2. 福建省预防兽医学与兽医生物技术重点实验室, 龙岩 364012
2. Fujian Provincial Key Laboratory for the Prevention and Control of Animal Infectious Diseases and Biotechnology, Longyan 364012, China
猪圆环病毒2型(porcine circovirus type 2,PCV2)可引起仔猪断奶后多系统衰竭综合征(postweaning multisystemic wasting syndrome,PMWS),目前PMWS已成为全球范围内危害猪群健康的重要疾病[1-2]。PCV2感染猪后主要入侵免疫器官,存在于猪的腹股沟淋巴结、肺、扁桃体、胸腺等组织的淋巴细胞和巨噬细胞中。PCV2感染以淋巴组织的细胞凋亡和组织细胞替换为特征,淋巴细胞破坏和白细胞数量减少可导致感染猪的免疫抑制[3-4]。
外泌体是活细胞分泌到细胞外的一种膜性小囊泡,为脂质双分子层的扁平或球形小体,直径在30~200 nm,该囊泡存在于多种体液中,包括尿液、血浆、腹水、唾液、乳汁、支气管肺泡灌洗液和羊水等[5],囊泡内包含细胞源的蛋白质、mRNA、miRNA、lncRNA等,具有调节免疫功能、介导病毒感染、转运细胞内物质等功能[6-8]。
研究显示外泌体在多种病毒的感染过程中起重要作用,如HIV、ALV-J、REV和HCV等,这些病毒包裹在外泌体内,逃避机体免疫监视,随着外泌体进行传播,引起机体持续性、隐蔽性感染[9-12]。虽然PCV2与这些病毒的感染机制不尽相同,但这些病毒包含在外泌体内形成潜在的免疫逃逸机制可能与PCV2感染具有相似性,所以笔者推测外泌体可能参与PCV2的传播与感染。本试验通过分离感染PCV2的PK-15细胞分泌的外泌体接种淋巴细胞,检测淋巴细胞感染率、淋巴细胞增殖率和淋巴细胞凋亡率,探讨PCV2-外泌体在PCV2感染淋巴细胞及诱导机体免疫抑制的作用。
1 材料与方法 1.1 细胞和病毒及主要试剂材料PK-15细胞系(无PCV污染)由本实验室保存,PCV2-SH(AY686763)病毒滴度为1×10-5.5TCID50·mL-1,pT-SH质粒由南京农业大学吕英军副教授赠送[13];兔抗TSG101多克隆抗体和PKH67荧光染料购自Sigma公司;兔抗CD81多克隆抗体购自北京博奥森公司;外泌体提取试剂盒购自上海UMIBIO公司;RIPA裂解液、CCK-8试剂盒、辣根过氧化物酶标记山羊抗兔IgG(H+L)抗体、BCA蛋白浓度测定试剂盒购自碧云天生物技术公司;普通PCR试剂、RNA提取、反转录试剂盒和荧光定量试剂购自TaKaRa公司;PCV2阳性血清为韩国金诺MEDIAN/JBT猪圆环病毒(PCV2) ELISA检测试剂盒产品;猪淋巴细胞分离液购自北京达科为公司;刀豆球蛋白A(ConA)和脂多糖(LPS)购自Sigma公司;Annexin V-FITC/PI Kit凋亡检测试剂盒购自北京四正柏生物有限公司;透射电镜为日本JEOL JEM1230,纳米颗粒跟踪分析测定仪为德国ZetaView (Particle Metri)。
1.2 外泌体的提取PK-15细胞在直径100 mm培养皿中培养12 h,融合度达到80%,用MOI=1.0的PCV2病毒接种细胞,感染1 h后,更换成含2%无外泌体血清的维持液继续培养60 h,收集细胞上清液。外泌体提取按照试剂盒说明书操作主要步骤如下:在4 ℃下2 000×g离心30 min去除死细胞,10 000×g离心30 min去除细胞碎片,上清液用0.22 μm滤器过滤去除微囊泡,将上清液转移至新的离心管中,按照体积比Exosomes Concentration Solution试剂:细胞上清液=1:4加入,旋涡振荡充分混匀,于4 ℃静置2 h。将混合液10 000×g离心60 min,收集沉淀,用PBS充分混匀,将混悬液12 000×g离心30 min,上清液即富含外泌体颗粒,外泌体颗粒粗品转入Exosomes Purafication Filter中,3 000×g离心10 min,收集管底滤液即为外泌体,纯化后的外泌体-80 ℃冰箱保存备用。未接毒PK-15细胞作为对照,细胞融合度达到80%时更换无外泌体血清,48 h后收集细胞培养上清,提取外泌体。将沉淀重新悬浮在100 μL PBS中,BCA试剂盒测定外泌体蛋白质浓度。
1.3 透射电镜观察将外泌体滴在2 mm铜网片上,用滤纸沿铜网片边缘轻轻擦拭,吸收多余水分。样品在室温下用醋酸双氧铀溶液染色5 min,干燥10 min,在80 kV下用透射电镜观察样品形态。
1.4 Western blot外泌体使用RIPA裂解液(含1%PMSF)裂解[14],与5×SDS变性缓冲液混合煮沸变性5 min,10%聚丙烯酰胺凝胶电泳分离,转移到0.22 μm PVDF膜,在37 ℃温箱中5%脱脂乳封闭2 h后,在4 ℃冰箱孵育一抗(TSG101和CD81抗体, 1:1 000稀释)过夜,室温孵育二抗(山羊抗兔IgG)2 h,用ECL显影液检测,具体步骤参照文献[15]。
