增大,并在向质膜转运的过程中包裹磷脂和多种蛋白后形成脂滴,脂滴运输到细胞顶浆质膜,通过出胞过程分泌到乳汁中形成乳脂肪球[24-25]。本研究采用激光粒度仪检测发现,CLA的处理显著降低了乳脂肪的球直径,同时伴随着小脂肪球比例的增加和大脂肪球比例的减少。Altenhofer等[26]已经发现了在乳脂肪含量与脂肪球直径之间的相关关系,结合本研究中CLA引起的脂肪降低,推测可能是饲喂CLA改变了奶牛乳腺分泌到乳中脂肪球的大小比例,引起了脂肪球平均粒径的减少,表现在乳成分上是乳脂肪含量的显著降低。
目前,CLA对于奶牛的作用机制主要认为是下调了乳腺脂肪合成基因的表达[27-29],而本研究发现,CLA也会对脂肪球的大小产生影响。在乳腺上皮细胞中,细胞脂滴作为乳脂肪球合成的前体,脂滴在细胞内可以相互融合来形成大脂滴[30-31];脂肪球的粒径会受到乳中磷脂的影响[32]。脂肪酸的饲喂能够影响奶牛的代谢[33],因此,这种CLA引起的乳脂肪球粒径的变化,究竟是由细胞内的脂滴融合引起[34],还是由乳腺分泌乳脂肪球本身所导致的,未来还需要更多深入研究。
4 结论本研究通过给奶牛饲喂CLA发现,乳脂肪发生明显降低,同时乳脂球直径D[3, 2]和D[4, 3]均显著降低,并且伴随着小脂肪球比例的增加和大脂肪球比例的减少。
| [1] | BERNARD L, LEROUX C, CHILLIARD Y. Expression and nutritional regulation of lipogenic genes in the ruminant lactating mammary gland[J]. Adv Exp Med Biol, 2008, 606(1): 67–108. |
| [2] | BAUMAN D E, GRⅡNARI J M. Regulation and nutritional manipulation of milk fat: low-fat milk syndrome[J]. Livest Prod Sci, 2001, 70(1-2): 15–29. DOI: 10.1016/S0301-6226(01)00195-6 |
| [3] | LOOR J J, HERBEIN J H. Exogenous conjugated linoleic acid isomers reduce bovine milk fat concentration and yield by inhibiting de novo fatty acid synthesis[J]. J Nutr, 1998, 128(12): 2411–2419. DOI: 10.1093/jn/128.12.2411 |
| [4] | HUANG Y, SCHOONMAKER J P, BRADFORD B J, et al. Response of milk fatty acid composition to dietary supplementation of soy oil, conjugated linoleic acid, or both[J]. J Dairy Sci, 2008, 91(1): 260–270. DOI: 10.3168/jds.2007-0344 |
| [5] | LOOR J J, HERBEIN J H. Reduced fatty acid synthesis and desaturation due to Exogenus trans10, cis12-CLA in cows fed oleic or linoleic oil[J]. J Dairy Sci, 2003, 86(4): 1354–1369. DOI: 10.3168/jds.S0022-0302(03)73720-5 |
| [6] | HARVATINE K J, BAUMAN D E. SREBP1 and thyroid hormone responsive spot 14(S14) are involved in the regulation of bovine mammary lipid synthesis during diet-induced milk fat depression and treatment with CLA[J]. J Nutr, 2006, 136(10): 2468–2474. DOI: 10.1093/jn/136.10.2468 |
| [7] | LEE H, PADHI E, HASEGAWA Y, et al. Compositional dynamics of the milk fat globule and its role in infant development[J]. Front Pediatr, 2018, 6: 313. DOI: 10.3389/fped.2018.00313 |
| [8] | MICHALSKI M C, BRIARD V, MICHEL F, et al. Size distribution of fat globules in human colostrum, breast milk, and infant formula[J]. J Dairy Sci, 2005, 88(6): 1927–1940. DOI: 10.3168/jds.S0022-0302(05)72868-X |
| [9] | ARGOV-ARGAMAN N, MIDA K, COHEN B C, et al. Milk fat content and DGAT1 genotype determine lipid composition of the milk fat globule membrane[J]. PLoS One, 2013, 8(7): e68707. DOI: 10.1371/journal.pone.0068707 |
| [10] | MESILATI-STAHY R, ARGOV-ARGAMAN N. The relationship between size and lipid composition of the bovine milk fat globule is modulated by lactation stage[J]. Food Chem, 2014, 145: 562–570. DOI: 10.1016/j.foodchem.2013.08.077 |
