2. 重庆市高校兽医科学工程研究中心中兽药创新研发实验室, 重庆 402460;
3. 无锡正大生物股份有限公司动物保健品厂, 新吴 214112
2. Chinese Veterinary Herbal Drugs Innovation Research Lab, University Veterinary Science Engineering Research Center in Chongqing, Chongqing 402460, China;
3. Wuxi Zhengda Biological Co., Ltd. Animal Health Products Factory, Xinwu 214112, China
传染性支气管炎(infectious bronchitis, IB)是鸡的一种急性、高度接触传染的病毒性呼吸道和泌尿生殖道疾病,其特征是咳嗽、喷嚏、气管啰音和呼吸道黏膜呈浆液性卡他性炎症[1]。雏鸡常表现呼吸困难、流鼻液等呼吸道症状,有时会发生死亡;产蛋鸡则以产蛋量减少和蛋白品质下降较为常见[2]。本病一年四季都可发生,但以气候寒冷的季节较为严重,在现代养殖业中,由于养殖密度大、通风不良、过热、过冷、缺乏维生素和矿物质、饲料中的营养成分配比失当及其他不良应激因素会促进本病的发生,IB倍受兽医工作者的重视[3]。雏鸡感染传染性支气管炎病毒(infectious bronchitis virus, IBV)后,容易出现畏寒扎堆、甩头、打喷嚏、流鼻涕、双翅下垂、气管啰音等现象。雏鸡细胞免疫和体液免疫常发生一定程度的降低[4]。感染过程中伴随着炎症反应和免疫病理反应,涉及多种炎性细胞、炎症介质、免疫细胞和细胞因子,其中T淋巴细胞是主要的免疫细胞,其数量与比例是衡量机体免疫水平的重要指标[5]。IFN-γ、IL-6、IL-10在免疫调节、抗病毒和介导炎症反应等方面发挥着重要的生物学功能[6-7]。中兽医认为该病主要是由于感受风热,内侵于肺致肺失宣发和肃降,宜清热解毒、宣肺止咳、祛痰散结。板芩桔甘合剂(BQJGHJ)主要由板蓝根、黄芩、桔梗、甘草等中药提取制成,具有清肺化痰、止咳平喘的功能。本试验拟通过探究BQJGHJ对人工感染IBV-M41雏鸡疗效与血清中IFN-γ、IL-6、IL-10含量的影响,探讨板芩桔甘合剂治疗鸡传染性支气管炎的可能作用机制,为其临床应用提供理论依据。
1 材料与方法 1.1 试验材料21日龄健康三黄雏鸡,120羽,分笼饲养,饲养舍温度28~30 ℃,湿度55%~65%,自由饮水,试验前适应性饲养一周。饲喂以雏鸡配合饲料(510),产品标准为GB/T 10262-1996,主要成分:粗蛋白质18.0%、粗灰分8.0%、粗纤维6.0%、粗脂肪2.5%、钙1.2%、赖氨酸0.8%、氯化钠0.8%、总磷0.5%、蛋氨酸0.3%。BQJGHJ:由无锡正大生物股份有限公司动物保健品厂提供,批号161215,包装100 mL·瓶-1,规格1 mL相当于原生药1.08 g。阳性对照药物:甘胆口服液,批号1807021B,北京生泰尔科技股份有限公司生产,包装200 mL·瓶-1,规格100 mL相当于原生药24.4 g。IFN-γ、IL-6、IL-10检测试剂盒购自厦门惠嘉生物技术有限公司,批号201811。试验毒株: IBV-M41毒株购自中国兽医微生物菌种保藏中心,批号AV1511。