1.5 PCR使用Trizol方法提取外泌体总RNA[14],根据说明书使用反转录试剂盒将RNA反转录成cDNA,以cDNA为模板,反应体系:2×Taq Master Mix 12.5 μL,模板2 μL,上下游引物各1 μL,ddH2O补足25 μL,引物序列如表 1所示。PCR反应程序:94 ℃ 5 min;94 ℃ 30 s,55 ℃ 30 s,72 ℃ 60 s,35个循环;72 ℃ 7 min。1%核酸电泳检测结果。
|
|
表 1 引物序列 Table 1 Sequence of primers |
根据PKH67试剂盒说明书,首先将外泌体加入到1 mL稀释剂C中,将PKH67浓度为4×10-6mol·L-1的PKH67加入到1.0 mL稀释剂C中,室温下与外泌体溶液孵育5 min,轻轻颠倒混匀。用1 mL 0.5% BSA溶液孵育1 min终止染色反应,标记的外泌体100 000 ×g离心1 h,沉淀用PBS悬浮,悬液加到PK-15细胞中孵育6 h,荧光显微镜观察PK-15细胞摄取外泌体情况。
1.7 间接免疫荧光PK-15细胞接种于带爬片的24孔板中,待细胞融合度至70%时,PCV2(MOI=1.0)和外泌体(质量浓度10 μg·mL-1)接种细胞[16],孵育2 h后更换成含2%血清的维持液培养72 h后,收取样品。爬片PBS洗3次,用4%的多聚甲醛固定,PBS洗3次,0.5% Triton X-100室温孵育细胞20 min,PBS洗3次,5% BSA 37 ℃下封闭1 h,一抗(PCV2阳性血清)4 ℃孵育过夜,二抗37 ℃孵育2 h,DAPI室温孵育10 min,抗荧光淬灭剂封片,置于荧光显微镜下观察、拍照。
1.8 猪脾淋巴细胞分离3头经检测PCV2抗原阴性的35日龄断奶仔猪,颈静脉放血致死,无菌采集新鲜脾,去除表面血污及纤维组织,机械法剥离分离脾淋巴细胞,经200目不锈钢网筛过滤分离细胞,1 500 r·min-1离心5 min,收集细胞沉淀,加入红细胞裂解液(0.15 mol·L-1 NH4CI,0.01 mol·L-1 KHCO3,0.000 1 mol·L-1 EDTA-Na2)裂解红细胞,37 ℃放置10 min,1 500 r·min-1,离心5 min,用1640培养液(含10%胎牛血清,1%双抗)重悬细胞,经淋巴细胞分离液分离淋巴细胞,用10% RPMI1640培养基悬浮细胞,将细胞在37 ℃二氧化碳培养箱中培养2 h除去贴壁细胞,收集上清液即为淋巴细胞,将细胞密度调整为1×106个·mL-1备用。
1.9 绝对定量PCR取“1.8”分离的淋巴细胞加入12孔板中,每孔1 mL,随机分为4组:对照组、PCV2组、外泌体组和外泌体裂解组,其中对照组为正常淋巴细胞,PCV2组、外泌体组37℃ 5% CO2培养箱培养2 h分别接种PCV2病毒(MOI=1.0)和PCV2-PK15外泌体(10 μg·mL-1),外泌体裂解组将外泌体用超声波破碎后再接种淋巴细胞,37 ℃ 5% CO2培养箱培养48 h,收取样品。提取样品RNA,按照“1.5”操作获得样品cDNA,备用。将提取好的pT-SH质粒进行梯度稀释,备用;将梯度稀释好的质粒与提取好的病毒DNA加入到PCR 8连管中,每个样品加3个重复,进行荧光定量PCR,PCV2引物为PCV2-F:CCAGGAGGGCGTTCTGAC,PCV2-R:CGTTACCGCTGGAGAAGGAA;根据梯度稀释的质粒浓度与3个重复样品的平均Ct值绘制标准曲线,并根据标准曲线计算病毒的拷贝数,拷贝数公式如下:
| $ 拷贝数 = \frac{{6.02 \times {{10}^{23}} \times {\rm{样品浓度}}\left( {{\rm{ng}} \cdot \mu {{\rm{L}}^{ - 1}}} \right)}}{{4646 \times 600}} $ |
取“1.8”分离的淋巴细胞加入96孔板中,每孔0.1 mL,随机分为4组,每组4个重复:对照组、PCV2组、外泌体组和外泌体裂解组,与“1.9”相同;将四组淋巴细胞在37 ℃ 5% CO2培养箱培养2 h后分别加入ConA(终质量浓度为5 mg·L-1)或LPS (终质量浓度为10 mg·L-1),继续培养至44 h,每孔加入10 μL CCK8,继续培养1 h,酶标仪测定OD450 nm值。
1.11 猪脾淋巴细胞凋亡试验按照“1.9”方法处理淋巴细胞,37 ℃ 5% CO2培养箱培养48 h,1 500 r·min-1离心5 min,收取样品,弃上清,加入100 μL 1×Binding Buffer轻轻重悬细胞,分别加入5和10 μL的Annexin Ⅴ-FITC和PI,室温避光孵育30 min,加入400 μL 1×Binding Buffer,BD Accur C6 Plus流式细胞仪检测与分析。
1.12 数据统计试验组与对照组的差异均用Spss 20.0软件进行分析,通过单因数方差分析法进行分析,结果以平均值±标准差(x±s)表示,*.P < 0.05表示差异显著,**.P < 0.01表示差异极显著,ns表示无显著差异。
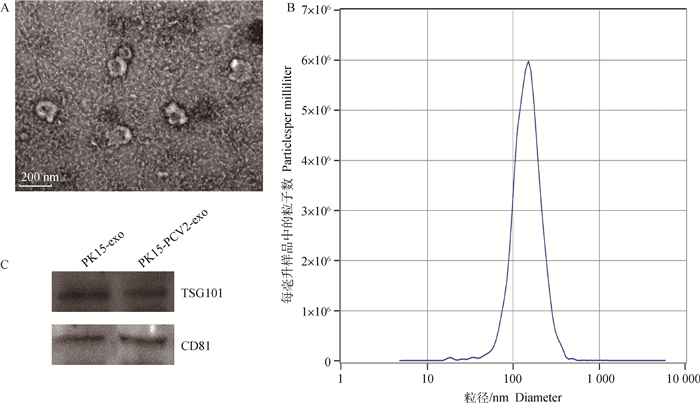
