1978年,Gilbert[1]提出假设,内含子通过特定的重组可以将外显子聚集,形成特殊的分化产物,这些特殊的基因产物可以启动特定新的剪切模式来解释免疫球蛋白重链现象,进而提出可变剪接这一概念。研究表明,可变剪接可能起源于外显子改组、可转座元件的外显子化以及组成性剪接外显子这3种机制[2]。可变剪接可以使基因在不同时间、不同环境中能够制造出的蛋白质类型更多,生理状况下机体系统的复杂性或适应性更强。而且,目前有研究表明,可变剪接可以直接参与RNA从转录前事件到转录后事件加工本身,并影响下游蛋白质与配体之间的结合、核酸或膜的结合、定位和酶学性质等[3]。虽然可变剪接这种“基因表达的中心元素”的作用十分重要,但是由于检测技术的限制,其研究发展并不是特别迅速。近几年,随着大规模深度测序等技术的飞速发展,可变剪接的鉴定越来越方便、准确,其研究也逐渐成为热点。
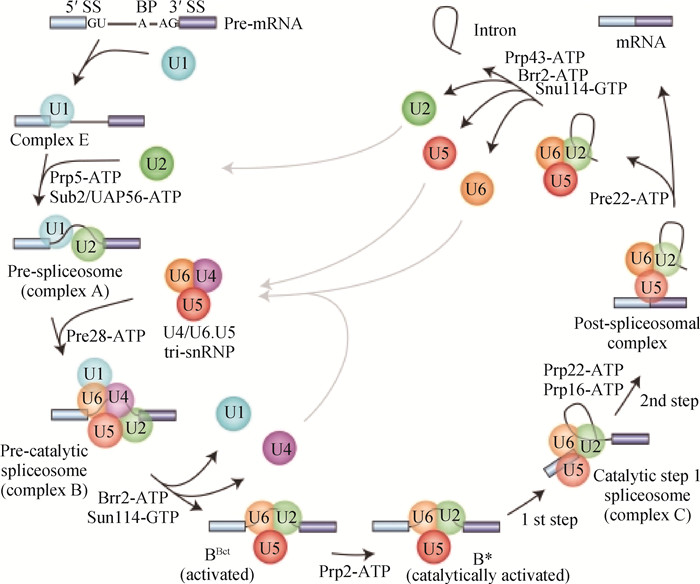
1 可变剪接事件发生的过程及调控机制可变剪接事件产生过程分为可变剪接体的组装以及pre-mRNA的转录加工剪接。剪接体就像一个大型核糖核蛋白(RNP)机器,主要由U1、U2、U4/U6和U5 snRNPs组装而成[4]。剪接体的功能是在1个复杂而动态的组装、反应和拆卸循环中进行的[5],在这个循环中,5个小的核糖核蛋白(SnRNP)复合物(U1、U2、U4/U6和U5)识别并组装在每个内含子上,最终形成1个具有催化活性的剪接体(图 1)[6]。
前体mRNA经历可变剪接形成的成熟mRNA的过程由顺式作用元件和反式作用元件的相互作用进行调控[7]。如果这些元件从外显子位置起促进或抑制它们所在外显子的包含的作用,则通常将其分类为外显子剪接增强子(例如SR蛋白)或沉默子(例如异质性核糖核蛋白(hnRNPs)),如果它们增强或抑制来自内含子位置的相邻剪接位点或外显子的使用,则将其分类为内含子剪接增强子(例如G三联体)或沉默子(例如与神经元特异性剪接因子Nova家族结合的YCAY基序簇)[8]。顺式作用元件和反式作用元件的功能通常都是相加的,增加元件的数量的同时也会影响剪接的调控,这可能与不同的元件会以协同的方式进行可变剪接的调节有关;同时沉默子较增强子在对可变剪接的调控中起到的作用更大[9]。
研究表明,剪接过程与pre-mRNA链的编辑过程是共转录的,RNA聚合酶Ⅱ的最大亚基的C末端结构域(CTD)是RNA加工事件的主要调控元件,当剪接发生并可能与编辑竞争时,CTD将允许编辑优先剪接进行[10]。CTD参与与基因表达相关的功能,包括5′帽化、剪接、聚腺苷酸化和染色质重塑[11],已成为控制转录和剪接之间相互作用的核心要素,功能性偶联似乎在可变剪接中起决定性的生理变化的驱动作用[12]。同时在后生动物中,启动子结构可以影响可变剪接的过程[13],染色质的调节也会导致可变剪接的改变[14]。
2 可变剪接的类型目前,已发现的可变剪接主要有7种类型(图 2)。分别为外显子跳跃、5′端可变剪接、3′端可变剪接、内含子保留、外显子选择性跳跃、第一个外显子可变剪接和最后一个外显子可变剪接。
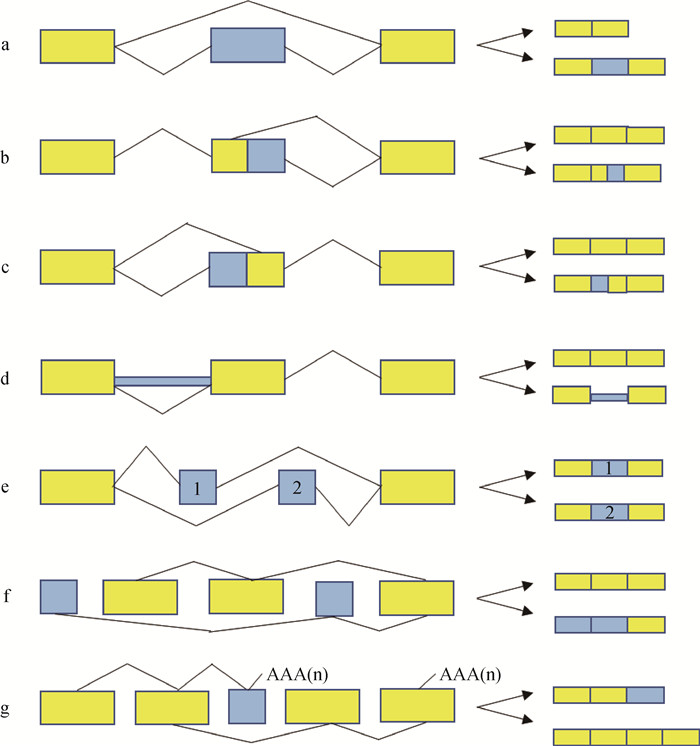
|
a.外显子跳跃;b.5′端可变剪接;c.3′端可变剪接;d.内含子保留;e.外显子选择性跳跃;f.第一个外显子可变剪接;g.最后一个外显子可变剪接。图中,构成外显子显示为黄色,剪接区显示为蓝色 a. Exon skipping; b. Alternative 5' splice site; c. Alternative 3' splice site; d. Retained intron; e. Mutually exclusive exon; f. Alternative first exon; g. Alternative last exon. In the figure, constitutive exons are shown in yellow and alternatively spliced regions in blue 图 2 可变剪接的类型 Fig. 2 Types of alternative splicing |