1.2 主要仪器设备高速离心机(THERMO公司ST16R);隔水式恒温培养箱(上海精宏实验设备有限公司GNP-9080);荧光定量PCR仪(Roche公司LightCycler 96);-86 ℃超低温冷冻冰箱(中科美菱低温科技股份有限公司DW-HL678);酶标仪(BIO-RAD公司iMark)等仪器。
1.3 试验方法 1.3.1 雏鸡分组及处理将适应性饲养一周的雏鸡120只随机分为空白组、模型组、阳性药物组和BQJGHJ高、中、低剂量组,每组20只。适应性喂养结束后,除空白组外,其余各组鸡给予IBV-M41株进行造模[8-11];造模4 d后,除空白组、模型组自由饮水外,BQJGHJ高、中、低剂量组雏鸡分别进行饮水给药,每升饮水添加2.0、1.0和0.5 mL BQJGHJ;阳性药物组雏鸡饮水给药,每升饮水添加0.7 mL甘胆口服液,1次·d-1,连续给药5 d。各组雏鸡喂以基础日粮,自由采食。各组雏鸡在造模后、给药第3、5天及停药后第2天分别自翅下静脉采集血液(空白组同时采血),每组均采集同号10只雏鸡,3 500 r·min-1离心10 min,分离血清,于-80 ℃条件下保存,备用。
1.3.2 临床症状观察试验期间,根据临床诊断标准,观察试验鸡采食、饮水以及精神状况,有无畏寒扎堆,甩头,流鼻涕,打喷嚏等现象,双翅有无下垂,是否伴有咳嗽症状,气管是否有啰音(尤其夜间最为明显),呼吸是否正常等,并记录。
1.3.3 板芩桔甘合剂对传染性支气管炎雏鸡临床症状评分与疗效评定参考《兽医传染病学》及临床病例纳入标准,制定实验性临床评价记分标准,见表 1;疗效及疗效判定标准见表 2,观察鸡的临床症状变化,对其症状评分,进行记分计算,评价板芩桔甘合剂对鸡传染性支气管炎的疗效,计算总有效率,有效率=(痊愈数+显效数+好转数)/总发病数×100%。
|
|
表 1 传染性支气管炎实验性临床评价记分 Table 1 The experimental clinical evaluation score of infectious bronchitis |
|
|
表 2 疗效判定标准 Table 2 The criteria for judging curative effect |
RT-PCR法检测支气管组织是否有特异性条带,根据GenBank中参照IBV-M41的N基因设计一对引物,正向引物: 5′-ATACGCCTACTCAATCGC-3′,反向引物: 5′-TTTCCAGTTGCCTTACCG-3′,扩增子长度239 bp,引物由上海生物工程有限公司合成。
试验结束后,各组分别随机选取3只雏鸡采集支气管,严格按照试剂盒使用说明书要求进行总RNA的提取、cDNA的合成和PCR扩增[12],取5 μL DNA Marker和8 μL PCR产物,用1%琼脂糖凝胶电泳检测目的条带,于凝胶成像系统拍照记录,根据扩增条带,判断模型组雏鸡支气管上是否有IBV-M41病毒。
1.3.5 BQJGHJ对人工感染IBV-M41雏鸡血清中IFN-γ、IL-6、IL-10含量的影响采用酶联免疫吸附法(ELISA)检测IL-6、IL-10和IFN-γ的含量,操作步骤严格按照试剂盒说明书进行,以空白孔调零,在450 nm波长检测各孔的OD值,计算各样本的含量。
1.3.6 统计分析用SPSS20.0软件进行数据处理。试验数据采用图表和柱状图形式表示,采用单因素方差分析和多重比较。
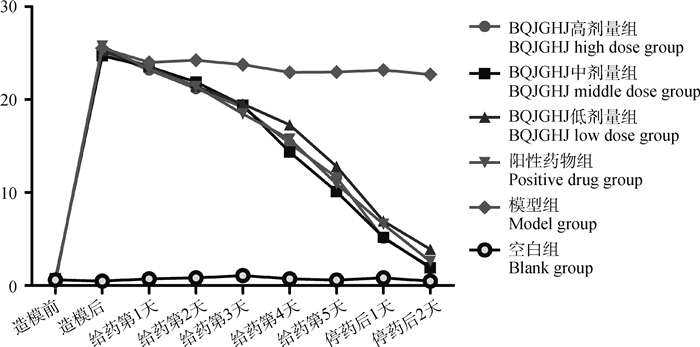
