蜱是一类以吸血为生的体外寄生虫,其生活史包括卵、幼蜱、若蜱和成蜱4个阶段。在卵期,受精卵经过卵裂、囊胚、原肠胚、分节等过程发育成幼蜱,并破壳而出,其间发生的反应众多而有序、参与的物质复杂而有定,尚能探明对了解蜱类胚胎发育规律,创新控蜱策略具有重要意义。
人们很早就已开始了蜱卵原生质组成和胚胎发育过程的研究。1943年,Wigglesworth[1]首先关注蜱卵颜色与血红素的关系。随后,Bremner[2]证实了蜱卵中的色素取决于球蛋白与血红素结合而成的复合物。迄今为止,人们已经从安氏矩头蜱[3]、毛白钝缘蜱[4]、变异矩头蜱[5-6]、肩突硬蜱[7]、微小扇头蜱[8]、长角血蜱[9]卵中分离鉴定了多种卵黄蛋白,以及催化卵黄蛋白和血红蛋白分解的微小扇头蜱的heme-binding aspartic proteinase[10]、Boophilus Yolk pro-Cathepsin[11]、vitellin-degrading cysteine endopeptidase[12]、毛白钝缘蜱的cathepsin L-like proteinase[13]。最近,人们又发现了参与糖类代谢的GSK-3[14]、能清除自由基的GST[15]、具抑制作用的serine proteinase inhibitor[16]、kallikrein inhibitor[17]和抑制细菌生长发育的抗菌肽[18]。人们对蜱卵蛋白成分及其相互关系知之尚少,本研究拟以LC/MS/MS法对豪猪血蜱卵原生质中蛋白质成分进行鉴定。
豪猪血蜱广泛分布于温带和亚热带地区,我国鲁、苏、闽、台、粤、琼、湘、鄂、滇、甘等地均有发现[19],常寄生于野猪、豪猪、猪獾,偶尔也见于狗、水牛、小麂和人类[20]。该蜱携带锥虫[21]、埃立克体[22]、立克次氏体[23]、包柔氏螺旋体、吉氏巴贝斯虫[24]等多种病原微生物,并有人被叮咬后发生无形体病[25]、日本斑点热[26]的报道,具有一定的公共卫生意义。
1 材料与方法 1.1 材料1.1.1 试剂与仪器 氯仿、甲醛、乙腈、甲酸系国药集团化学试剂有限公司产品;胰蛋白酶购于Promega(北京)生物技术有限公司;Q Exactive质谱仪为Thermo Fisher生产;液相色谱仪购于Agilent Technologies。
1.1.2 豪猪血蜱 豪猪血蜱采自湖南省湘西猪獾体表,由湖南农业大学动物医学院程天印教授鉴定。
1.2 方法1.2.1 蜱卵的收集与处理 将饱血雌蜱置于昆虫培养箱,于28 ℃,85%RH下培养。每日定时收集蜱卵。按徐律和程天印[27]的方法去除卵表腊质。
1.2.2 蛋白的提取与消化 取去腊质卵50 mg,加入0.1 mol·L-1 Tris-HCl溶液0.4 mL,研碎;转移至洁净离心管,加入20%SDS和1M DTT各25 μL,混匀,95 ℃孵育3 min;25 ℃下,16 000 g离心10 min,取上清;冻干,备用。
1.2.3 样品处理 取卵蛋白干粉50 g,加入100 mmol·L-1 DTT 50 μL,沸水浴5 min,冷却至室温;加入8 mol·L-1尿素缓冲液200 μL,混匀,转入10 ku超滤离心管,14 000 g离心15 min。加入100的IAA液,混匀,14 000 g离心10 min。尿素缓冲液洗涤2次。加胰蛋白酶消化液40 μL至滤膜上,600 r·min-1振荡1 min,37 ℃ 16~18 h。14 000 g离心滤液10 min,移至C18小柱,过柱脱盐后冷冻干燥。
1.2.4 质谱分析 以0.1%三氟乙酸溶解上述肽段样粉,自动进样器进样,经捕集柱、色谱柱分离后,质谱仪检测离子。检测方式:正离子;采集方法:每次采集10个碎片图谱。
1.2.5 数据分析 将质谱数据和自建的褐黄血蜱卵巢转录组翻译文库(PRJNA600997)、唾液腺转录组翻译文库(PRJNA280697)和中肠转录组翻译文库(PRJNA286260)肽序列导入Mascot2.2,设定搜索参数,搜索特异性肽段及其匹配多肽。
1.2.6 蛋白序列分析 上传各高可信蛋白序列至Uniprot数据库,进行搜库、注释。
2 结果 2.1 卵总蛋白检测采用BCA法测定卵蛋白粗提液浓度,提取液蛋白含量5.26 μg·μL-1。卵蛋白粗提液的SDS-PAGE结果表明,豪猪血蜱卵原生质所含蛋白多在15~170 ku,且分布不均,相对质量近55、65、80、120和170 ku者丰度尤高。
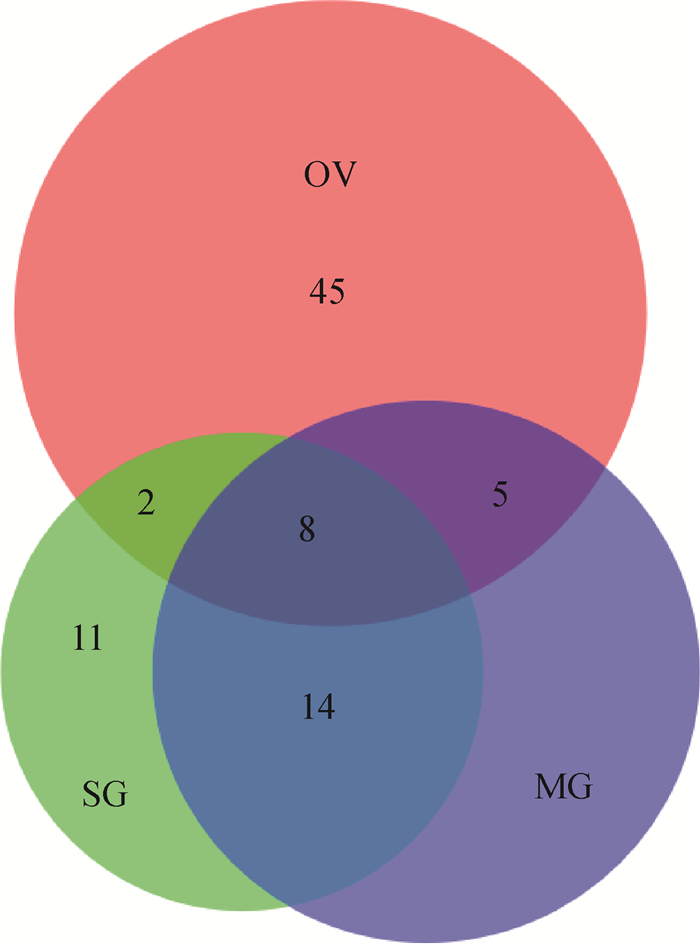
2.2 卵蛋白鉴定以卵蛋白酶解物质谱检测数据搜索卵巢、唾液腺、中肠转录组文库,分别检出特异性肽段359、312、357条,鉴定蛋白108、170、161种,其中高可信的(unique peptides≥2)依次为60、35、45种(图 2,有部分重叠)。基于序列比对,合并由同一mRNA编码的,最终鉴定高可信蛋白103种,但其中36种尚无相关研究资料(表 1)。

|
M. 蛋白质相对分子质量标准;1.样品 M. Protein marker; 1. Sample 图 1 豪猪血蜱卵总蛋白提取液SDS-PAGE图 Fig. 1 Proteins extracted from the eggs of Haemaphysalis hystricis |
|
