动物骨骼肌生长发育分为两个阶段,第一阶段是胚胎发育早期,即骨骼肌细胞增殖时期;第二阶段是胚胎发育后期,主要是肌纤维增粗时期。因此,胚胎发育期对动物出生后骨骼肌结构及生长至关重要[1-2]。羊骨骼肌结构特征研究表明,胚胎期是肌纤维生长发育的关键时期,直接决定肌纤维的种类、数量和状态[3]。大量研究发现,胚胎发育过程中一些转录因子和基因对胚胎骨骼肌的发育具有重要作用,如BHLH转录因子、Pax3和Pax7等在不同发育阶段的表达具有特异性。蛋白质是基因功能的执行者和生命活动的直接体现者,在骨骼肌生长发育中发挥重要作用[4]。因此,研究胚胎不同发育阶段上、下调差异蛋白质表达,分析候选蛋白质的理化性质、潜在修饰位点及三级结构有利于解析绵羊胚胎骨骼肌成肌机制,阐明骨骼肌生长发育机理[5]。
绵羊胚胎骨骼肌蛋白质组数据深入分析有助于解析绵羊胚胎骨骼肌生长发育机理及相关蛋白质表达规律。PRM靶向蛋白质组学技术具有无需抗体、特异性高、灵敏度高和通量高等优势,可以在复杂条件下定量和分析相对较小的蛋白质[6],已成为验证生长发育标记蛋白质的理想手段[7-9]。本试验将在前期研究的基础上,利用KEGG和PRM等技术分析绵羊胚胎骨骼肌上、下调蛋白质功能及富集通路,为揭示绵羊胚胎骨骼肌生长发育机理,筛选调控蛋白质奠定基础。
1 材料与方法 1.1 前期蛋白质组学定量结果前期选择体况良好、体重55~60 kg的成年中国美利奴母羊,进行同期发情与人工输精。并采集妊娠D85、D105和D135母羊的胚胎相同部位背最长肌为样品进行TMT蛋白质组学定量。结果共鉴定到5 520种蛋白质,其中差异丰度蛋白质1 316种,724种上调,592种下调[10]。基于以上结果,本试验利用KEGG和PRM等技术对上调和下调差异丰度蛋白质分别进行分析和验证,并对候选蛋白质进行生物信息学分析。
1.2 PRM靶向验证1.2.1 主要试剂和仪器 主要试剂:TMT标记试剂盒购自赛默飞世尔科技公司;乙腈购自Fisher Chemical公司;胰蛋白酶购自Promega公司;BCA试剂盒购自碧云天生物技术有限公司;二硫苏糖醇购自Sigma Aldrich公司;蛋白酶抑制剂购自Calbiochem公司;尿素(urea)购自Sigma公司;其他:乙二胺四乙酸(EDTA)和三乙基碳酸氢铵(TEAB)。主要仪器:Orbitrap LumosTM质谱和EASY-nLC 1200超高效液相系统购自赛默飞世尔科技公司。
1.2.2 验证蛋白质提取和酶解 将前期采集的样品从-80 ℃冰箱中取出,称取适量组织样品至液氮预冷的研钵中,加液氮充分研磨至粉末。将100 mg粉末样品转移到5 mL离心管中进行蛋白质提取,并使用胰蛋白酶将提取的蛋白质进行酶解。
1.2.3 高效液相色谱(high performance liquid chromatography, HPLC)分级和液相色谱-质谱分析 使用EASY-nLC 1200超高效液相系统对肽段进行分离,分离后的肽段被注入NSI离子源中进行电离,然后进Orbitrap LumosTM质谱进行分析,其离子碎片将使用高分辨的Orbitrap进行检测和分析,使用数据非依赖型扫描(DIA)程序进行数据采集。最后,使用Maxquant(v1.5.2.8)进行检索[11]。
1.2.4 PRM数据处理 利用Skyline(v.3.6)处理验证蛋白质谱数据。肽段参数:蛋白酶设置为Trypsin(KR/P),最大漏切位点数设置为0,肽段长度设置为7~25个氨基酸残基,设置半胱氨酸烷基化为固定修饰。Transition参数:母离子电荷设置为2,子离子电荷设置为1,离子类型设置为b、y和p。碎片离子选择从第三个开始到最后一个,离子匹配的质量误差设置为0.02 Da。
1.3 绵羊胚胎骨骼肌上调和下调差异丰度蛋白质KEGG分析利用KAAS v.2.0 (http://www.genome.jp/kaas-bin/kaas_main)、KEGG mapper V2.5 (http://www.kegg.jp/kegg/mapper.html)和Perl module (v.1.31https://metacpan.org/pod/Text::NSP::Measures::2D::Fisher)等数据库和软件对上调和下调差异丰度蛋白质分别进行富集分析[12]。