其中,外显子跳跃是动物中最常见的可变剪接现象,至少占已知可变剪接数量的三分之一[15]。外显子跳跃是指外显子在前体mRNA剪接形成成熟mRNA过程中被跳过,与其两侧的内含子被剪切掉,最终没有出现在某些成熟mRNA上,只保留其上游和下游的外显子被直接连在一起,保留在剪切的产物中。外显子序列内5′或3′端剪切位点的替换剪切,这两种可变剪接的发生也十分频繁,加起来约占已知可变剪接数量的四分之一。5′端可变剪接是指在前体mRNA剪接形成成熟mRNA的过程中,5′端边界发生不同方式的剪接,导致5′端外显子有所延长。3′端可变剪接则是在前体mRNA剪接形成成熟mRNA的过程中,3′端边界发生不同方式的剪接,导致3′端外显子有所延长。内含子保留指前体mRNA在剪接形成成熟mRNA的过程中,部分内含子被保留下来,使得某一段核苷酸序列在一个剪切体中是外显子的一部分,而在与之对照的剪切体中却是内含子而被剪切掉[16]。外显子选择性跳跃是在形成的两种不同的转录本中,两转录本之间不同的外显子以相互排斥的方式剪切,不能同时存在于同一转录本中,只能分别存在于不同转录本中[17]。除了以上的可变剪接类型外,还存在着转录本中第一个外显子可变剪接和最后一个外显子可变剪接[18]。
尽管可变剪接有这么多类型,但是已经证明每种类型的可变剪接都可以以随机方式进行,并且在所有生物界中,不同的剪接位点识别和处理机制不一定以相同的频率发生[19]。这些可变剪接事件附近的基因组序列显示出更高的序列保守性。可变剪接的外显子和近端的内含子都表现出比组成性剪接的外显子更高的基因组保守性。对于外显子跳跃事件,外显子两侧的两个内含子区域都是保守的,而对于选择性5′和3′剪接事件,可变剪接位点附近的保守性更大[20]。
3 可变剪接的鉴定方法对于可变剪接的鉴定涉及到对已知可变剪接的验证及对未知可变剪接的预测两方面。目前,对已知可变剪接的鉴定主要利用RT-PCR及RACE技术对CDS进行克隆扩增等。其基本原理就是对不同的剪接体进行扩增,对扩增结果进行测序后即可确定不同的剪接形式。对于未知可变剪接的预测则经历了表达序列标签(EST)与基因组比对、基因芯片分型预测、利用RNA-seq数据与基因组对比等几个过程。尤其目前RNA三代测序技术日益成熟,可变剪接的预测也越来越准确。
3.1 利用RNA-seq数据鉴定可变剪接的基本原理利用RNA-seq进行可变剪接鉴定的基本原理就是利用读段定位进行差异剪接体鉴定。经过建库测序,RNA-seq数据会被测定为若干条固定长度的碱基序列(即读段)。把读段匹配到基因组DNA(参考序列)的过程就称为读段定位。当把读段匹配回基因组DNA后,如果读段足够多地定位到参考序列上,那么就可以确定剪接位点的位置。剪接位点是内含子与外显子的边界,准确找出剪接位点即可准确识别与鉴定可变剪接。
3.2 利用RNA-seq数据鉴定可变剪接的算法及工具目前,用于对RNA-seq数据进行读段定位的软件基本上采用以下几种算法:1)BWT(Burrows-Wheeler Transform)算法,例如Bowtie和BWA等;2)Smith-Waterman算法,例如BFAST,SHRiMP等;3)spaced-seed空位种子算法,例如MAQ等;4)2way-BWT算法,例如SOAPaligner等。其中,Bowtie和BWA相对比较高效,但SOAPaligner、BFAST和MAQ等具有良好的容错能力(mismatches),其算法优势不同。而读段定位后,常用的剪接位点预测的软件有ERANGE、TopHat、MapSplice、SpliceMap、HMMSplicer等。
3.3 可变剪接鉴定数据库目前,基于各种数据已鉴定出了数以百万计的可变剪接。在畜禽上,2020年,Liu等[21]首次建立一个关于5个主要畜禽动物物种(牛、羊、猪、马和鸡)的可变剪接事件的数据库。该数据库包含可变剪接事件的多种类型的信息,包括诸如基因组位置、基因和事件类型等基本信息、可变剪接百分比(PSI)形式的定量测量、已知DNA变体的重叠以及跨不同谱系组的同源事件的数据库。为动物基因组的功能注释提供了一个有用的探索性工具。
4 可变剪接在畜禽育种中的研究与应用研究显示,在猪、牛、鸡和家鸭中,分别有80.6%、60%、66.7%和46.12%的基因发生了可变剪接[22-24],可变剪接在畜禽发育的不同阶段、不同组织或器官都有发生,对畜禽的各种经济性状都有影响。