OV、SG、MG分别是卵巢转录组文库、唾液腺转录组文库、中肠转录组文库鉴定到的蛋白 OV, SG and MG represent the proteins identified in ovarian transcriptome library, salivary gland transcriptome library and midgut transcriptome library respectively 图 2 卵巢、唾液腺和中肠转录组文库中鉴定到的高可信蛋白及其重叠情况 Fig. 2 Overlap of highly confidence proteins identified in the ovary, salivary glands, and midgut transcriptome libraries |
|
|
表 1 豪猪血蜱卵蛋白功能分类 Table 1 Functional classification of Haemaphysalis hystricis egg proteins |
基于既有文献,按活性或功能对本研究检出的、有文献参考的67种高可信蛋白进行分类,结果见表 1。表 1列出的67种高可信蛋白分别属于酶类、蛋白酶抑制剂、转运蛋白、细胞骨架蛋白、热休克蛋白和免疫相关蛋白等6类,其中酶类最多(25种),其次是蛋白酶抑制剂(18种)和转运蛋白(11种)。
2.3 103种高可信蛋白生物信息学分析借助Omics Bean对高可信蛋白进行GO分析,结果表明(图 3),本次鉴定的高可信蛋白多为细胞外蛋白,参与免疫调节,并且在酶活性调节方面发挥作用。

|
图 3 豪猪血蜱卵103种高可信蛋白GO分析结果 Fig. 3 GO analysis of 103 highly confident proteins in Haemaphysalis hystricis eggs |
由于目前尚无豪猪血蜱的基因或蛋白文库,故本研究便基于笔者构建的其近缘蜱种(褐黄血蜱)的卵巢、唾液腺和中肠转录组文库进行其卵蛋白成分鉴定。与笔者所做的褐黄血蜱卵蛋白鉴定结果一致,暗示这一做法可行、可靠。在分析过程中,发现有些蛋白可从不止一个文库搜出,为此,我们首先借助序列比对厘清它们的关系,然后进行取舍或拼接,如SG27993长149 aa,MG1301长373 aa,两者相同部分138 aa,对其进行拼接,然后搜库、注释;MG4254长316 aa、OVN53697长673 aa,MG4254包含于OVN53697,故舍弃MG4254,以卵巢序列OVN53697搜库、注释。
从本次鉴定的高可信蛋白看,无论是酶,还是酶抑制剂和转运蛋白都存在同一家族多个成员并存的情况,笔者称之为家族性。在卵原生质蛋白中比较突出的是Cathepsin、Aspartic protease、Serpins 9、Kunitz、Vitellogenin、Microtubule associated complex、HSP70等。同家族蛋白在胚胎发育过程中如何协调、互补地发挥作用尚不得而知。比对显示,同家族蛋白间的一级结构极其相似,如OVN53099和MG241(Cathepsin L-like cysteine protein)对应部分的相似性达93.16%。
组织蛋白酶类是酶类最多的,且主要是天冬氨酸蛋白酶和半胱氨酸蛋白酶两类,其中前者14种,后者7种。业已证明,Cathepsin D、Heme-binding aspartic peptidase、Cathepsin L-like cysteine protein以及Vitellin-degrading cysteine endopeptidase均可催化卵黄蛋白和血红蛋白水解[12-13, 28-30], 但活性有所差异,如微小扇头蜱的Cathepsin D对卵黄蛋白的活性较高[28],Vitellin-degrading cysteine endopeptidase对血红蛋白的活性更高[12]。
在蜱卵原生质中还存在大量的蛋白酶抑制剂,其中Serpin类数量最多,其次是Cystatin。人们已从长角血蜱、篦子硬蜱等蜱鉴定到数种Serpin[31-35],大多数serpin既能抑制丝氨酸蛋白酶,进而影响到血红蛋白的降解[36],又可抑制糜蛋白酶、弹性蛋白酶和木瓜蛋白酶样半胱氨酸蛋白酶等,起到调节宿主免疫系统的作用[37-38]。Waxman等[39]证实,蜱类的Serpin具有抗凝活性,但机制不尽相同,如毛白钝缘蜱的serpin抑制Xa因子,长角血蜱的serpin 2抑制凝血酶活性[40]。Cystatin是半胱氨酸蛋白酶抑制剂,可抑制Cathepsin L-like cysteine protein[41]以及Vitellin-degrading cysteine endopeptidase[42]等酶的活性,暗示蜱类胚胎发育过程中发生的酶促反应也受控制、调节。
Vg是豪猪血蜱卵中的高丰度成分,种类也较多,如Vitellogenin-1、Vitellogenin-2、Vitellogenin-B以及Vitellogenin。Vg主要由饱血雌蜱的中肠和脂肪体合成,通过淋巴转移至卵巢,然后转化为Vn,作为胚胎发育必须的营养贮存于卵原生质[43]。另外,本次还鉴定了6个脂蛋白和1个脂肪酸结合蛋白。Kluck等[44]证实,微小扇头蜱血淋巴中的一种脂蛋白RmLCP可结合大多数脂肪酸,发挥运输脂类的作用。Campos等[45]认为,脂类和碳水化合物是微小牛蜱胚胎发育的主要能源物质。此次鉴定到的葡萄糖脱氢酶、甘油醛-3-磷酸脱氢酶、转酮醇酶和ATP合酶等4种糖代谢相关酶的存在说明胚胎发育过程中既有糖酵解反应[46],也有戊糖磷酸途径[47]。