2 结果 2.1 PK-15细胞分泌外泌体特征从PK-15细胞上清液中分离外泌体,透射电镜观察外泌体的超微结构,结果如图 1A所示,外泌体呈典型圆形或椭圆形,具有凹盘状膜结构,直径在30~200 nm;为了进一步研究外泌体的大小分布,笔者使用NTA进行了粒径检测,显示在147.9 nm具有峰值(图 1B);Western blot结果如图 1C所示,外泌体存在特异性标记蛋白TSG101和CD81。这些结果表明PK-15细胞向培养基中释放了外泌体。
|
A.透射电镜观察外泌体的超微结构,bar=200 nm;B. NTA检测外泌体的粒径分布;C. Western blot检测外泌体的标志蛋白TSG101和CD81 A. The ultrastructure of exos by transmission electron microscopy, bar size 200 nm; B. The size distribution profile of exos by nanoparticle tracking analysis (NTA); C. Western blot analysis of surface markers TSG101 and CD81 in exosomes released by PK-15 cells 图 1 PK-15细胞分泌的外泌体形态 Fig. 1 Characterization of exosomes derived from PK-15 cells |
为了检测分离得到的外泌体是否具有进入细胞的活性,PKH67绿色荧光染料标记外泌体,观察外泌体进入细胞的情况。结果如图 2所示,PK-15细胞质内呈现绿色荧光颗粒,细胞核呈蓝色,试验结果表明外泌体在体外可以被细胞所摄取。

|
PK-15细胞孵育PKH67标记的外泌体(绿色)和细胞核DAPI(蓝色)。Bar=20 μm PK-15 cells were incubated with PKH67-labelled exosomes (green) and nuclei with DAPI (blue). Bar=20 μm 图 2 PK-15摄取外泌体 Fig. 2 The uptake of exosomes by PK-15 |
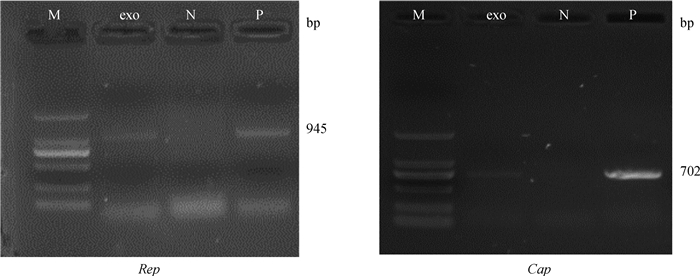
PCR检测PCV2感染细胞分泌的外泌体是否含有病毒DNA,其中positive是PCV2感染PK-15细胞DNA,negative是正常PK-15细胞DNA,对PCV2的基因组两个特异性片段(Rep和Cap)进行扩增,结果如图 3所示,从PCV2感染的PK-15细胞中分离的外泌体中可以扩增出两个片段,表明外泌体含有PCV2基因组DNA。
|
M. 2000 bp DNA相对分子质量标准;N.阴性对照;P.阳性对照 M. 2000 bp DNA marker; N. Negative; P. Positive 图 3 外泌体中PCV2基因组成分检测 Fig. 3 Components of exosomes derived from PCV2-infected cells |
为检测感染PCV2的PK-15细胞分泌的外泌体是否具有感染细胞能力,将从PCV2感染PK-15细胞中分离的外泌体孵育PK-15细胞,用间接免疫荧光法(IFA)检测感染情况。以PCV2感染细胞为阳性对照,PBS接种细胞为阴性对照。结果如图 4所示,感染PCV2的PK-15细胞分泌的外泌体重新被PK-15细胞摄取后,PK-15细胞中可见明显的阳性信号。这些结果表明,外泌体携带PCV2基因组可在细胞内建立阳性感染。

|
PCV2用抗PCV2抗体染色(绿色),细胞核用DAPI染色(蓝色)。A. DAPI; B. PCV2; C.合成图 The PCV2 was stained with an anti-PCV2 antibody (green) and the nucleus was stained with DAPI (blue). A. DAPI; B. PCV2; C. Merge 图 4 IFA检测PCV2-外泌体对PK-15细胞的感染 Fig. 4 The PK-15 cells infected with PCV2 |
为比较PCV2-Exo和PCV2感染淋巴细胞后细胞内病毒含量的差异,绝对荧光定量PCR结果如图 5所示,与PCV2组和PCV2-Exo lysate组相比,PCV2-Exo处理组的淋巴细胞内PCV2病毒拷贝数显著升高(P<0.01),结果表明外泌体能够促进PCV2对淋巴细胞的感染和复制。

|
**.P < 0.01 图 5 淋巴细胞内PCV2病毒的拷贝数检测 Fig. 5 Detection of PCV2 virus copy in lymphocyte |
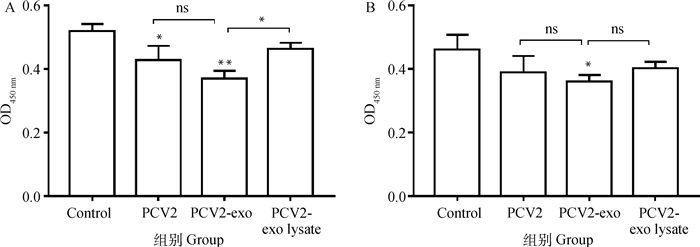