| [11] | LOPEZ C, BRIARD-BION V, MENARD O, et al. Phospholipid, sphingolipid, and fatty acid compositions of the milk fat globule membrane are modified by diet[J]. J Agric Food Chem, 2008, 56(13): 5226–5236. DOI: 10.1021/jf7036104 |
| [12] | MESILATI-STAHY R, MIDA K, ARGOV-ARGAMAN N. Size-dependent lipid content of bovine milk fat globule and membrane phospholipids[J]. J Agric Food Chem, 2011, 59(13): 7427–7435. DOI: 10.1021/jf201373j |
| [13] | LIANG L, QI C, WANG X G, et al. Influence of homogenization and thermal processing on the gastrointestinal fate of bovine milk fat: in vitro digestion study[J]. J Agric Food Chem, 2017, 65(50): 11109–11117. DOI: 10.1021/acs.jafc.7b04721 |
| [14] | ARGOV-ARGAMAN N, MIDA K, COHEN B C, et al. Milk fat content and DGAT1 genotype determine lipid composition of the milk fat globule membrane[J]. PLoS One, 2013, 8(7): e68707. DOI: 10.1371/journal.pone.0068707 |
| [15] | LU J, ARGOV-ARGAMAN N, ANGGREK J, et al. The protein and lipid composition of the membrane of milk fat globules depends on their size[J]. J Dairy Sci, 2016, 99(6): 4726–4738. DOI: 10.3168/jds.2015-10375 |
| [16] | LOPEZ C, MÉNARD O. Human milk fat globules: polar lipid composition and in situ structural investigations revealing the heterogeneous distribution of proteins and the lateral segregation of sphingomyelin in the biological membrane[J]. Colloids Surf B Biointerfaces, 2011, 83(1): 29–41. DOI: 10.1016/j.colsurfb.2010.10.039 |
| [17] | MICHALSKI M C, GASSI J Y, FAMELART M H, et al. The size of native milk fat globules affects physic-chemical and sensory properties of Camembert cheese[J]. Dairy Sci Technol, 2003, 83(2): 131–143. DOI: 10.1051/lait:2003003 |
| [18] | AVRAMIS C A, WANG H, MCBRIDE B W, et al. Physical and processing properties of milk, butter, and Cheddar cheese from cows fed supplemental fish meal[J]. J Dairy Sci, 2003, 86(8): 2568–2576. DOI: 10.3168/jds.S0022-0302(03)73851-X |
| [19] | MICHALSKI M C, OLLIVON M, BRIARD V, et al. Native fat globules of different sizes selected from raw milk: thermal and structural behavior[J]. Chem Phys Lipids, 2004, 132(2): 247–261. DOI: 10.1016/j.chemphyslip.2004.08.007 |
| [20] | ARGOV N, LEMAY D G, GERMAN J B. Milk fat globule structure and function: nanoscience comes to milk production[J]. Trends Food Sci Tech, 2008, 19(12): 617–623. DOI: 10.1016/j.tifs.2008.07.006 |
| [21] | PERFIELD Ⅱ J W, BERNAL-SANTOS G, OVERTON T R, et al. Effects of dietary supplementation of rumen-protected conjugated linoleic acid in dairy cows during established lactation[J]. J Dairy Sci, 2002, 85(10): 2609–2617. DOI: 10.3168/jds.S0022-0302(02)74346-4 |
| [22] | VISWANADHA S, GIESY J G, HANSON T W, et al. Dose response of milk fat to intravenous administration of the trans-10, cis-12 isomer of conjugated linoleic acid[J]. J Dairy Sci, 2003, 86(10): 3229–3236. DOI: 10.3168/jds.S0022-0302(03)73926-5 |