蜱卵原生质蛋白具有开发应用价值。Maria以微小扇头蜱卵源Cathepsin免疫牛,激发了显著的免疫应答[48]。以微小扇头蜱卵源Vitellin-degrading enzyme免疫牛使其获得了保护性[49]。
4 结论经FASP法消化豪猪血蜱卵蛋白提取物,LC/MS/MS法检测结果显示:搜索褐黄血蜱卵巢转录组文库、唾液腺转录组文库和中肠转录组文库,分别检出特异性肽段359、312、357条,鉴定多肽108、170、161条,高可信蛋白103种。豪猪血蜱蜱卵原生质中富含酶类、蛋白酶抑制剂、转运蛋白、细胞骨架蛋白、蛋白质合成与修饰、分泌蛋白以及未鉴定的蛋白等。
| [1] |
WIGGLESWORTH V B. The fate of haemoglobin in Rhodnius prolixus (Hemiptera) and other blood-sucking arthropods[J]. Proc Roy Soc B-Biol Sci, 1943, 131: 313-339. |
| [2] |
BREMNER K C. Studies on "Haemixodovin", the pigment in the eggs of the cattle tick Boophilus microplus (Acarina: Ixodidae)[J]. Aust J Biol Sci, 1959, 12(3): 263-273. DOI:10.1071/BI9590263 |
| [3] |
BOCTOR F N, KAMEL M Y. Purification and characterization of two lipovitellins from eggs of the tick, Dermacentor andersoni[J]. Insect Biochem, 1976, 6(3): 233-240. DOI:10.1016/0020-1790(76)90088-3 |
| [4] |
CHINZEI Y, CHINO H, TAKAHASHI K. Purification and properties of vitellogenin and vitellin from a tick, Ornithodoros moubata[J]. J Comp Physiol, 1983, 152(1): 13-21. DOI:10.1007/BF00689722 |
| [5] |
ROSELL R, COONS L B. Purification and partial characterization of vitellin from the eggs of the hard tick, Dermacentor variabilis[J]. Insect Biochem, 1991, 21(8): 871-885. DOI:10.1016/0020-1790(91)90094-U |
| [6] |
THOMPSON D M, KHALIL S M S, JEFFERS L A, et al. Sequence and the developmental and tissue-specific regulation of the first complete vitellogenin messenger RNA from ticks responsible for heme sequestration[J]. Insect Biochem Mol Biol, 2007, 37(4): 363-374. DOI:10.1016/j.ibmb.2007.01.004 |
| [7] |
JAMES A M, OLIVER JR J H. Purification and partial characterization of vitellin from the black-legged tick, Ixodes scapularis[J]. Insect Biochem Mol Biol, 1997, 27(7): 639-649. DOI:10.1016/S0965-1748(97)00038-6 |
| [8] |
LOGULLO C, MORAES J, DANSA-PETRETSKI M, et al. Binding and storage of heme by vitellin from the cattle tick, Boophilus microplus[J]. Insect Biochem Mol Biol, 2002, 32(12): 1805-1811. DOI:10.1016/S0965-1748(02)00162-5 |
| [9] |
YANG X L, YU Z J, HE Y J, et al. Purification of vitellin and dynamics of vitellogenesis in the parthenogenetic tick Haemaphysalis longicornis (Acari: Ixodidae)[J]. Exp Appl Acarol, 2015, 65(3): 377-388. DOI:10.1007/s10493-014-9866-z |
| [10] |