由图 6A结果可知,与空白对照组相比,脾淋巴细胞孵育PCV2-外泌体后,接受LPS刺激增殖能力极显著下降(P<0.01),PCV2组淋巴细胞增殖能力显著下降(P<0.05),PCV2-外泌体裂解后对淋巴细胞增殖能力有降低趋势,但无显著差异;与外泌体裂解组相比,PCV2-外泌体组淋巴细胞受LPS刺激增殖能力显著下降(P<0.05)。图 6B结果显示,与空白对照组相比,脾淋巴细胞孵育PCV2-外泌体后,接受Con A刺激增殖能力显著下降(P<0.05),PCV2组和PCV2-外泌体裂解组淋巴细胞增殖能力降低,但与对照组无显著差异;PCV2-外泌体组淋巴细胞增殖能力与PCV2组和外泌体裂解组相比有降低趋势,但无显著差异。
|
A. LPS刺激组;B.Con A刺激组。*.P < 0.05;**.P < 0.01; ns.P>0.05 A. LPS stimulate; B. Con A stimulate. *.P < 0.05;**.P < 0.01; ns.P>0.05 图 6 外泌体对猪脾淋巴细胞增殖活性影响 Fig. 6 Effects of exosomes on proliferation activity of swine splenic lymphocyte |
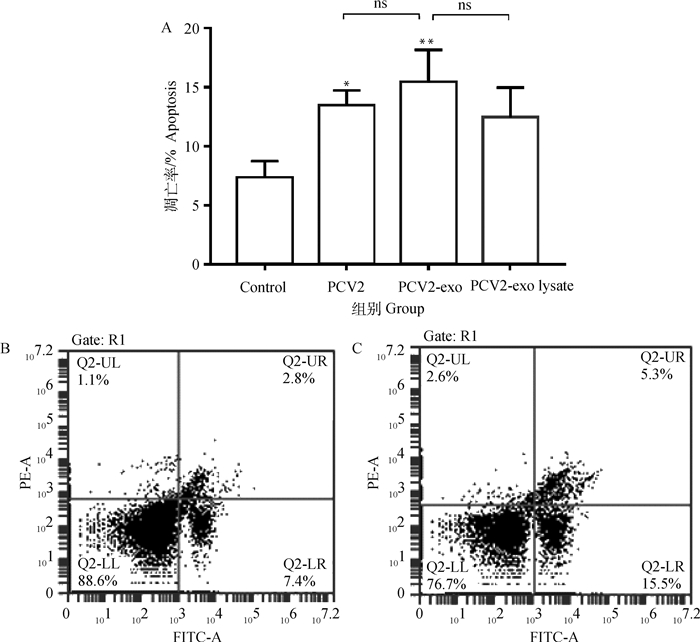
由图 7A结果可知,脾淋巴细胞感染PCV2后细胞凋亡率升高(图 7B),但与对照组无显著差异;与空白对照组相比,PCV2感染淋巴细胞后细胞凋亡率显著升高(P<0.05),PCV2-外泌体感染淋巴细胞后(图 7C)细胞凋亡率极显著升高(P<0.05);外泌体裂解后再孵育淋巴细胞可引起细胞凋亡率升高,但与对照组相比无显著差异。
|
A.淋巴细胞凋亡比例结果;B.对照组;C. PCV2-exo组。*.P < 0.05;**.P < 0.01; ns.P>0.05 A. Lymphocyte apoptosis results; B. Control group; C. PCV2 exo group. *.P < 0.05;**.P < 0.01; ns.P>0.05 图 7 外泌体对猪脾淋巴细胞凋亡影响 Fig. 7 Effects of Exosomes on apoptosis of swine splenic lymphocyte |
PCV2对猪群的危害主要是引起猪机体免疫抑制,感染组织中淋巴细胞和树突状细胞数量减少,CD4+和CD8+细胞比例失衡,细胞因子释放失衡,TNF-α、IL-2、IL-6等表达下调,IL-10等表达上调,严重影响猪机体免疫功能,使猪群免疫功能紊乱从而造成猪群免疫抑制,增加猪对其他病原菌的易感性,在临床中常常出现PCV2与其他疾病混合感染[17-18]。PCV2的主要靶细胞是巨噬细胞,PCV2感染猪后主要存在于猪的腹股沟淋巴结、肺、扁桃体、胸腺等组织和器官中的淋巴细胞和巨噬细胞[19-20]。有研究表明PCV2存在于树突状细胞中,但不影响这些细胞的活性和成熟,在此类细胞中出现大量的PCV2或病毒基因组,说明PCV2逃避了巨噬细胞的吞噬和降解,PCV2感染机体后可能处于免疫逃逸状态[21-22]。课题组前期对PCV2免疫抑制机制的研究表明,PCV2的靶细胞是巨噬细胞而发生凋亡的是脾淋巴细胞,功能研究表明,巨噬细胞能够介导PCV2诱导的淋巴细胞凋亡,去除巨噬细胞后,PCV2对细胞因子分泌及细胞损伤明显减弱[23],那么巨噬细胞感染PCV2后产生的相关信号分子是如何影响脾淋巴细胞呢?外泌体可能在这一过程中起到了至关重要的作用。
外泌体在细胞外环境中稳定存在,这些释放到胞外的外泌体可将其携带的膜受体和胞质成分转运至邻近或机体较远部位的受体细胞,从而介导细胞间通讯,调节靶细胞的代谢通路[24-25]。外泌体表面表达有CD55和CD59,避免了调理素、补体或凝血因子的激活,可在体液中稳定存在;同时由于其体积微小(< 100 nm),可有效避免单核巨噬细胞的吞噬,并能较自由地穿过血管壁及细胞外基质,因此可以在体液中广泛分布,外泌体最终可与周围细胞发生膜融合,实现生物膜循环[26-27]。目前,外泌体研究主要集中于分离、鉴定和功能分析。为去除血清内源性外泌体的影响,本试验中所用胎牛血清进行100 000×g离心12 h[28],采用试剂盒分离细胞上清液中的外泌体,透射电镜观察外泌体形态,动态光散射分析粒径,同时选择外泌体常见的标志蛋白(CD81、TSG-101)进行Western blot检测。试验结果显示,提取的外泌体呈双层膜结构,直径在30~200 nm,外泌体粒径分布曲线呈单峰,峰值顶点为147.9 nm,外泌体表面含有CD81、TSG-101标志蛋白,结果与其他文献描述的外泌体特征相一致[29]。PK-15细胞外泌体经过PKH67荧光染料染色后,观察外泌体能够被细胞所摄取。