1.4 关键候选蛋白质生物信息学分析使用NCBI网站提供的BLAST程序(http://blast.ncbi.nlm.nih.gov/Blast.cgi)对中国美利奴绵羊PRKACA和GLUT4蛋白进行同源检索,并使用ExPASy网站的ProtParam(http://web.expasy.org/protparam/)预测和分析蛋白质的分子量、等电点等物理参数[13];使用ExPASy(http://www.expasy.org/proteomics)软件分析蛋白质潜在的磷酸化和糖基化等位点[14-16];利用Protein Homology/analogY Recognition Engine V 2.0(Phyre2,http://www.sbg.bio.ic.ac.uk/phyre2/html/page.cgi?id=index)预测蛋白质的三级结构。
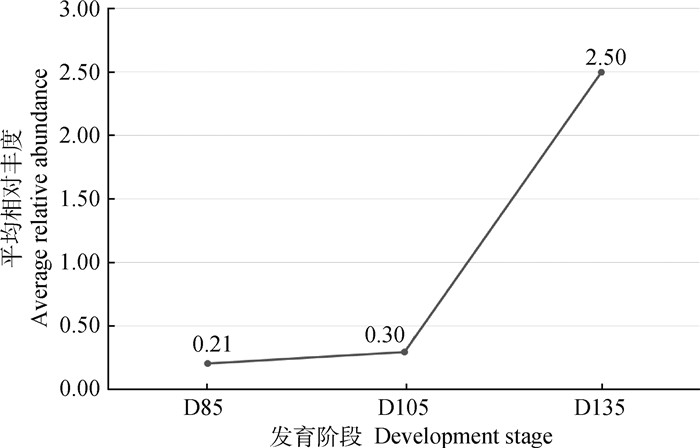
2 结果 2.1 PRM靶向验证利用PRM靶向定量肌纤维成熟分化标志性蛋白质MYH的两个特异性肽段,比较分析该蛋白TMT和PRM定量准确性及其表达变化趋势(表 1)。结果表明,MYH表达丰度呈现显著增加趋势(图 1)。
|
|
表 1 PRM靶向验证肽段及TMT和PRM定量结果比较 Table 1 PRM targeted validation protein and the comparison of quantification results between TMT and PRM |
|
图 1 不同发育阶段肌球蛋白重链(MYH)TMT定量平均丰度趋势图 Fig. 1 The TMT quantification trend of average relative abundance of myosin heavy chain (MYH) in different development stages |
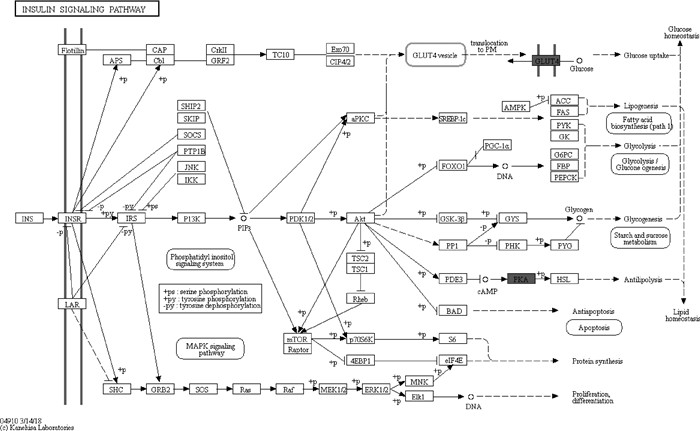
在前期鉴定到的5 520种蛋白质中,差异丰度蛋白质1 316种,724种上调,592种下调[10]。本试验针对上调和下调差异丰度蛋白分别进行了KEGG分析。结果显示,D105/D85比较组中上调差异丰度蛋白质显著富集于胰岛素信号通路,D135/D105和D135/D85比较组中下调差异丰度蛋白质显著富集于DNA复制和蛋白质消化吸收等信号通路,而在D105/D85比较组中没有富集到与生长发育相关的信号通路(图 2)。同时,差异丰度蛋白质cAMP依赖性蛋白激酶催化亚基α(PRKACA)和葡萄糖转运蛋白成员4(GLUT4)在D105/D85比较组中丰度较高,并显著富集于胰岛素信号通路(图 3)。由此可见,绵羊胚胎骨骼肌的3个发育阶段存在较大生物学差异。

|
a.D105/D85;b.D135/D105;c. D135/D85 图 2 上调和下调差异丰度蛋白质KEGG富集分析 Fig. 2 The KEGG enrichment analysis of up-regulation and down-regulation differential abundance proteins |
|
图 3 差异丰度蛋白质显著富集的胰岛素信号通路 Fig. 3 Insulin signaling pathway significantly enriched by differential abundance proteins |
2.3.1 PRKACA和GLUT4蛋白的理化性质 PRKACA和GLUT4蛋白分别具有351和190个氨基酸。使用ExPASy网站的ProtParam在线软件分析中国美利奴绵羊PRKACA和GLUT4蛋白的理化性质,推测其分子式分别为C5580H8567N1455 O1552S27和C971H1493N231O247S9;分子量分别为121.73和20.64 ku;理论等电点(pI)分别为8.81和6.54;半衰期分别为30和1.9 h;不稳定系数分别为30.87和29.34,均属于稳定蛋白;脂肪系数分别为81.73和109.32;亲水性平均系数(GRAVY)分别为-0.408和0.811,PRKACA属于亲水性蛋白;负电荷(Asp+Glu)氨基酸残基分别为135和12个,正电荷(Arg+Lys)氨基酸残基分别为147和12个。
2.3.2 PRKACA和GLUT4蛋白潜在磷酸化和糖基化预测 使用NetNGlyc 1.0 Server和NetPhos 3.1 Server分别预测PRKACA和GLUT4蛋白N-端糖基化和磷酸化情况,结果显示,PRKACA蛋白没有N-糖基化位点,同时预测该蛋白有45个磷酸化位点,其中有16个丝氨酸(Ser)磷酸化位点、15个苏氨酸(Thr)磷酸化位点、14个酪氨酸(Tyr)磷酸化位点。GLUT4蛋白在第112氨基酸残基处有一个N-糖基化位点,、11个苏氨酸(Thr)磷酸化位点、5个酪氨酸(Tyr)磷酸化位点(图 4)。

|