SORGINE M H F, LOGULLO C, ZINGALI R B, et al. A heme-binding aspartic proteinase from the eggs of the hard tick Boophilus microplus[J]. J Biol Chem, 2000, 275(37): 28659-28665. DOI:10.1074/jbc.M005675200 |
| [11] |
LOGULLO C, DA SILVA VAZ I, SORGINE M H F, et al. Isolation of an aspartic proteinase precursor from the egg of a hard tick, Boophilus microplus[J]. Parasitology, 1998, 116(6): 525-532. DOI:10.1017/S0031182098002698 |
| [12] |
SEIXAS A, DOS SANTOS P, VELLOSO F F, et al. A Boophilus microplus vitellin-degrading cysteine endopeptidase[J]. Parasitology, 2003, 126(Pt 2): 155-163. |
| [13] |
FAGOTTO F. Yolk degradation in tick eggs: I. Occurrence of a cathepsin L-like acid proteinase in yolk spheres[J]. Arch Insect Biochem Physiol, 1990, 14(4): 217-235. DOI:10.1002/arch.940140403 |
| [14] |
LOGULLO C, WITOLA W H, ANDRADE C, et al. Expression and activity of glycogen synthase kinase during vitellogenesis and embryogenesis of Rhipicephalus (Boophilus) microplus[J]. Vet Parasitol, 2009, 161(3-4): 261-269. DOI:10.1016/j.vetpar.2009.01.029 |
| [15] |
FREITAS D R J, ROSA R M, MORAES J, et al. Relationship between glutathione S-transferase, catalase, oxygen consumption, lipid peroxidation and oxidative stress in eggs and larvae of Boophilus microplus (Acarina: Ixodidae)[J]. Comp Biochem Physiol A Mol Integr Physiol, 2007, 146(4): 688-694. DOI:10.1016/j.cbpa.2006.04.032 |
| [16] |
WILLADSEN P, MCKENNA R V. Trypsin-chymotrypsin inhibitors from the tick, Boophilus microplus[J]. Aust J Exp Biol Med Sci, 1983, 61(2): 231-238. DOI:10.1038/icb.1983.21 |
| [17] |
ABREU P A, SOARES T S, BUARQUE D S, et al. RmKK, a tissue kallikrein inhibitor from Rhipicephalus microplus eggs[J]. Biochem Biophys Res Commun, 2014, 449(1): 69-73. DOI:10.1016/j.bbrc.2014.04.154 |
| [18] |
ESTEVES E, FOGACA A C, MALDONADO R, et al. Antimicrobial activity in the tick Rhipicephalus (Boophilus) microplus eggs: cellular localization and temporal expression of microplusin during oogenesis and embryogenesis[J]. Dev Comp Immunol, 2009, 33(8): 913-919. DOI:10.1016/j.dci.2009.02.009 |
| [19] |
陈泽, 杨晓军, 杨晓红, 等. 中国蜱类地理分布及区系分析[J]. 四川动物, 2008, 27(5): 820-823. CHEN Z, YANG X J, YANG X H, et al. Geographical distribution and fauna of Chinese ticks[J]. Sichuan Journal of Zoology, 2008, 27(5): 820-823. (in Chinese) |
| [20] |