4.1 可变剪接对胚胎及器官发育的作用在畜禽中,可变剪接内含子保留事件在鸡胚胎发育过程中表现出明显的特征和动态调节[25],猪胚胎伸长的形态发生过程也受到了可变剪接的调控[26]。母牛长非编码RNA基因Meg8和Meg9之间的非编码RNA(LINC24061)的两个可变剪接的转录本可能与器官的形成有关[27]。另一个非编码RNA(LINC24065)剪接异构体在成年牛组织中表现出组织特异性表达模式[28]。Xu等[29]对山羊4个发育阶段的代表 5个组织60个样本进行测序发现,可变剪接主要参与器官功能和发育,这为研究山羊转录组的复杂性和基因调控提供了有价值的资源。山羊睾丸Boule基因的剪接异构体在减数分裂过程中发挥了重要的作用[30]。可变剪接参与了鸡视网膜突触的发育过程[31],鸡染色域蛋白死亡因子4样蛋白1(Morf4L1)的剪接异构体也参与鸡视网膜的形成[32],从鸡视网膜中分离出Reelin-Disabled 1(Dab1)信号通路的剪接异构体对神经细胞在大脑中的定位起着至关重要的作用,也是调节混合细胞群体中Dab1信号通路的一种有效和灵活的方式[33]。
4.2 可变剪接对生长性状的作用猪糖原合成酶激酶3(GSK3)基因的鉴定不仅对猪的遗传改良具有重要意义,而且有助于理解和发展猪的人类疾病防治模型。研究发现,不同GSK3β剪接异构体在胰岛素信号转导途径作用也不同,对糖原合成酶也表现出不同的活性[34]。猪中DUOX2基因异常可变剪接会使甲状腺激素产生严重缺陷,这种突变猪可能是一种潜在的人类先天性甲状腺功能减退的大型动物模型[35]。猪生长激素释放激素受体(GHRHR)的剪接异构体(SVs)对原代垂体细胞生长激素合成和细胞增殖有调节作用[36]。2020年,He等[37]发现了陕北白绒山羊CMTM2基因变异剪接体CMTM2-AS2,其在肌肉和肝组织中的表达量较高,表明其在生长性状中发挥了有效的作用,以及对CMTM2基因启动子区域缺失的研究都对今后的山羊育种具有直接的指导意义。Han等[38]在鸡中发现类锌指蛋白(ZNF764L)基因的两个剪接异构体,显著影响了4周龄鸡的初生重、胸宽、体斜长、皮下脂肪重等胴体性状和生长性状,可以作为促进鸡的繁殖力的靶基因。
4.3 可变剪接对繁殖性状的作用可变剪接在母猪发情期间的基因表达调控中起着重要作用,许多与性腺发育、激素代谢、昼夜节律相关的基因都通过可变剪接进行差异调控[39]。在60 d猪睾丸中,内含子保留是最常见的可变剪接形式,而在180 d的睾丸中,内含子保留、5′端可变剪接、3′端可变剪接和外显子跳跃的频率相对较高[40]。第一个外显子可变剪接和最后一个外显子可变剪接与猪睾丸素合成和分泌密切相关[22]。在寒羊和多赛特绵羊品种的卵巢中,外显子跳跃是最为常见的可变剪接类型[41]。在毛驴睾丸和附睾中,外显子跳跃和3′端可变剪接事件最为普遍[42]。可变剪接参与了猪睾丸特异基因TSGs转录的调控[43],这也为进一步提高猪的经济效益以及未来男性不育的治疗提供了有价值的见解。猪睾丸的间质细胞中羟基类固醇17-β脱氢酶3 (Hsd17b3)基因的两个剪接异构体可以作为潜在的DNA标记用于猪的标记辅助选择,这也将丰富猪遗传育种的研究[44]。通过对编码精子运动调节分子cAMP的合成酶的ADCY10 mRNA片段的研究发现,精子mRNA片段可用于检测公牛睾丸mRNA的剪接错误[45]。可变剪接可能在牦牛睾丸乳酸脱氢酶C(ldhc)基因的表达中起一定的调节作用,这可能是牦牛杂种不育的原因之一[46]。可变剪接参与了陕北白绒山羊睾丸中连环蛋白β1 (CTNNB1)基因的表达调控,4种剪接异构体中CTNNB1-C的表达量最高,可能在转录或生理过程中发挥重要作用[47]。研究发现,可变剪接使得奶牛中黄体生成素/绒毛膜促性腺激素受体(LHCGR)具有很高多态性[48]。跨膜蛋白95(TMEM95)基因的变异剪接体与顶体反应密切相关,可以成为提高黄牛雄性的繁殖力新靶点[49]。
4.4 可变剪接对肉品质性状的作用可变剪接参与脂肪酸代谢、甘油三酯代谢、葡萄糖反应以及脂肪细胞因子信号信号等生物学过程和通路。蔡兆伟等[50]发现,可变剪接可能在雄激素调控高脂诱导的非酒精性脂肪肝病(NAFLD)发病过程中发挥重要作用。可变剪接参与猪调控脂肪滴发育和脂肪沉积的关键因子Cidec[51]、脂肪质量和肥胖相关基因(FTO)[52]、胆固醇和类固醇合成急性调节蛋白(StAR)的结合和转运的过程[53],以及控制CGI-58激活脂肪甘油三酯脂肪酶的能力而参与脂解的调节[54], 这些都有可能成为提高猪肉品质的新靶点。