2 结果 2.1 BQJGHJ对雏鸡传染性支气管炎不同时间段治疗症状记分的影响由图 1可知,模型组雏鸡症状记分一直处于较高水平,BQJGHJ高、中、低剂量组和阳性药物组雏鸡症状记分随着治疗进程而降低,BQJGHJ高、中、低剂量组与阳性药物组雏鸡治疗症状记分,与模型组相比,差异显著(P < 0.05);BQJGHJ高、中、低剂量组雏鸡治疗症状记分,与阳性药物组相比,无显著性差异(P>0.05)。BQJGHJ高、中、低剂量组与阳性药物组临床治疗效果相一致。
|
图中未标注差异性 Difference is not marked in the figure 图 1 BQJGHJ对传染性支气管炎雏鸡不同时间段治疗症状记分 Fig. 1 The symptom score of infectious bronchitis chicks treated with BQJGHJ in different periods |
在造模第4天,模型组雏鸡陆续出现食欲下降,畏寒扎堆、皮温升高、饮水增加,甩头,流鼻涕,双翅下垂,伴有咳嗽、呼吸急促,气管出现明显啰音(尤其夜间最为明显)等现象;模型组雏鸡在造模第4天后,无药物治疗第2天死亡2只,第4天死亡1只;BQJGHJ高剂量组雏鸡在药物治疗第2天死亡2只,BQJGHJ低剂量组和阳性药物组雏鸡在药物治疗第3天各死亡2只,BQJGHJ中、低剂量组雏鸡在药物治疗第4天各死亡1只。阳性药物组雏鸡治愈率、有效率分别为70%、80%;BQJGHJ高剂量组雏鸡治愈率、有效率分别为75.0%、90.0%;BQJGHJ中剂量组雏鸡治愈率、有效率分别为80.0%、95.0%;BQJGHJ低剂量组雏鸡治愈率、有效率分别为65.0%、80.0%;BQJGHJ高、中剂量组雏鸡治愈率和有效率高于阳性药物组,详见表 3。随机采集各组3只雏鸡支气管,模型组雏鸡支气管样品的PCR扩增显示明显核苷酸条带,阳性药物组、BQJGHJ高、中、低剂量组和空白组雏鸡支气管样品未出现条带,详见图 2。该结果表明,雏鸡经过药物治疗后能有效降低其支气管中IBV-M41病毒水平。
|
|
表 3 BQJGHJ对传染性支气管炎雏鸡的临床治疗效果 Table 3 The clinical effect of BQJGHJ on chicks with infectious bronchitis |

|
M.DNA相对分子质量标准;1~3.模型组;4~5.阳性药物组;6~8.BQJGHJ高剂量组;9~11.BQJGHJ中剂量组;12~14.BQJGHJ低剂量组;15.空白组 M.DNA marker; 1-3. Model group; 4-5. Positive drug group; 6-8. BQJGHJ high dose group; 9-11. BQJGHJ middle dose group; 12-14. BQJGHJ low dose group; 15. Blank group 图 2 各组雏鸡的IBV-M41检测 Fig. 2 IBV-M41 detection of each group |
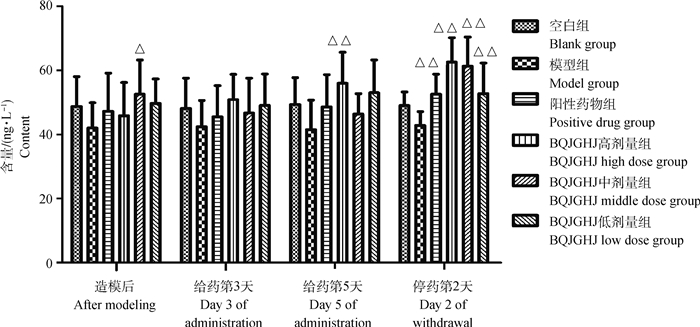