a.PRKACA没有N-糖基化位点;b. PRKACA有45个磷酸化位点;c. GLUT4有一个N-糖基化位点; d. GLUT4有25个磷酸化位点 a. No N-glycosylation site in PRKACA; b. 45 phosphorylation sites in PRKACA; c.One N-glycosylation site in GLUT4;d. 25 phosphorylation sites in GLUT4 图 4 PRKACA和GLUT4蛋白糖基化和磷酸化位点预测 Fig. 4 Predicted glycosylation and phosphorylation sites in PRKACA and GLUT4 |
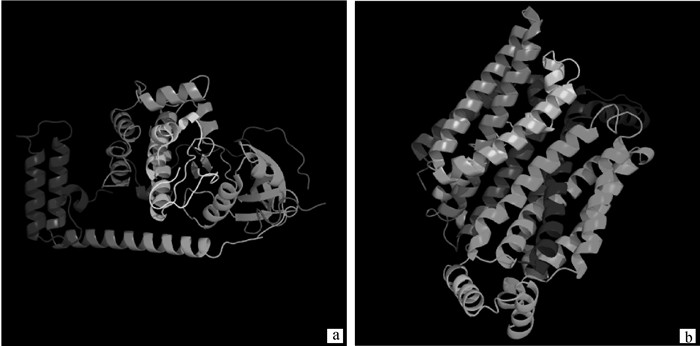
2.3.3 PRKACA和GLUT4蛋白三级结构预测 由PHYER2预测结果可知,PRKACA蛋白三级结构整体呈晶体结构,与CAMP-2依赖蛋白激酶a嵌合晶体结构相似度85%;GLUT4蛋白三级结构整体呈晶体结构,与人葡萄糖转运蛋白GLUT1的相似性为78%(图 5)。
|
图 5 PRKACA(a)和GLUT4(b)蛋白三级结构预测 Fig. 5 Predicted tertiary structure of PRKACA (a) and GLUT4 (b) proteins |
胚胎骨骼肌生长发育已成为研究热点[17],PRM技术为探索、识别和量化其蛋白质提供了有效方法[18-19]。该技术在复杂背景下具有更好的抗干扰能力和检测灵敏度[20-22]。本试验使用PRM验证了肌纤维成熟分化标志性蛋白质MYH,其变化趋势与绵羊骨骼肌生长发育规律一致,表明该技术与传统验证方法相比具有一定的优势。
本研究中,上调差异丰度蛋白质KEGG富集分析发现,在D105/D85比较组中胰岛素信号通路显著富集,该通路具有调节物质代谢、细胞增殖及生长发育的作用[23]。该信号通路通过磷酸化胰岛素受体信号转导蛋白(IRS),激活MAPK通路和磷脂酰肌醇3激酶(PI3K)途径来调控细胞增殖[24]。在此过程中,胰岛素受体(INSR)磷酸化SHC蛋白酪氨酸位点,并与下游含有SH2/3结合域的GRB2结合或由IRS直接激活Ras和Raf,从而使MAPK激酶1/2(MEK1/2)上的两个丝氨酸位点磷酸化,激活MAPK信号通路调控细胞增殖[25-29]。除可以通过MAPK途径进行信号转导外,研究发现,PI3K途径可以通过激活下游丝/苏氨酸蛋白激酶,调节下游mTOR、PDE3、GSK-3β和FOXO1等基因表达,从而实现对胚胎骨骼肌细胞增殖、蛋白质合成代谢、脂肪代谢和肌肉葡萄糖转运的调节。这表明PI3K途径在胰岛素信号通路调控肌细胞发育及增殖中也具有重要作用[30-34]。而显著富集于胰岛素信号通路的PRKACA编码丝/苏氨酸蛋白激酶催化亚基α(PRKACA),也称为cAMP依赖蛋白激酶,是PKA蛋白质的一种亚型,其能够调节生长、分化及糖原分化相关基因的表达,诱导细胞信号传导,且敲除该基因的小鼠存在生长迟缓现象[35-36]。另一个显著富集于胰岛素信号通路的GLUT4蛋白具有调控骨骼肌细胞和脂肪细胞葡萄糖摄取的作用。而GLUT4的激活不仅与MAPK信号通路相关,还与成肌细胞形成密切相关[37]。综上,在D105/D85比较组中胰岛素信号通路对绵羊胚胎骨骼肌细胞增殖及成肌细胞形成过程具有重要作用,PRKACA和GLUT4蛋白是调控胚胎骨骼肌增殖及成肌细胞形成的候选蛋白。
下调差异蛋白质KEGG富集分析表明,在D135/D105和D135/D85比较组中的下调差异蛋白质显著富集于DNA复制和蛋白质消化吸收等信号通路,以上富集信号通路对骨骼肌卫星细胞增殖凋亡及胚胎骨骼肌生长发育有一定的调控作用[38-39]。这些信号通路下调表明,在绵羊胚胎骨骼肌发育中后期可能存在肌纤维细胞增殖分化减弱的过程。猪肌肉生长与增粗的多组学整合研究也发现,蛋白质及转录因子主要参与细胞增殖凋亡、能量代谢、肌纤维构成和收缩等通路[40]。以上结果与本试验结果基本一致,强调了DNA复制和蛋白质消化吸收等信号通路与绵羊胚胎骨骼肌生长发育的相关性。
生物信息学分析表明,PRKACA和GLUT4具有丰富的磷酸化位点,表明可逆磷酸化调控在实现PRKACA和GLUT4蛋白质功能中起到重要作用。且候选蛋白质的三级结构整体紧凑,折叠结构较为复杂,推测这两种蛋白质具有多种生物功能。而蛋白的磷酸化过程与细胞间的级联反应和酶的生物活性等生物过程密切相关,并具有调控骨骼肌生长发育重要信号分子活性的功能[41-42]。此外,肌纤维成熟分化标志性蛋白MYH的验证结果变化趋势与绵羊胚胎骨骼肌生长发育趋势一致。综上,胰岛素信号通路分别在绵羊胚胎骨骼肌增殖分化及增粗中具有重要调控作用,PRKACA和GLUT4可作为调节胰岛素信号通路的关键候选蛋白。