猪肌神经原蛋白(MYNN)基因的剪接异构体可能在消化吸收和骨骼肌生长中起重要作用[55],猪MEF2A基因4种剪接异构体在马身猪和大白猪背最长肌中的表达谱存在差异[56]。在胎牛、成年公牛、成年小母牛和成年公牛背部脂肪中38.85%的基因发生了可变剪接事件[57],在前脂肪细胞和脂肪细胞中分别检测到4 534个可变剪接事件和5 153个可变剪接事件[58]。可变剪接的参与影响牛肉品质的PRKAG3基因的转录过程,从而在该基因翻译的蛋白质群体中引入了异质性[59],抑制了牛成肌细胞增殖相关基因lnc9141的剪接异构体,从而抑制牛成肌细胞的增殖[60],对牛血管生成素蛋白6(bANGPTL6)基因的可变剪接研究有助于研究胰岛素抵抗事件的分子机制和ANGPTL6基因的功能,这可能成为治疗肥胖及肥胖相关疾病的新靶点[61]。可变剪接对鸡肌肉发育调控的重要性已经被证实[62],禽类肌肉生长抑制素(MSTN)的异构体可以负向调节肌肉细胞中肌抑制素原的加工,并阻止MSTN介导的对禽类肌肉发生的抑制[63]。
4.5 可变剪接对抗病性状的作用在抗病性状中,可变剪接也参与了很多过程。例如,热休克可能通过调节巴马小型猪Toll样受体4(TLR4)及其剪接异构体的表达来调节宿主的免疫应答[64],由可变剪接产生的两种不同形式的鸡不变链(Ⅱ)蛋白在主要免疫器官中强烈表达[65]。转录因子3(TCF3)基因的两个剪接异构体TCF3A和TCF3B可能在淋巴细胞成熟过程中起重要作用,同时可能与猪瘟病毒(CSFV)抵抗有关[66]。成对的免疫球蛋白样2型受体(PILR)β的异构体可以调控对病原体感染的炎症反应,揭示PILRβ在宿主免疫应答中的作用,有助于通过分子育种方法培育抗病毒病的猪[67]。人工感染猪繁殖与呼吸综合征病毒(PRRSV)抗病性与可变剪接之间也存在潜在的关系[68],同时猪免疫球蛋白Fc区受体FcrRI通过可变性剪接机制在PRRSV增殖和炎症过程中发挥双重调节作用,有望成为PRRSV防治的新靶点[69]。对于牛的乳腺炎,有很多关于可变剪接的研究。例如,α-2-巨球蛋白(A2M)可以通过可变剪接机制发挥对乳腺炎的抗炎作用[70],牛乳铁蛋白(bLF)基因可能通过不同的剪接机制发挥抗乳腺炎的作用[71],牛血中性粒细胞(PMN)中分化簇14(CD14)基因剪接异构体 CD14-SV可能是影响奶牛乳腺炎抗性的候选功能标志物[72],可变剪接是与乳腺炎感染有关免疫相关基因C-C基序趋化因子配体5(CCL5)基因表达调控的机制之一[73],大肠杆菌乳腺炎抗性研究的候选基因LGR4基因外显子5的DNA甲基化通过可变剪接来影响LGR4基因的表达[74],这些研究都可以为奶牛抗乳腺炎育种提供新的靶基因和表观遗传标记。牛白细胞介素-6受体-α(IL6R)基因的剪接异构体IL6R-TV在正常乳腺组织的表达水平较低,可能在炎症感染过程中有着潜在的作用[75]。在肿瘤与癌症方面,Patel等[76]研究发现,可变剪接异构体可能参与牛的角癌的发展,鸡中脑源性神经营养因子cBDNF基因的变异剪接体在MD肿瘤耐药性和敏感性中的也有潜在作用[77],同时,可变剪接也与网状内皮组织增生症病毒(REV)感染的鸡胚成纤维细胞(CEFs)的凋亡和肿瘤发生密切相关[78]。作为鸭先天免疫信号的重要调节因子,鸭肿瘤坏死因子受体相关因子3(duTRAF3)基因的变异剪接体duTRAF3-S与该基因的发生竞争,导致干扰素-β产量下降[79]。SPSF10通过与剪接顺式元件结合来调节鸡ANP32A内含子4的可变剪接,从而在禽流感病毒的聚合酶活性和复制中发挥负调控作用[80]。Douaud等[81]报道了SV2A第二内含子(编码突触囊泡糖蛋白2A的基因)受体位点的异常可变剪接导致一种独特的脊椎动物模型-费皮鸡株发生了光敏反射性癫痫,显著降低纯合子携带者SV2A mRNA水平。
4.6 可变剪接对其它性状的作用宋艳芳等[82]在民猪hnRNPUL1的基因编码区发现了丰富的可变剪接事件,这也为揭示猪hnRNPUL1基因的功能及转录调控机制提供了研究基础。牛重要产奶性状候选基因GPIHBP1的剪接异构体(X5)可能对GPIHBP1的蛋白质功能产生巨大影响,其在泌乳奶牛乳腺组织中的mRNA表达量明显高于其他组织[83]。U2 snRNP辅助因子65(U2AF65)可以通过控制牛奶合成和BMECs生长的信号分子(mTOR和SREBP-1c)mRNA的可变剪接,从而对牛乳腺上皮细胞的乳汁合成和增殖起正向调节作用[84]。山羊乳腺癌1(DBC1)基因剪接异构体在产奶量中起着重要作用[85]。绒山羊的被毛底层纤维具有商业价值,其来源于次级毛囊(HF),而可变剪接调控胚胎中原发和继发次级毛囊的形成[86]。在其他方面,例如,发生在鸡沿着耳蜗正位轴的特异性可变剪接有助于频率调谐[87],编码酪氨酸酶TYR基因剪接异构体在黑色和白色羽鳞茎、肌肉以及皮肤中的表达水平均有显著差异,它们的mRNA表达水平与鸡皮肤黑素合成密切相关[88]。