由图 3可知,模型组雏鸡血清中IFN-γ含量降低,但与空白组相比,无显著性差异(P>0.05);在造模后到给药第5天,BQJGHJ高剂量组雏鸡血清中IFN-γ含量升高,与模型组相比,在给药后第5天差异极显著(P < 0.01);BQJGHJ高、中、低剂量组雏鸡血清中IFN-γ含量,与阳性药物组相比,无显著性差异(P>0.05)。在停药后第2天,BQJGHJ高、中、低剂量组、阳性药物组雏鸡血清中IFN-γ含量升高,与模型组相比,差异极显著(P < 0.01);BQJGHJ高、中、低剂量组雏鸡血清中IFN-γ含量,与阳性药物组相比,无显著性差异(P>0.05)。
|
模型组vs空白组,*. P < 0.05,**. P < 0.01;各药物组(阳性药物组及不同剂量BQJGHJ)vs模型组,Δ. P < 0.05,ΔΔ. P < 0.01。下同 The model group vs the blank group, *. P < 0.05, **. P < 0.01;the drug groups (positive drug and different doses of BQJGHJ) vs the model group, Δ. P < 0.05, ΔΔ. P < 0.01. The same as below 图 3 不同时间段BQJGHJ对人工感染IBV-M41雏鸡血清中IFN-γ含量的影响 Fig. 3 The effect of BQJGHJ on the content of IFN- γ in serum of chicks artificially infected with IBV-M41 at different time points |
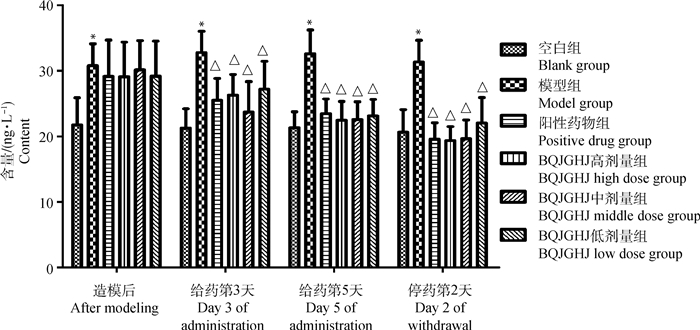
由图 4可知,模型组雏鸡血清中IL-6含量升高,与空白组相比,差异显著(P < 0.05);在给药第3、5天以及停药后第2天,BQJGHJ高、中、低剂量组、阳性药物组雏鸡血清中IL-6含量降低,与模型组相比,差异显著(P < 0.05);BQJGHJ高、中、低剂量组雏鸡血清中IL-6含量,与阳性药物组相比,无显著性差异(P>0.05)。
|
图 4 不同时间段QJGHJ对人工感染IBV-M41雏鸡血清中IL-6含量的影响 Fig. 4 The effect of QJGHJ on IL-6 content in serum of chicks artificially infected with IBV-M41 at different time points |
由图 5可知,模型组雏鸡血清中IL-10含量降低,与空白组相比,差异显著或极显著(P < 0.05或P < 0.01)。在给药第3天,BQJGHJ高、中、低剂量组、阳性药物组鸡血清中IL-10含量升高,与模型组相比,无显著性差异(P>0.05)。在给药第5天和停药后第2天,BQJGHJ高、中、低剂量组、阳性药物组雏鸡血清中IL-10含量升高,与模型组相比,差异极显著(P < 0.01);BQJGHJ高、中、低剂量组雏鸡血清中IL-10含量,与阳性药物组相比,无显著性差异(P>0.05)。

|
图 5 不同时间段BQJGHJ对人工感染IBV-M41雏鸡血清中IL-10含量的影响 Fig. 5 The effect of BQJGHJ on IL-10 content in serum of chicks artificially infected with IBV-M41 at different time points |