DURDEN L A, MERKER S, BEATI L. The tick fauna of Sulawesi, Indonesia (Acari: Ixodoidea: Argasidae and Ixodidae)[J]. Exp Appl Acarol, 2008, 45(1-2): 85-110. DOI:10.1007/s10493-008-9144-z |
| [21] |
THEKISOE O M M, HONDA T, FUJITA H, et al. A trypanosome species isolated from naturally infected Haemaphysalis hystricis ticks in Kagoshima Prefecture, Japan[J]. Parasitology, 2007, 134(7): 967-974. DOI:10.1017/S0031182007002375 |
| [22] |
LI J J, LIU X X, MU J Q, et al. Emergence of a Novel Ehrlichia minasensis strain, harboring the major immunogenic glycoprotein trp36 with unique tandem repeat and C-terminal region sequences, in Haemaphysalis hystricis ticks removed from free-ranging sheep in Hainan Province, China[J]. Microorganisms, 2019, 7(9): 369. DOI:10.3390/microorganisms7090369 |
| [23] |
MAHARA F. Japanese spotted fever: report of 31 cases and review of the literature[J]. Emerg Infect Dis, 1997, 3(2): 105-111. DOI:10.3201/eid0302.970203 |
| [24] |
JONGEJAN F, SU B L, YANG H J, et al. Molecular evidence for the transovarial passage of Babesia gibsoni in Haemaphysalis hystricis (Acari: Ixodidae) ticks from Taiwan: a novel vector for canine babesiosis[J]. Parasite Vector, 2018, 11(1): 134. DOI:10.1186/s13071-018-2722-y |
| [25] |
YU Z J, WANG H, WANG T H, et al. Tick-borne pathogens and the vector potential of ticks in China[J]. Parasite Vector, 2015, 8: 24. DOI:10.1186/s13071-014-0628-x |
| [26] |
SEKI M, IKARI N, YAMAMOTO S, et al. Severe Japanese spotted fever successfully treated with fluoroquinolone[J]. Intern Med, 2006, 45(22): 1323-1326. DOI:10.2169/internalmedicine.45.1831 |
| [27] |
徐律, 程天印. 褐黄血蜱卵蛋白质组分析[J]. 畜牧兽医学报, 2019, 50(3): 627-636. XU L, CHENG T Y. Proteomics Analysis of Haemaphysalis flava eggs[J]. Acta Veterinaria et Zootechnica Sinica, 2019, 50(3): 627-636. (in Chinese) |
| [28] |
ABREU L A, VALLE D, MANSO P P A, et al. Proteolytic activity of Boophilus microplus Yolk pro-Cathepsin D (BYC) is coincident with cortical acidification during embryogenesis[J]. Insect Biochem Mol Biol, 2004, 34(5): 443-449. DOI:10.1016/j.ibmb.2004.01.006 |
| [29] |
ZHANG T T, QIU Z X, LI Y, et al. The mRNA expression and enzymatic activity of three enzymes during embryonic development of the hard tick Haemaphysalis longicornis[J]. Parasite Vector, 2019, 12(1): 96. DOI:10.1186/s13071-019-3360-8 |