5 目前存在问题及展望随着测序技术的发展以及人们对基因转录过程研究的深入,转录本的鉴定和组注释工作得到了极大的发展。然而,虽然目前各种数据库及相关研究提供了丰富的转录本,但是大多数研究集中在稳态RNA丰度的差异上,并未捕捉转录后调控的差异,各转录本对于畜禽中很多经济性状表型变异的具体作用机制的研究还尚未明确。此外,目前虽有部分整体基因组层面的可变剪切多态与表型的关联,但是研究并不多。未来对畜禽重要经济性状相关可变剪接的研究及应用可以从以下几个方面进行:1)可变剪接在不同畜禽品种及组织中的分布及差异研究。通过可变剪接分布的不同,结合品种特异性,可推测或验证可变剪接可能的作用,并为其在基因聚合育种中的应用提供参考;2)可变剪接与不同表型个体的基因组关联研究。通过eQTL等方法,对影响表型性状的可变剪接进行鉴定并进行功能分析,鉴定对表型有影响的剪接位点,为在育种中进行应用提供标记;3)可变剪接影响经济性状的分子作用通路研究。通过共表达,免疫共沉降等方法对可变剪接的剪接异构体进行上、下游分子通路研究,进而对可变剪接的分子作用机制进行探讨;4)利用获得的可变剪接位点,进行连锁分子标记开发并在基因组育种中进行应用。
总之,虽然可变剪接对畜禽的各种生长过程及性状有着显著的影响,但其在畜禽探索并不深入。将来通过对畜禽中可变剪接更全面的理解,必定可为畜禽育种的改良提供更多新的方向。
| [1] | GILBERT W. Why genes in pieces?[J]. Nature, 1978, 271(5645): 501. |
| [2] | KIM E, GOREN A, AST G. Alternative splicing:current perspectives[J]. Bioessays, 2008, 30(1): 38–47. |
| [3] | KELEMEN O, CONVERTINI P, ZHANG Z Y, et al. Function of alternative splicing[J]. Gene, 2013, 514(1): 1–30. |
| [4] | WAHL M C, WILL C L, LUHRMANN R. The spliceosome:design principles of a dynamic RNP machine[J]. Cell, 2009, 136(4): 701–718. |
| [5] | WILL C L, LUHRMANN R. Spliceosome structure and function[J]. Cold Spring Harb Perspect Biol, 2011, 3(7): a003707. |
| [6] | LEE Y, RIO D C. Mechanisms and regulation of alternative pre-mRNA splicing[J]. Annu Rev Biochem, 2015, 84(1): 291–323. |
| [7] | WANG Y, LIU J, HUANG B O, et al. Mechanism of alternative splicing and its regulation[J]. Biomed Rep, 2015, 3(2): 152–158. |
| [8] | WANG Z, BURGE C B. Splicing regulation:from a parts list of regulatory elements to an integrated splicing code[J]. RNA, 2008, 14(5): 802–813. |
| [9] | JIN Y F, YANG Y, ZHANG P. New insights into RNA secondary structure in the alternative splicing of pre-mRNAs[J]. RNA Biol, 2011, 8(3): 450–457. |