许多病毒都能随外泌体进行传播,病毒可将病毒蛋白、病毒基因或完整的病毒颗粒载入其中,从而感染机体[30-32]。因此,外泌体途径在一定程度上补充了传统上对病毒传播机制的认识。外泌体携带病毒相关蛋白、病毒基因或病毒完整粒子感染细胞,在人类嗜T淋巴细胞1型感染的细胞系中发现包含使免疫调节异常的多效反式激活蛋白Tax的外泌体,Tax通过泛素化靶向进入外泌体,继而感染外周细胞[33];在HTLV-1阳性细胞系中分泌出的外泌体中发现病毒mRNA和miRNA,如tax和hbz[34];研究发现甲型肝炎病毒(HAV)借助类似于外泌体合成的途径,挟持内体膜包裹子代病毒,并介导其释放,这样释放的病毒颗粒具备完整的感染性[35]。这些研究表明病毒可以利用外泌体将自身伪装成有“包膜”的病毒颗粒,这些颗粒既能够促进病毒潜伏,又避免被机体中和抗体中和,从而协助病毒逃避机体免疫监视[36]。PCV2的传播和感染是否也利用了该途径,成为阐明PCV2免疫抑制致病机制新的研究思路。本试验中用PCV2感染PK-15细胞后,分离上清液中的外泌体,提取外泌体中的基因组,PCR检测结果显示外泌体基因组中包含了PCV2的Rep和Cap基因;将PCV2-外泌体重新孵育PK-15细胞后进行间接免疫荧光检测,结果在PCV2组和PCV2-外泌体组均出现阳性信号,试验结果表明PCV2-外泌体具有感染性。为进一步检测PCV2-外泌体对淋巴细胞的感染率,分离外周血淋巴细胞,分别孵育PCV2和PCV2-外泌体,绝对定量PCR结果显示,与PCV2组和外泌体裂解组相比,PCV2-外泌体孵育淋巴细胞后,淋巴细胞内PCV2含量显著升高,PCV2-外泌体裂解后再去孵育淋巴细胞,结果淋巴细胞内PCV2含量与PCV2组无显著差异,结果表明PCV2对淋巴细胞感染率低,外泌体在PCV2感染淋巴细胞中起重要作用。PCV2可在单核巨噬细胞、上皮细胞、内皮细胞和淋巴细胞等多种细胞内存在,淋巴细胞并非是PCV2的靶细胞,但在胞内也能检测到PCV2,课题组前期试验结果发现PCV2只有在淋巴细胞和巨噬细胞共存时才对淋巴细胞有感染率[14],本试验结果证实外泌体在这一过程中发挥重要作用。
刀豆球蛋白A(concanavalin A, ConA)和脂多糖(lipopolysaccharide, LPS)常用于诱导脾淋巴细胞增殖,分别用于评价T淋巴细胞和B淋巴细胞的增殖能力[37]。本研究发现淋巴细胞孵育PCV2-外泌体再接受LPS刺激后的增殖能力极显著降低,表明PCV2-外泌体能够抑制B淋巴细胞增殖,抑制分泌抗体的浆细胞的生成,抑制机体体液免疫功能;PCV2和裂解的外泌体也能够抑制B淋巴细胞增殖,但效果不如PCV2-外泌体显著;T淋巴细胞增殖结果显示,PCV2-外泌体可抑制T淋巴细胞增殖,抑制机体细胞免疫功能;将外泌体裂解后再孵育淋巴细胞,结果显示T/B淋巴细胞增殖都与PCV2组无显著差异,试验结果表明PCV2通过外泌体抑制淋巴细胞增殖。细胞凋亡检测结果显示,淋巴细胞感染PCV2后细胞凋亡率升高,孵育PCV2-外泌体后凋亡率显著升高,达到12.3%;外泌体裂解后诱导淋巴细胞凋亡作用降低,表明PCV2通过外泌体诱导淋巴细胞凋亡。
PCV2可引起仔猪体内淋巴细胞的耗竭,导致仔猪出现免疫抑制,严重影响仔猪健康。对PCV2免疫抑制的致病机制目前仍不清楚,有研究发现仔猪感染PCV2后一方面淋巴细胞凋亡增加,另一方面周围淋巴细胞增殖受抑制,且淋巴细胞衰竭主要是细胞增殖能力降低引起的,而不是细胞凋亡[38]。而本研究结果显示,PCV2-外泌体既显著抑制淋巴细胞增殖,又诱导淋巴细胞凋亡,表明外泌体在PCV2引起机体免疫抑制过程中起重要作用。
4 结论PK-15细胞感染PCV2后能够分泌外泌体,且符合外泌体的特征;PCV2-外泌体内包含病毒基因组,具有感染性,在感染淋巴细胞中起重要作用;PCV2-外泌体可抑制T/B淋巴细胞增殖,诱导淋巴细胞凋亡,在PCV2造成机体免疫抑制中起重要作用。
| [1] | RESENDES A R, SEGALÉS J. Characterization of vascular lesions in pigs affected by porcine circovirustype 2-systemic disease[J]. Vet Pathol, 2015, 52(3): 497–504. DOI: 10.1177/0300985814540542 |
| [2] | GRAU-ROMA L, HJULSAGER C K, SIBILA M, et al. Infection, excretion and seroconversion dyna-mics of porcine circovirus type 2(PCV2) in pigs from post-weaning multisystemic wasting syndrome (PMWS) affected farms in Spain and Denmark[J]. Vet Microbiol, 2009, 135(3-4): 272–282. DOI: 10.1016/j.vetmic.2008.10.007 |
| [3] | SHI L J, HAN H L, ZHANG S X. Virus location and lymphocyte apoptosis in lymph nodes of piglets infected with PCV-2[J]. Agric Sci China, 2008, 7(4): 507–512. DOI: 10.1016/S1671-2927(08)60096-6 |