4 结论本研究利用PRM技术高效、准确地验证了MYH表达趋势与绵羊胚胎骨骼肌生长发育趋势相符,为高通量蛋白质组学提供了有效验证手段。本研究对绵羊胚胎骨骼肌上调差异丰度蛋白质和下调差异丰度蛋白质数据进行了分析表明,上调差异丰度蛋白质显著富集于胰岛素信号通路,cAMP依赖性蛋白激酶催化亚基α (PRKACA)和葡萄糖转运蛋白成员4(GLUT4)具有丰富的磷酸化位点,参与调控胰岛素信号通路,是重要候选蛋白。
| [1] | WHITE R B, BIÉRINX A S, GNOCCHI V F, et al. Dynamics of muscle fibre growth during postnatal mouse development[J]. BMC Dev Biol, 2010, 10(1): 21. DOI: 10.1186/1471-213X-10-21 |
| [2] | BENTZINGER C F, WANG Y X, RUDNICKI M A. Building muscle:molecular regulation of myogenesis[J]. Cold Spring Harb Perspect Biol, 2012, 4(2): a008342. DOI: 10.1101/cshperspect.a008342 |
| [3] | ASHMORE C R, ROBINSON D W, RATTRAY P, et al. Biphasic development of muscle fibers in the fetal lamb[J]. Exp Neurol, 1972, 37(2): 241–255. DOI: 10.1016/0014-4886(72)90071-4 |
| [4] | HINDI S M, TAJRISHI M M, KUMAR A. Signaling mechanisms in mammalian myoblast fusion[J]. Sci Signal, 2013, 6(272): re2. |
| [5] | MURPHY M, KARDON G. Origin of vertebrate limb muscle:the role of progenitor and myoblast populations[J]. Curr Top Dev Biol, 2011, 96: 1–32. DOI: 10.1016/B978-0-12-385940-2.00001-2 |
| [6] | GALLIEN S, BOURMAUD A, KIM S Y, et al. Technical considerations for large-scale parallel reaction monitoring analysis[J]. J Proteomics, 2014, 100: 147–159. DOI: 10.1016/j.jprot.2013.10.029 |
| [7] | ALEKSANDR A D R G. Targeted proteomics:bench to bedside[J]. J Proteomics, 2018, 189: 3–4. |
| [8] | DOERR A. Targeting with PRM[J]. Nat Methods, 2012, 9(10): 950. DOI: 10.1038/nmeth.2193 |
| [9] | PICOTTI P, AEBERSOLD R. Selected reaction monitoring-based proteomics:workflows, potential, pitfalls and future directions[J]. Nat Methods, 2012, 9(6): 555–566. DOI: 10.1038/nmeth.2015 |
| [10] | WANG X Y, SHI T P, ZHAO Z D, et al. Proteomic analyses of sheep (ovis aries) embryonic skeletal muscle[J]. Sci Rep, 2020, 10(1): 1750. |
| [11] | COX J, NEUHAUSER N, MICHALSKI A, et al. Andromeda:a peptide search engine integrated into the MaxQuant environment[J]. J Proteome Res, 2011, 10(4): 1794–1805. |