| [10] | RYMAN K, FONG N, BRATT E, et al. The C-terminal domain of RNA Pol Ⅱ helps ensure that editing precedes splicing of the GluR-B transcript[J]. RNA, 2007, 13(7): 1071–1078. |
| [11] | PHATNANI H P, GREENLEAF A L. Phosphorylation and functions of the RNA polymerase Ⅱ CTD[J]. Genes Dev, 2006, 20(21): 2922–2936. |
| [12] | KALSOTRA A, COOPER T A. Functional consequences of developmentally regulated alternative splicing[J]. Nat Rev Genet, 2011, 12(10): 715–729. |
| [13] | KORNBLIHTT A R, DE LA MATA M, FEDEDA J P, et al. Multiple links between transcription and splicing[J]. RNA, 2004, 10(10): 1489–1498. |
| [14] | SHUKLA S, OBERDOERFFER S. Co-transcriptional regulation of alternative pre-mRNA splicing[J]. Biochim Biophys Acta, 2012, 1819(7): 673–683. |
| [15] | BLENCOWE B J. Alternative splicing:new insights from global analyses[J]. Cell, 2006, 126(1): 37–47. |
| [16] | OHLER U, SHOMRON N, BURGE C B. Recognition of unknown conserved alternatively spliced exons[J]. PLoS Comput Biol, 2005, 1(2): e15. |
| [17] | AST G. How did alternative splicing evolve?[J]. Nat Rev Genet, 2004, 5(10): 773–782. |
| [18] | KORNBLIHTT A R. Chromatin, transcript elongation and alternative splicing[J]. Nat Struct Mol Biol, 2006, 13(1): 5–7. |
| [19] | MASTRANGELO A M, MARONE D, LAIDÒ G, et al. Alternative splicing:enhancing ability to cope with stress via transcriptome plasticity[J]. Plant Sci, 2012, 185-186: 40–49. |
| [20] | SUGNET C W, KENT W J, ARES M, et al. Transcriptome and genome conservation of alternative splicing events in humans and mice[J]. Pac Symp Biocomput, 2004: 66–77. |
| [21] | LIU J D, TAN S X, HUANG S Q, et al. ASlive:a database for alternative splicing atlas in livestock animals[J]. BMC Genomics, 2020, 21(1): 97. |
| [22] |
冉茂良, 陈斌, 李智, 等. 基于RNA-seq测序数据鉴定和分析猪基因组可变剪接事件[J]. 中国科学:生命科学, 2016, 46(3): 274–284.