| [4] | DOSTER A R, SUBRAMANIAM S, YHEE J Y, et al. Distribution and characterization of IL-10-secreting cells in lymphoid tissues of PCV2-infected pigs[J]. J Vet Sci, 2010, 11(3): 177–183. DOI: 10.4142/jvs.2010.11.3.177 |
| [5] | KELLER S, SANDERSON M P, STOECK A, et al. Exosomes:from biogenesis and secretion to biological function[J]. Immunol Lett, 2006, 107(2): 102–108. DOI: 10.1016/j.imlet.2006.09.005 |
| [6] | FANG J H, ZHANG Z J, SHANG L R, et al. Hepatoma cell-secreted exosomal microRNA-103 increases vascular permeability and promotes metastasis by targeting junction proteins[J]. Hepatology, 2018, 68(4): 1459–1475. DOI: 10.1002/hep.29920 |
| [7] | XIA T, LIAO Q, JIANG X M, et al. Long noncoding RNA associated-competing endogenous RNAs in gastric cancer[J]. Sci Rep, 2014, 4: 6088. DOI: 10.1038/srep06088 |
| [8] | SKOG J, WVRDINGER T, VAN RIJN S, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers[J]. Nat Cell Biol, 2008, 10(12): 1470–1476. DOI: 10.1038/ncb1800 |
| [9] | NARAYANAN A, IORDANSKIY S, DAS R, et al. Exosomes derived from HIV-1-infected cells contain trans-activation response element RNA[J]. J Biol Chem, 2013, 288(27): 20014–20033. DOI: 10.1074/jbc.M112.438895 |
| [10] | WANG G H, WANG Z Z, ZHUANG P P, et al. Exosomes carring gag/env of ALV-J possess negative effect on immunocytes[J]. Microb Pathog, 2017, 112: 142–147. DOI: 10.1016/j.micpath.2017.09.013 |
| [11] | ZHOU D F, XUE J W, HE S H, et al. Reticuloendotheliosis virus and avian leukosis virus subgroup J synergistically increase the accumulation of exosomal miRNAs[J]. Retrovirology, 2018, 15(1): 45. DOI: 10.1186/S12977-018-0427-0 |
| [12] | ZHANG C, YANG X, QI Q, et al. lncRNA-HEIH in serum and exosomes as a potential biomarker in the HCV-related hepatocellular carcinoma[J]. Cancer Biomark, 2018, 21(3): 651–659. DOI: 10.3233/CBM-170727 |
| [13] | HUANG B, ZHANG L L, LU M Q, et al. PCV2 infection activates the cGAS/STING signaling pathway to promote IFN-β production and viral replication in PK-15 cells[J]. Vet Microbiol, 2018, 227: 34–40. DOI: 10.1016/j.vetmic.2018.10.027 |
| [14] | GAO W, LIU H B, YUAN J, et al. Exosomes derived from mature dendritic cells increase endothelial inflammation and atherosclerosis via membrane TNF-α mediated NF-κB pathway[J]. J Cell Mol Med, 2016, 20(12): 2318–2327. DOI: 10.1111/jcmm.12923 |