| [12] |
李琳, 王磊, 王文静, 等. 基于串联质谱标签法和平行反应监测技术的氟喹诺酮耐药沙门菌蛋白质组学分[J]. 微生物学通报, 2018, 45(7): 1535–1545.
LI L, WANG L, WANG W J, et al. Fluoroquinolone resistant salmonella proteomics analysis based on tandem mass tag and parallel reaction monitoring techniques[J]. Microbiology China, 2018, 45(7): 1535–1545. (in Chinese) |
| [13] | GASTEIGER E, HOOGLAND C, GATTIKER A, et al.Protein identification and analysis tools on the ExPASy server[M]//WALKER J M.The Proteomics Protocols Handbook.Totowa: Humana Press, 2005. |
| [14] | BLOM N, SICHERITZ-PONTÉN T, GUPTA R, et al. Prediction of post-translational glycosylation and phosphorylation of proteins from the amino acid sequence[J]. Proteomics, 2004, 4(6): 1633–1649. |
| [15] | STEENTOFT C, VAKHRUSHEV S Y, JOSHI H J, et al. Precision mapping of the human O-GalNAc glycoproteome through simple cell technology[J]. EMBO J, 2013, 32(10): 1478–1488. DOI: 10.1038/emboj.2013.79 |
| [16] | BLOM N, GAMMELTOFT S, BRUNAK S. Sequence and structure-based prediction of eukaryotic protein phosphorylation sites[J]. J Mole Biol, 1999, 294(5): 1351–1362. |
| [17] | BUCKINGHAM M E. Muscle:the regulation of myogenesis[J]. Curr Opin Genet Dev, 1994, 4(5): 745–751. DOI: 10.1016/0959-437X(94)90142-P |
| [18] | MARX V. Targeted proteomics[J]. Nat Methods, 2013, 10(1): 19–22. DOI: 10.1038/nmeth.2285 |
| [19] | URISMAN A, LEVIN R S, GORDAN J D, et al. An optimized chromatographic strategy for multiplexing in parallel reaction monitoring mass spectrometry:insights from quantitation of activated kinases[J]. Mol Cell Proteomics, 2017, 16(2): 265–277. DOI: 10.1074/mcp.M116.058172 |
| [20] | PETERSON A C, RUSSELL J D, BAILEY D J, et al. Parallel reaction monitoring for high resolution and high mass accuracy quantitative, targeted proteomics[J]. Mol Cell Proteomics, 2012, 11(11): 1475–1488. DOI: 10.1074/mcp.O112.020131 |
| [21] |
王素兰, 高华萍, 张菁, 等. 基于稳定同位素标记和平行反应监测的蛋白质组学定量技术用于肝癌生物标志物的筛选和验证[J]. 色谱, 2017, 35(9): 934–940.