RAN M L, CHEN B, LI Z, et al. Identification and analysis of alternative splicing events in Sus scrofa using RNA-Seq data[J]. Scientia Sinica Vitae, 2016, 46(3): 274–284. (in Chinese) |
| [23] | WU W W, ZONG J, WEI N, et al. CASH:a constructing comprehensive splice site method for detecting alternative splicing events[J]. Brief Bioinform, 2018, 19(5): 905–917. |
| [24] |
徐铁山, 顾丽红, 侯水生, 等. 应用RNA-seq数据开展鸭基因组可变剪接的鉴定与分析[J]. 中国家禽, 2016, 38(17): 10–16.
XU T S, GU L H, HOU S S, et al. Identification and analysis of alternative splicing in duck genome using RNA-seq Data[J]. China Poultry, 2016, 38(17): 10–16. (in Chinese) |
| [25] | REN J X, SUN C J, CLINTON M, et al. Dynamic transcriptional landscape of the early chick embryo[J]. Front Cell Dev Biol, 2019, 7: 196. |
| [26] | WILSON M E, SONSTEGARD T S, SMITH T P, et al. Differential gene expression during elongation in the preimplantation pig embryo[J]. Genesis, 2000, 26(1): 9–14. |
| [27] | ZHANG M Y, ZHAO Y P, WANG G N, et al. An imprinted long noncoding RNA located between genes Meg8 and Meg9 in the cattle Dlk1-Dio3 domain[J]. Genetica, 2017, 145(1): 1–7. |
| [28] | ZHANG C, XU D, CHEN W N, et al. LINC24065 is a monoallelically expressed long intergenic noncoding RNA located in the cattle DLK1-DIO3 cluster[J]. J Genet, 2019, 98(1): 30. |
| [29] | XU T S, XU F, GU L H, et al. Landscape of alternative splicing in Capra_hircus[J]. Sci Rep, 2018, 8(1): 15128. |
| [30] | ZHANG X Y, YU S, YANG Q, et al. Goat Boule:Isoforms identification, mRNA expression in testis and functional study and promoter methylation profiles[J]. Theriogenology, 2018, 116: 53–63. |
| [31] | WAHLIN K J, HACKLER L J R, ADLER R, et al. Alternative splicing of neuroligin and its protein distribution in the outer plexiform layer of the chicken retina[J]. J Comp Neurol, 2010, 518(24): 4938–4962. |
| [32] | BOIJE H, RING H, FARD S S, et al. Alternative splicing of the chromodomain protein Morf4l1 pre-mRNA has implications on cell differentiation in the developing chicken retina[J]. J Mol Neurosci, 2013, 51(2): 615–628. |
| [33] | KATYAL S, GODBOUT R. Alternative splicing modulates Disabled-1(Dab1) function in the developing chick retina[J]. EMBO J, 2004, 23(8): 1878–1888. |
| [34] | WANG L J, ZUO B, XU D Q, et al. Alternative splicing of the porcine glycogen synthase kinase 3β (GSK-3β) gene with differential expression patterns and regulatory functions[J]. PLoS One, 2012, 7(7): e40250. |
| [35] | CAO C W, ZHANG Y, JIA Q T, et al. An exonic splicing enhancer mutation in DUOX2 causes aberrant alternative splicing and severe congenital hypothyroidism in Bama pigs[J]. Dis Model Mech, 2019, 12(1): dmm036616. |
| [36] | CHENG Y Y, CHEN T, SONG J, et al. Pituitary miRNAs target GHRHR splice variants to regulate GH synthesis by mediating different intracellular signalling pathways[J]. RNA Biol, 2020: 1–13. |
| [37] | HE L B, KANG Z H, KANG Y X, et al. Goat CMTM2:mRNA expression profiles of different alternative spliced variants and associations analyses with growth traits[J]. 3 Biotech, 2020, 10(3): 131. |
| [38] | HAN R L, WANG X N, WANG X L, et al. Chicken ZNF764L gene:mRNA expression profile, alternative splicing analysis and association analysis between first exon indel mutation and economic traits[J]. Gene, 2019, 695: 92–98. |
| [39] | TANG L T, RAN X Q, MAO N, et al. Analysis of alternative splicing events by RNA sequencing in the ovaries of Xiang pig at estrous and diestrous[J]. Theriogenology, 2018, 119: 60–68. |
| [40] | SONG H B, ZHU L H, LI Y, et al. Exploiting RNA-sequencing data from the porcine testes to identify the key genes involved in spermatogenesis in Large White pigs[J]. Gene, 2015, 573(2): 303–309. |
| [41] | MIAO X Y, LUO Q M, ZHAO H J, et al. Ovarian transcriptomic analysis reveals the alternative splicing events associated with fecundity in different sheep breeds[J]. Anim Reprod Sci, 2018, 198: 177–183. |