| [15] | CHEN H B, ADAM A, CHENG Y F, et al. Localization and expression of heat shock protein 70 with rat myocardial cell damage induced by heat stress in vitro and in vivo[J]. Mol Med Rep, 2015, 11(3): 2276–2284. DOI: 10.3892/mmr.2014.2986 |
| [16] |
庄萍萍, 王小满, 孟薇, 等. 网状内皮增生症病毒感染改变外泌体蛋白质组成和免疫调节功能[J]. 中国细胞生物学学报, 2016, 38(6): 682–690.
ZHUANG P P, WANG X M, MENG W, et al. The infection of reticuloendotheliosis virus changed the protein composition and immunomodulation of exosome[J]. Chinese Journal of Cell Biology, 2016, 38(6): 682–690. (in Chinese) |
| [17] | FIGUEIREDO M M, AMORIM I F G, PINTO A J W, et al. Expression of Toll-like receptors 2 and 9 in cells of dog jejunum and colon naturally infected with Leishmania infantum[J]. BMC Immunol, 2013, 14(1): 22. DOI: 10.1186/1471-2172-14-22 |
| [18] |
刘月月, 吴家强, 任庆海, 等. 鲁中地区猪高热性疾病的病理变化及病原学调查研究[J]. 畜牧兽医学报, 2012, 43(12): 1917–1924.
LIU Y Y, WU J Q, REN Q H, et al. Pathological changes and etiological study of the Swine High Fever Syndrome (SHFS) in central region of Shandong Province[J]. Acta Veterinaria et Zootechnica Sinica, 2012, 43(12): 1917–1924. (in Chinese) |
| [19] | BECSKEI Z, NOVOSEL D, POLAČEK V, et al. Tissue distribution and tropism of PCV2 in first detected cases of porcine circovirus-associated disease (PCVAD) of wild boars in Serbia[J]. J Comp Pathol, 2015, 152(1): 86. DOI: 10.1016/j.jcpa.2014.10.181 |
| [20] | ROVIRA A, BALASCH M, SEGALÉS J, et al. Experimental inoculation of conventional pigs with porcine reproductive and respiratory syndrome virus and porcine circovirus 2[J]. J Virol, 2002, 76(7): 3232–3239. DOI: 10.1128/JVI.76.7.3232-3239.2002 |
| [21] | MCCULLOUGH K C, RUGGLI N, SUMMERFIELD A. Dendritic cells-at the front-line of pathogen attack[J]. Vet Immunol Immunopathol, 2009, 128(1-3): 7–15. DOI: 10.1016/j.vetimm.2008.10.290 |
| [22] | VINCENT I E, CARRASCO C P, HERRMANN B, et al. Dendritic cells harbor infectious porcine circovirus type 2 in the absence of apparent cell modulation or replication of the virus[J]. J Virol, 2003, 77(24): 13288–13300. DOI: 10.1128/JVI.77.24.13288-13300.2003 |
| [23] |
华利忠, 陈耿, 张书霞. 巨噬细胞介导PCV2诱导体外培养仔猪淋巴细胞凋亡的研究[J]. 南京农业大学学报, 2010, 33(3): 94–98.