肺主一身之表,外合皮毛,当外邪侵犯机体时,主要由皮毛侵入,肺先受之[13-14]。雏鸡感染IBV后,热毒内蕴,热盛雍肺,热伤津液,痰涎阻肺,肺失肃降引发咳喘、呼吸困难,鼻腔有鼻汁,气管有啰音等[15-16],治疗宜清热解毒、止咳祛痰。中草药中,用于防治鸡传染性支气管炎的药物主要包括清热药、解表药、化痰止咳平喘药等三类[17-18],清热药主要有板蓝根、连翘、金银花、生石膏[19],解表药主要有麻黄、白芷、荆芥、防风[20],化痰止咳平喘药主要有桔梗、贝母、百部[21]。BQJGHJ主要由板蓝根、黄芩、桔梗、甘草等中药提取制成,具有清肺化痰、止咳平喘的功能。方中板蓝根清热解毒,消肿利咽,为治咽要药,咽通则气顺;桔梗辛散,止咳祛痰、宣肺,与黄芩同用,有清热燥湿,宽胸利膈,祛胸膈之热功效[22-23];且方中加入甘草,能缓能解,用于气虚诸证及减毒。全方配伍合理,标本兼治,清热为本,兼以化痰、止咳平喘之功效。本试验通过点眼滴鼻接种IBV-M41活化的鸡胚尿囊液0.4 mL·只-1造模发病,BQJGHJ治疗高、中、低剂量组的治愈率分别为75.0%、80.0%、65.0%,有效率分别为90.0%、95.0%、80.0%,阳性药物组的治愈率、有效率分别为70%、80%。张文彬等[24]用牛黄、板蓝根等作主耍药材制成的中药粒剂,其对传染性支气管炎的治愈率高达95%。本试验结果与文献[25]报道相一致。
IBV-M41感染雏鸡后,细胞免疫和体液免疫功能处于低下与失衡状态[26-27]。当机体受病毒刺激后,T淋巴细胞可产生多种细胞因子。根据CD4+Th细胞分泌的细胞因子不同,Th细胞分为Th0、Th1和Th2共3个亚群[28]。受细胞因子、抗原特性和激素等的影响,Th0细胞向Th1细胞或Th2细胞分化。Th1细胞主要分泌IFN-γ,可增强机体细胞免疫功能,在抗病毒感染中发挥积极作用[29]。Th2细胞主要分泌IL-6、IL-10等细胞因子,IL-6与炎症反应密切相关。在无病理状态下,机体内Th1/Th2处于平衡状态,对免疫调节起重要作用。IL-10是一种多细胞源、多功能的细胞因子,调节细胞的生长与分化,参与炎性反应和免疫反应,是目前公认的炎症与免疫抑制因子,IFN-γ和IL-6可分别作为Th1和Th2细胞的特征性细胞因子[30],在机体发生炎症时,IL-6大量分泌,使血液和淋巴循环中的炎性细胞在病变组织或周围浸润积聚,进一步释放炎性因子等活性产物,加重感染症状并延长了病程[31]。通过检测这两种细胞因子可以了解Th1和Th2功能状态,更好地了解IBV-M41的发病机制[32]。本试验结果显示,BQJGHJ高、中、低剂量组雏鸡血清中IL-6水平降低,IFN-γ、IL-10水平升高,且BQJGHJ低剂量组IFN-γ含量也较模型组明显升高,而其对上述细胞因子的影响与阳性药物组比较无统计学差异,但BQJGHJ高剂量组明显优于低剂量组。表明BQJGHJ对人工感染IBV-M41模型雏鸡血清中IFN-γ、IL-6、IL-10的影响存在一定的量效关系,以BQJGHJ高剂量组效果最佳。以上研究结果提示,BQJGHJ可通过调节促炎与抗炎细胞因子动态平衡而发挥免疫调节作用,缓解IBV-M41对机体破坏,从而达到治疗传染性支气管炎的目的。
4 结论板芩桔甘合剂对人工感染IBV-M41雏鸡有治疗效果,其作用机制与其降低传染性支气管炎雏鸡血清中IL-6的含量,升高雏鸡血清中IFN-γ、IL-10的含量,增强免疫有关。
| [1] |
刘帆, 任广彩, 闫圆圆, 等. 鸡传染性支气管炎病毒的分离鉴定及其致病性的研究[J]. 中国兽医科学, 2019, 49(7): 861–870.