| [23] | MCMANAMAN J L, RUSSELL T D, SCHAACK J, et al. Molecular determinants of milk lipid secretion[J]. J Mammary Gland Biol Neop, 2007, 12(4): 259–268. DOI: 10.1007/s10911-007-9053-5 |
| [24] | WALTHER T C, FARESE JR R V. Lipid droplets and cellular lipid metabolism[J]. Annu Rev Biochem, 2012, 81(1): 687–714. DOI: 10.1146/annurev-biochem-061009-102430 |
| [25] | ARGOV-ARGAMAN N. Symposium review: Milk fat globule size: Practical implications and metabolic regulation[J]. J Dairy Sci, 2019, 102(3): 2783–2795. DOI: 10.3168/jds.2018-15240 |
| [26] | ALTENHOFER C, HOLZMÜLLER W, WOLFERTSTETTER F, et al. Temporal variation of milk fat globule diameter, fat and cholesterol content and milk epithelial cell gene expression in dairy cows[J]. Int J Dairy Technol, 2015, 68(4): 519–526. DOI: 10.1111/1471-0307.12220 |
| [27] | GERVAIS R, MCFADDEN J W, LENGI A J, et al. Effects of intravenous infusion of trans-10, cis-1218:2 on mammary lipid metabolism in lactating dairy cows[J]. J Dairy Sci, 2009, 92(10): 5167–5177. DOI: 10.3168/jds.2009-2281 |
| [28] | HAN L Q, PANG K, LI H J, et al. Conjugated linoleic acid-induced milk fat reduction associated with depressed expression of lipogenic genes in lactating Holstein mammary glands[J]. Genet Mol Res, 2012, 11(4): 4754–4764. |
| [29] | HARVATINE K J, BOISCLAIR Y R, BAUMAN D E. Time-dependent effect of trans-10, cis-12 conjugated linoleic acid on gene expression of lipogenic enzymes and regulators in mammary tissue of dairy cows[J]. J Dairy Sci, 2018, 101(8): 7585–7592. DOI: 10.3168/jds.2017-13935 |
| [30] | WALTHER T C, CHUNG J, FARESE JR R V. Lipid droplet biogenesis[J]. Annu Rev Cell Dev Biol, 2017, 33: 491–510. DOI: 10.1146/annurev-cellbio-100616-060608 |
| [31] | COHEN B C, RAZ C, SHAMAY A, et al. Lipid droplet fusion in mammary epithelial cells is regulated by phosphatidylethanolamine metabolism[J]. J Mammary Gland Biol Neop, 2017, 22(4): 235–249. DOI: 10.1007/s10911-017-9386-7 |
| [32] | ARGOV-ARGAMAN N, MESILATI-STAHY R, MAGEN Y, et al. Elevated concentrate-to-forage ratio in dairy cow rations is associated with a shift in the diameter of milk fat globules and remodeling of their membranes[J]. J Dairy Sci, 2014, 97(10): 6286–6295. DOI: 10.3168/jds.2014-8174 |
| [33] |
张海波. 日粮补充棕榈酸对泌乳早期荷斯坦奶牛泌乳性能、血液生化指标和激素水平的影响[J]. 畜牧兽医学报, 2019, 50(1): 203–210.
ZHANG H B. Effects of dietary supplementation of palmitic acid on lactation performance, blood biochemical indexes and hormone concentration in early lactation holstein dairy cows[J]. Acta Veterinaria et Zootechnica Sinica, 2019, 50(1): 203–210. (in Chinese) |
| [34] |
邢智洋, 张梦璐, 张菡, 等. 沉默SCAP基因对奶牛乳腺上皮细胞脂滴的影响[J]. 畜牧兽医学报, 2019, 50(3): 507–516.
XING Z Y, ZHANG M L, ZHANG H, et al. Effect of silencing SCAP gene on lipid droplets in dairy mammary epithelial cells[J]. Acta Veterinaria et Zootechnica Sinica, 2019, 50(3): 507–516. (in Chinese) |