| [30] |
POHL P C, SORGINE M H, LEAL A T, et al. An extraovarian aspartic protease accumulated in tick oocytes with vitellin-degradation activity[J]. Compar Biochem Phys B-Biochem Mol Biol, 2008, 151(4): 392-399. DOI:10.1016/j.cbpb.2008.08.008 |
| [31] |
SUGINO M, IMAMURA S, MULENGA A, et al. A serine proteinase inhibitor (serpin) from ixodid tick Haemaphysalis longicornis; cloning and preliminary assessment of its suitability as a candidate for a tick vaccine[J]. Vaccine, 2003, 21(21-22): 2844-2851. DOI:10.1016/S0264-410X(03)00167-1 |
| [32] |
MULENGA A, TSUDA A, ONUMA M, et al. Four serine proteinase inhibitors (serpin) from the brown ear tick, Rhiphicephalus appendiculatus; cDNA cloning and preliminary characterization[J]. Insect Biochem Mol Biol, 2003, 33(2): 267-276. DOI:10.1016/S0965-1748(02)00240-0 |
| [33] |
PREVOT P P, BESCHIN A, LINS L, et al. Exosites mediate the anti-inflammatory effects of a multifunctional serpin from the saliva of the tick Ixodes ricinus[J]. FEBS J, 2009, 276(12): 3235-3246. DOI:10.1111/j.1742-4658.2009.07038.x |
| [34] |
RODRIGUEZ-Valle M, VANCE M, MOOLHUIJZEN P M, et al. Differential recognition by tick-resistant cattle of the recombinantly expressed Rhipicephalus microplus serine protease inhibitor-3 (RMS-3)[J]. Ticks Tick Borne Dis, 2012, 3(3): 159-169. DOI:10.1016/j.ttbdis.2012.03.002 |
| [35] |
YU Y F, CAO J, ZHOU Y Z, et al. Isolation and characterization of two novel serpins from the tick Rhipicephalus haemaphysaloides[J]. Ticks Tick Borne Dis, 2013, 4(4): 297-303. DOI:10.1016/j.ttbdis.2013.02.001 |
| [36] |
MOTOBU M, TSUJI N, MIYOSHI T, et al. Molecular characterization of a blood-induced serine carboxypeptidase from the ixodid tick Haemaphysalis longicornis[J]. FEBS J, 2007, 274(13): 3299-3312. DOI:10.1111/j.1742-4658.2007.05852.x |
| [37] |
BLISNICK A A, FOULON T, BONNET S I. Serine protease inhibitors in ticks: an overview of their role in tick biology and tick-borne pathogen transmission[J]. Front Cell Infect Microbiol, 2017, 7: 199. DOI:10.3389/fcimb.2017.00199 |
| [38] |
黄钰, 潘保良. 外寄生虫丝氨酸蛋白酶抑制剂的研究进展[J]. 中国兽医科学, 2021, 51(2): 219-225. HUANG Y, PAN B L. Research advances in serine proteinase inhibitor of ectoparasite[J]. Chinese Veterinary Science, 2021, 51(2): 219-225. (in Chinese) |
| [39] |