WANG S L, GAO H P, ZHANG J, et al. Stable isotope labeling and parallel reaction monitoring-based proteomic quantification for biomarker screening and validation of hepatocellular carcinoma[J]. Chinese Journal of Chromatography, 2017, 35(9): 934–940. (in Chinese) |
| [22] | TIMSON D J, TRAYER H R, SMITH K J, et al. Size and charge requirements for kinetic modulation and actin binding by alkali 1-type myosin essential light chains[J]. J Biol Chem, 1999, 274(26): 18271–18277. DOI: 10.1074/jbc.274.26.18271 |
| [23] | HAMELIN M, SAYD T, CHAMBON C, BOUIX J, et al. Proteomic analysis of ovine muscle hypertrophy[J]. J Anim Sci, 2006, 84(12): 3266–3276. DOI: 10.2527/jas.2006-162 |
| [24] |
吴智春, 王浩, 于华芸. 与细胞增殖有关的胰岛素信号通路[J]. 中国老年学杂志, 2009, 29(15): 1988–1990.
WU Z H, WANG H, YU H Y. Insulin signaling pathways associated with cell proliferation[J]. Chinese Journal of Gerontology, 2009, 29(15): 1988–1990. DOI: 10.3969/j.issn.1005-9202.2009.15.062 (in Chinese) |
| [25] | LOWENSTEIN E J, DALY R J, BATZER A G, et al. The SH2 and SH3 domain-containing protein GRB2 links receptor tyrosine kinases to ras signaling[J]. Cell, 1992, 70(3): 431–442. |
| [26] | SKOLNIK E Y, LEE C H, BATZER A, et al. The SH2/SH3 domain-containing protein GRB2 interacts with tyrosine-phosphorylated IRS1 and Shc:implications for insulin control of ras signalling[J]. EMBO J, 1993, 12(5): 1929–1936. |
| [27] |
张勇, 马勇, 朱宇旌, 等. p38丝裂原活化蛋白激酶信号途径调节骨骼肌生长发育的机理[J]. 动物营养学报, 2012, 24(1): 14–19.