LIU F, REN G C, YAN Y Y, et al. Isolation, identification and pathogenicity of infectious bronchitis virus[J]. Chinese Veterinary Science, 2019, 49(7): 861–870. (in Chinese) |
| [2] |
黄梦姣, 张芸, 薛春宜, 等. 应对日益严峻的挑战:中国禽传染性支气管炎研究[J]. 微生物学通报, 2019, 46(7): 1837–1849.
HUANG M J, ZHANG Y, XUE C Y, et al. To meet the growing challenge: research of avian infectious bronchitis in China[J]. Microbiology China, 2019, 46(7): 1837–1849. (in Chinese) |
| [3] | SHIRVANI E, PALDURAI A, MANOHARAN V K, et al. A recombinant Newcastle disease virus (NDV) expressing S protein of infectious bronchitis virus (IBV) protects chickens against IBV and NDV[J]. Sci Rep, 2018, 8: 11951. |
| [4] |
薛洋.黄芩苷防治雏鸡传染性支气管炎的作用研究[D].郑州: 河南农业大学, 2017.
XUE Y. Study on prevention and treatment of avian infectious bronchitis by baicalin[D]. Zhengzhou: Henan Agricultural University, 2017. (in Chinese) http://cdmd.cnki.com.cn/Article/CDMD-10466-1017279024.htm |
| [5] |
李涛, 崔建东, 龙桂宁, 等. 蒽环类药物联合紫杉醇对局部晚期乳腺癌患者T淋巴细胞亚群功能的影响[J]. 中国当代医药, 2019, 26(11): 77–79.
LI T, CUI J D, LONG G N, et al. Effect of anthracyclines combined with purslanol on T lymphocyte subsets in patients with locally advanced breast cancer[J]. China Modern Medicine, 2019, 26(11): 77–79. (in Chinese) |
| [6] | FATHY S A, MOHAMED R M, ALI M A M, et al. Influence of IL-6, IL-10, IFN-γ and TNF-α genetic variants on susceptibility to diabetic kidney disease in type 2 diabetes mellitus patients[J]. Biomarkers, 2019, 24(1): 43–55. |
| [7] |
刁艳霞, 陈兰举. 轮状病毒致乳鼠肝脏IFN-γ/IL-10的变化研究[J]. 中国微生态学杂志, 2011, 23(7): 608–611.
DIAO Y X, CHEN L J. Study on the change of liver IFN-γ/IL-10 after rotavirus infection in sucking mice[J]. Chinese Journal of Microecology, 2011, 23(7): 608–611. (in Chinese) |
| [8] |
潘力, 林树乾, 于可响, 等. 鸡传染性支气管炎病毒M41株的致病性及排毒规律[J]. 中国兽医学报, 2019, 39(5): 842–847.
PAN L, LIN S Q, YU K X, et al. Pathogenicity and virus-shedding of infectious bronchitis virus M41 strain[J]. Chinese Journal of Veterinary Science, 2019, 39(5): 842–847. (in Chinese) |
| [9] |
张友敏.清肺止咳合剂对鸡传染性支气管炎的治疗研究[D].扬州: 扬州大学, 2018.
ZHANG Y M. Therapeutic effect of QFZKHJ on infectious bronchitis[D]. Yangzhou: Yangzhou University, 2018. (in Chinese) |
| [10] |
李清艳, 郭兵, 王海凤, 等. 鸡传染性支气管炎人工感染模型的中药保护作用[J]. 中国兽医杂志, 2009, 45(10): 61–63.
LI Q Y, GUO B, WANG H F, et al. Protective effect of Chinese herbal medicine on chickens experimentally infected with infectious bronchitis virus[J]. Chinese Journal of Veterinary Medicine, 2009, 45(10): 61–63. (in Chinese) |
| [11] |
王萌.中药复方制剂对人工感染雏鸡传染性支气管炎防治效果研究[D].兰州: 甘肃农业大学, 2012.
WANG M. Prevention and treatment experimentation of self-made Chinese herbal compound in chicken IBV-M41 challenged[D]. Lanzhou: Gansu Agricultural University, 2012. (in Chinese) http://cdmd.cnki.com.cn/Article/CDMD-10733-1012034508.htm |
| [12] |
闫延华.复方板青可溶性粉对IB的防治作用研究[D].郑州: 河南农业大学, 2017.