HUA L Z, CHEN G, ZHANG S X. Study on macrophages mediated apoptosis of lymphocytes induced by PCV2 in vitro[J]. Journal of Nanjing Agricultural University, 2010, 33(3): 94–98. (in Chinese) |
| [24] | MELO S A, LUECKE L B, KAHLERT C, et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer[J]. Nature, 2015, 523(7559): 177–182. DOI: 10.1038/nature14581 |
| [25] | SANTANGELO A, IMBRUCÈ P, GARDENGHI B, et al. A microRNA signature from serum exosomes of patients with glioma as complementary diagnostic biomarker[J]. J Neurooncol, 2018, 136(1): 51–62. DOI: 10.1007/s11060-017-2639-x |
| [26] | CLAYTON A, HARRIS C L, COURT J, et al. Antigen-presenting cell exosomes are protected from complement-mediated lysis by expression of CD55 and CD59[J]. Eur J Immunol, 2003, 33(2): 522–531. DOI: 10.1002/immu.200310028 |
| [27] | TUCCI M, PASSARELLI A, MANNAVOLA F, et al. Serum exosomes as predictors of clinical response to ipilimumab in metastatic melanoma[J]. Oncoimmunology, 2018, 7(2): e1387706. DOI: 10.1080/2162402X.2017.1387706 |
| [28] | LÄSSER C, ELDH M, LÖTVALL J. Isolation and characterization of RNA-containing exosomes[J]. J Vis Exp Jove, 2012(59): 3037. DOI: 10.3791/3037 |
| [29] | WANG T, FANG L R, ZHAO F W, et al. Exosomes mediate intercellular transmission of porcine reproductive and respiratory syndrome virus[J]. J Virol, 2018, 92(4): e01734-17. |
| [30] | Longatti A. The dual role of exosomes in hepatitis A and C virus transmission and viral immune activation[J]. Viruses, 2015, 7(12): 6707–6715. DOI: 10.3390/v7122967 |
| [31] | CHAHAR H S, BAO X Y, CASOLA A. Exosomes and their role in the life cycle and pathogenesis of RNA viruses[J]. Viruses, 2015, 7(6): 3204–3225. DOI: 10.3390/v7062770 |
| [32] | COSSET F L, DREUX M. HCV transmission by hepatic exosomes establishes a productive infection[J]. J Hepatol, 2014, 60(3): 674–675. DOI: 10.1016/j.jhep.2013.10.015 |
| [33] | JAWORSKI E, NARAYANAN A, VAN DUYNE R, et al. Human T-lymphotropic virus type 1-infected cells secrete exosomes that contain Tax protein[J]. J Biol Chem, 2014, 289(32): 22284–22305. DOI: 10.1074/jbc.M114.549659 |
| [34] | EL-SAGHIR J, NASSAR F, TAWIL N, et al. ATL-derived exosomes modulate mesenchymal stem cells:potential role in leukemia progression[J]. Retrovirology, 2016, 13(1): 73. DOI: 10.1186/S12977-0160-0307-4 |
| [35] | FENG Z D, HENSLEY L, MCKNIGHT K L, et al. A pathogenic picornavirus acquires an envelope by hijacking cellular membranes[J]. Nature, 2013, 496(7445): 367–371. DOI: 10.1038/nature12029 |
| [36] | GOULD S J, BOOTH A M, HILDRETH J E K. The Trojan exosome hypothesis[J]. Proc Natl Acad Sci U S A, 2003, 100(19): 10592–10597. DOI: 10.1073/pnas.1831413100 |
| [37] | KRUISBEEK A M. Age-related changes in ConA-and LPS-induced lymphocyte transformation. Ⅰ. Effect of culture conditions on mitogen responses of blood and spleen lymphocytes from young and aged rats[J]. Mech Ageing Dev, 1976, 5: 125–138. DOI: 10.1016/0047-6374(76)90013-0 |
| [38] | MANDRIOLI L, SARLI G, PANARESE S, et al. Apoptosis and proliferative activity in lymph node reaction in postweaning multisystemic wasting syndrome (PMWS)[J]. Vet Immunol Immunopathol, 2004, 97(1-2): 25–37. DOI: 10.1016/j.vetimm.2003.08.017 |