WAXMAN L, CONNOLLY T M, et al. Isolation of an inhibitor selective for collagen-stimulated platelet aggregation from the soft tick Ornithodoros moubata[J]. J Biol Chem, 1993, 268(8): 5445-5449. DOI:10.1016/S0021-9258(18)53341-X |
| [40] |
CHMELAR J, OLIVEIRA C J, REZACOVA P, et al. A tick salivary protein targets cathepsin G and chymase and inhibits host inflammation and platelet aggregation[J]. Blood, 2011, 117(2): 736-744. DOI:10.1182/blood-2010-06-293241 |
| [41] |
YAMAJI K, TSUJI N, MIYOSHI T, et al. Hlcyst-1 and Hlcyst-2 are potential inhibitors of HlCPL-A in the midgut of the ixodid tick Haemaphysalis longicornis[J]. J Vet Med Sci, 2010, 72(5): 599-604. DOI:10.1292/jvms.09-0561 |
| [42] |
LIMA C A, SASAKI S D, TANAKA A S. Bmcystatin, a cysteine proteinase inhibitor characterized from the tick Boophilus microplus[J]. Biochem Biophys Res Commun, 2006, 347(1): 44-50. DOI:10.1016/j.bbrc.2006.06.018 |
| [43] |
DIEHL P, AESCHLIMANN A, OBENCHAIN F. Tick reproduction: oogenesis and oviposition[J]. Phys Ticks, 1982, 1(9): 277-350. |
| [44] |
KLUCK G E G, CARDOSO L S, DE CICCO N N T, et al. A new lipid carrier protein in the cattle tick Rhipicephalus microplus[J]. Ticks Tick Borne Dis, 2018, 9(4): 850-859. DOI:10.1016/j.ttbdis.2018.03.010 |
| [45] |
CAMPOS E, MORAES J, FAÇANHA A R, et al. Kinetics of energy source utilization in Boophilus microplus (Canestrini, 1887) (Acari: Ixodidae) embryonic development[J]. Vet Parasitol, 2006, 138(3-4): 349-357. DOI:10.1016/j.vetpar.2006.02.004 |
| [46] |
XU L, LIU L, CHENG T Y. Cloning and expression profile of glyceraldehyde-3-phosphate dehydrogenase in Haemaphysalis flava (Acari: Ixodidae)[J]. Journal of Medical Entomology, 2019, 56(2): 569-575. DOI:10.1093/jme/tjy200 |
| [47] |
ZHAO J, ZHONG C J. A review on research progress of transketolase[J]. Neurosci Bull, 2009, 25(2): 94-99. DOI:10.1007/s12264-009-1113-y |
| [48] |
NASCIMENTO M C L, LEAL A T, DAFFRE S, et al. BYC, an atypical aspartic endopeptidase from Rhipicephalus (Boophilus) microplus eggs[J]. Compar Biochem Phys B-Biochem Mol Biol, 2008, 149(4): 599-607. DOI:10.1016/j.cbpb.2007.12.007 |
| [49] |
SEIXAS A, LEAL A T, NASCIMENTO-SILVA M C L, et al. Vaccine potential of a tick vitellin-degrading enzyme (VTDCE)[J]. Vet Immunol Immunopathol, 2008, 124(3-4): 332-340. DOI:10.1016/j.vetimm.2008.04.001 |
(编辑 白永平)