YAN Y H. Green soluble powder compound board role in prevention and cure of infectious bronchitis research[D]. Zhenzhou: Henan Agricultural University, 2017. (in Chinese) http://cdmd.cnki.com.cn/Article/CDMD-10466-1017281159.htm |
| [13] |
尧国荣, 许振华, 陈和生, 等. 中兽医辨证施治鸡肾型传染性支气管炎[J]. 中兽医学杂志, 2014(4): 39–40.
YAO G R, XU Z H, CHEN H S, et al. Treatment of infectious bronchitis of chicken kidney type by syndrome differentiation of traditional Chinese and veterinary medicine[J]. Journal of Chinese Traditional Veterinary Science, 2014(4): 39–40. (in Chinese) |
| [14] |
刘钟杰, 许剑琴.
中兽医学[M]. 4版. 北京: 中国农业出版社, 2011: 459.
LIU Z J, XU J Q. Chinese animal medicine[M]. 4th ed. Beijing: China Agricultural Publishing House, 2011: 459. (in Chinese) |
| [15] |
邓秀凤. 中草药防治鸡传染性支气管炎[J]. 中兽医学杂志, 2018(8): 52.
DENG X F. Prevention and treatment of infectious bronchitis of chicken with Chinese herbal medicine[J]. Journal of Chinese Traditional Veterinary Science, 2018(8): 52. (in Chinese) |
| [16] |
崔小七.柴黄颗粒对鸡传染性支气管炎的防治试验[D].杨凌: 西北农林科技大学, 2013.
CUI X Q. Prevention and treatment of Chaihuang granules on avian infectious brconchitis[D]. Yangling: Northwest A&F University, 2013. (in Chinese) http://cdmd.cnki.com.cn/article/cdmd-10712-1013347636.htm |
| [17] |
陈银山, 赵占景, 王辉. 黄芩、板蓝根治疗小儿咳嗽的临床研究[J]. 中医临床研究, 2013, 5(4): 3–4.
CHEN Y S, ZHAO Z J, WANG H. Clinical researches on treating infantile cough with Huanqin and Banlangen[J]. Clinical Journal of Chinese Medicine, 2013, 5(4): 3–4. (in Chinese) |
| [18] |
郑洪新, 李敬林. 张元素对中药分类、药性、归经报使理论的创新[J]. 中国中医基础医学杂志, 2013, 19(12): 1377–1378, 1387.
ZHENG H X, LI J L. Zhang element's innovation in the theory of classification and drug properties of traditional Chinese medicine[J]. Chinese Journal of Basic Medicine in Traditional Chinese Medicine, 2013, 19(12): 1377–1378, 1387. (in Chinese) |
| [19] |
杨秀伟. 中草药化学成分的研究[J]. 中草药, 2007, 38(7): 961–969.
YANG X W. Studies on chemical constituents in Chinese herbal medicine[J]. Chinese Traditional and Herbal Drugs, 2007, 38(7): 961–969. (in Chinese) |
| [20] |
陈亚乔, 侯林, 崔清华, 等. 中药抗病毒活性及作用机制研究进展[J]. 中医药导报, 2017, 23(22): 103–106.
CHEN Y Q, HOU L, CUI Q H, et al. Research progress of antiviral activity and mechanism of Chinese medicine[J]. Guiding Journal of Traditional Chinese Medicine and Pharmacy, 2017, 23(22): 103–106. (in Chinese) |
| [21] |
韩刚, 姬晓辉. 对中药抗病毒作用的研究进展[J]. 当代医药论丛, 2014, 12(8): 37.
HAN G, JI X H. Research progress on the antiviral effect of traditional Chinese medicine[J]. Contemporary Medicine Forum, 2014, 12(8): 37. (in Chinese) |
| [22] |
左军, 尹柏坤, 胡晓阳. 桔梗化学成分及现代药理研究进展[J]. 辽宁中医药大学学报, 2019, 21(1): 113–116.