ZHANG Y, MA Y, ZHU Y J, et al. Mechanism of p38 MAPK signalingpathway in regulating skeletal muscle growth and development[J]. Chinese Journal of Animal Nutrition, 2012, 24(1): 14–19. DOI: 10.3969/j.issn.1006-267x.2012.01.003 (in Chinese) |
| [28] | RAMOCKI M B, JOHNSON S E, WHITE M A, et al. Signaling through mitogen-activated protein kinaseand Rac/Rho does not duplicate the effects of activated Ras on skeletal myogenesis[J]. Mol Cell Biol, 1997, 17(7): 3547–3555. DOI: 10.1128/MCB.17.7.3547 |
| [29] | PAGE J L, WANG X, SORDILLO L M, et al. MEKK1signaling through p38 leads to transcriptionalinactivation of E47 and repression of skeletal myo-genesis[J]. J Biol Chem, 2004, 279(30): 30966–30972. DOI: 10.1074/jbc.M402224200 |
| [30] | TOBE K, ASAI S, MATUOKA K, et al. Cytoskeletal reorganization induced by insulin:involvement of Grb2/Ash, Ras and phosphatidylinositol 3-kinase signalling[J]. Genes Cells, 2003, 8(1): 29–40. |
| [31] | JENSEN J, BRENNESVIK E O, LAI Y C, et al. GSK-3β regulation in skeletal muscles by adrenaline and insulin:evidence that PKA and PKB regulate different pools of GSK-3[J]. Cell Signal, 2007, 19(1): 204–210. DOI: 10.1016/j.cellsig.2006.06.006 |
| [32] | AHMAD M, FLATT P R, FURMAN B L, et al. The role of the cyclic GMP-inhibited cyclic AMP-specific phosphodiesterase (PDE3) in regulating clonal BRIN-BD11 insulin secreting cell survival[J]. Cell Signal, 2000, 12(8): 541–548. DOI: 10.1016/S0898-6568(00)00093-0 |
| [33] | VANDER HAAR E, LEE S I, BANDHAKAVI S, et al. Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40[J]. Nat Cell Biol, 2007, 9(3): 316–323. DOI: 10.1038/ncb1547 |
| [34] |
张小宁, 陆健, 任航行, 等. Texel和乌珠穆沁绵羊妊娠中后期胎儿背最长肌中AKT基因的发育表达模式[J]. 中国畜牧兽医, 2012, 39(8): 20–25.
ZHANG X N, LU J, REN H X, et al. Developmental expression patterns of AKT gene in fetal longissimus dorsi muscle between texel and ujumqin sheep during the second half of gestation[J]. China Animal Husbandry & Veterinary Medicine, 2012, 39(8): 20–25. DOI: 10.3969/j.issn.1671-7236.2012.08.005 (in Chinese) |
| [35] | TURNHAM R E, SCOTT J D. Protein kinase a catalytic subunit isoform PRKACA; history, function and physiology[J]. Gene, 2016, 577(2): 101–108. DOI: 10.1016/j.gene.2015.11.052 |
| [36] | SKÅLHEGG B S, HUANG Y Z, SU T, et al. Mutation of the calpha subunit of PKA leads to growth retardation and spermdysfunction[J]. Mol Endocrinol, 2002, 16(3): 630–639. |
| [37] | NIU W Y, HUANG C, NAWAZ Z, et al. Maturation of the regulation of GLUT4 activity by p38 MAPK during L6 cell myogenesis[J]. J Biol Chem, 2003, 278(20): 17953–17962. DOI: 10.1074/jbc.M211136200 |
| [38] | MORIYA N, MIYAZAKI M. Akt1 deficiency diminishes skeletal muscle hypertrophy by reducing satellite cell proliferation[J]. Am J Physiol Regul Integr Comp Physiol, 2018, 314(5): R741–R751. DOI: 10.1152/ajpregu.00336.2017 |
| [39] |
郑琪, 睢梦华, 凌英会. 骨骼肌卫星细胞增殖与成肌分化过程中关键信号通路的作用[J]. 畜牧兽医学报, 2007, 48(11): 2005–2014.
ZHENG Q, SUI M H, LING Y H. The role of key signalingpathways in the proliferation and differentiation of skeletal muscle satellite cells[J]. Acta Veterinaria et Zootechnica Sinica, 2007, 48(11): 2005–2014. (in Chinese) |
| [40] | LIU S Q, HAN W P, JIANG S Y, et al. Integrativetranscriptomics and proteomics analysis of longissimus dorsi muscles of Canadian double-muscled large white pigs[J]. Gene, 2016, 577(1): 14–23. DOI: 10.1016/j.gene.2015.11.016 |
| [41] |
辛向博.Myostatin基因对骨骼肌代谢机制影响的定量蛋白组学与磷酸化蛋白组学研究[D].天津: 天津农学院, 2018.
XIN X B.Quantitative proteomics andphosphoproteomics study on the effect of Myostatin gene on skeletal muscle metabolism[D].Tianjin: Tianjin Agricultural University, 2018.(in Chinese) http://cdmd.cnki.com.cn/Article/CDMD-10061-1018193004.htm |
| [42] | YANG S P, LI X F, LIU X F, et al. Parallel comparative proteomics and phosphoproteomics reveal that cattle myostatin regulates phosphorylation of key enzymesin glycogen metabolism and glycolysis pathway[J]. Oncotarget, 2018, 9(13): 11352–11370. DOI: 10.18632/oncotarget.24250 |