ZUO J, YIN B K, HU X Y. Research progress in the chemical constituents and modern pharmacology of platycodon[J]. Journal of Liaoning University of TCM, 2019, 21(1): 113–116. (in Chinese) |
| [23] |
谢雄雄, 张迟, 曾金祥, 等. 中药桔梗的化学成分和药理活性研究进展[J]. 中医药通报, 2018, 17(5): 66–72.
XIE X X, ZHANG C, ZENG J X, et al. Research progress on chemical constituents and pharmacological activities of Platycodon grandiflorum[J]. Traditional Chinese Medicine Journal, 2018, 17(5): 66–72. (in Chinese) |
| [24] |
张文彬, 周其珍, 贾英科. 中成药"7811"粒剂对鸡传染性喉气管炎和传染性支气管炎治疗的研究[J]. 中国兽医科技, 1989(7): 5–8.
ZHANG W B, ZHOU Q Z, JIA Y K. Study on the treatment of infectious laryngotracheitis and infectious bronchitis with Chinese patent medicine "7811" granule[J]. Chinese Journal of Veterinary Science and Technology, 1989(7): 5–8. (in Chinese) |
| [25] |
曾豫娟. 参灵清瘟败毒口服液对鸡传染性支气管炎的疗效研究[J]. 当代畜牧, 2016(11): 112.
ZENG Y J. Study on the effect of Shen Lingqing blast and poisoning oral liquid on infectious bronchitis in chicken[J]. Contemporary Animal Husbandry, 2016(11): 112. (in Chinese) |
| [26] |
郭兵. 传染性支气管炎病毒感染对雏鸡血清IFN-γ和IL-4的影响[J]. 安徽农业科学, 2011, 39(26): 16134–16136.
GUO B. Effect of IBV infection on serum levels of IFN-γ and IL-4 in chickens[J]. Journal of Anhui Agricultural Sciences, 2011, 39(26): 16134–16136. (in Chinese) |
| [27] | YING X, LI J N, LIU H, et al. Induction of innate immune response following introduction of infectious bronchitis virus (IBV) in the trachea and renal tissues of chickens[J]. Microb Pathog, 2018, 116: 54–61. |
| [28] |
张虹, 魏红, 张卓, 等. Th细胞亚群失衡在多种血液疾病中的研究进展[J]. 中国肿瘤临床与康复, 2018, 25(6): 766–768.
ZHANG H, WEI H, ZHANG Z, et al. Research progress of Th subgroup imbalance in a variety of blood diseases[J]. Chinese Journal of Clinical Oncology and Rehabilitation, 2018, 25(6): 766–768. (in Chinese) |
| [29] | FOULDS K E, ROTTE M J, PALEY M A, et al. IFN-gamma mediates the death of Th1 cells in a paracrine manner[J]. J Immunol, 2008, 180(2): 842–849. |
| [30] | CHEN Y F, ZHENG J J, QU C, et al. Inonotus obliquus polysaccharide ameliorates dextran sulphate sodium induced colitis involving modulation of Th1/Th2 and Th17/Treg balance[J]. Artif Cells Nanomed Biotechnol, 2019, 47(1): 757–766. |
| [31] |
刘锦梅, 杜亚涛, 杨松, 等. CD4+Th细胞亚群与哮喘研究[J]. 临床和实验医学杂志, 2018, 17(21): 2350–2353.
LIU J M, DU Y T, YANG S, et al. Study on CD4 Th cell subsets and asthma[J]. Journal of Clinical and Experimental Medicine, 2018, 17(21): 2350–2353. (in Chinese) |
| [32] |
李文军, 范桂虹, 杨明, 等. 不同分期肺癌患者血清外周血Th1、Th2细胞因子、IL-18水平变化及其预后研究[J]. 实用医院临床杂志, 2018, 15(5): 187–189.
LI W J, FAN G H, YANG M, et al. Changes of serum Th1 and Th2 cytokines and IL-18 levels in patients with lung cancer at different stages and their correlation with prognosis[J]. Practical Journal of Clinical Medicine, 2018, 15(5): 187–189. (in Chinese) |