| [42] | TIAN F, WANG J P, LI Y H, et al. Integrated analysis of mRNA and miRNA in testis and cauda epididymidis reveals candidate molecular markers associated with reproduction in Dezhou donkey[J]. Livest Sci, 2020, 234: 103885. |
| [43] | YANG W J, ZHAO F Y, CHEN M Y, et al. Identification and characterization of male reproduction-related genes in pig (Sus scrofa) using transcriptome analysis[J]. BMC Genomics, 2020, 21(1): 381. |
| [44] | CHEN M Y, YANG W J, LIU N, et al. Pig Hsd17b3:Alternative splice variants expression, insertion/deletion (indel) in promoter region and their associations with male reproductive traits[J]. J Steroid Biochem Mol Biol, 2019, 195: 105483. |
| [45] | NODA T, SAKASE M, FUKUSHIMA M, et al. Novel approach for the detection of the vestiges of testicular mRNA splicing errors in mature spermatozoa of Japanese Black bulls[J]. PLoS One, 2013, 8(2): e57296. |
| [46] | HUANG L, JIN S Y, XU Y O, et al. Quantitation of alternative splicing variants of lactate dehydrogenase C gene in testes of adult yak, sexually immature yak calf and sterile male hybrid of yak[J]. Can J Anim Sci, 2012, 92(3): 291–296. |
| [47] | ZHANG X L, YAN H L, WANG K, et al. Goat CTNNB1:mRNA expression profile of alternative splicing in testis and association analysis with litter size[J]. Gene, 2018, 679: 297–304. |
| [48] | WOHLRES-VIANA S, ARASHIRO E K N, REIS D R L, et al. Polymorphisms and alternative splicing of the luteinizing hormone receptor of dairy cattle[J]. Genet Mol Res, 2016, 15(2): gmr.15027046. |
| [49] | ZHANG S H, CAI H F, YANG Q, et al. Identification of novel alternative splicing transcript and expression analysis of bovine TMEM9 gene[J]. Gene, 2016, 575(2): 531–536. |
| [50] |
蔡兆伟, 吕建敏, 凌云, 等. 雄激素缺乏对高脂高胆固醇饲喂小型猪肝mRNA可变剪接的影响[J]. 中国实验动物学报, 2018, 26(4): 424–430.
CAI Z W, LV J M, LING Y, et al. Testosterone deficiency regulates mRNA alternative splicing in the liver of miniature pigs fed a high-fat and high-cholesterol diet[J]. Acta Laboratorium Animalis Scientia Sinica, 2018, 26(4): 424–430. (in Chinese) |
| [51] |
褚毅, 俞林, 康会芳, 等. 猪Cidec两种可变剪切体的发现及组织分布[J]. 基因组学与应用生物学, 2016, 35(4): 838–844.
CHU Y, YU L, KANG H F, et al. Detection and tissue distribution of the two alternative splicing of Cidec in the pig[J]. Genomics and Applied Biology, 2016, 35(4): 838–844. (in Chinese) |
| [52] | HUANG J M, LIU G, LIU Y P, et al. Splice variant identification and expression analysis of the fat mass and obesity-associated (FTO) gene in intact and castrated male pigs[J]. DNA Cell Biol, 2010, 29(12): 729–733. |
| [53] | ZHANG Y H, CUI Y, ZHANG X L, et al. Pig StAR:mRNA expression and alternative splicing in testis and Leydig cells, and association analyses with testicular morphology traits[J]. Theriogenology, 2018, 118: 46–56. |
| [54] | LI X, SUH Y, KIM E, et al. Alternative splicing and developmental and hormonal regulation of porcine comparative gene identification-58(CGI-58) mRNA[J]. J Anim Sci, 2012, 90(12): 4346–4354. |
| [55] | GUO X H, LI M, GAO P F, et al. Novel splice isoforms of pig myoneurin and their diverse mRNA expression patterns[J]. Asian-Australas J Anim Sci, 2018, 31(10): 1581–1590. |
| [56] | GUO X H, ZHANG Q, LI M, et al. Novel alternatively spliced isoforms of MEF2A and their mRNA expression patterns in pigs[J]. J Genet, 2018, 97(4): 977–985. |
| [57] | ZHOU Y, SUN J J, LI C J, et al. Characterization of transcriptional complexity during adipose tissue development in bovines of different ages and sexes[J]. PLoS One, 2014, 9(7): e101261. |
| [58] | CAI H F, LI M X, SUN X M, et al. Global transcriptome analysis during adipogenic differentiation and involvement of transthyretin gene in adipogenesis in cattle[J]. Front Genet, 2018, 9: 463. |
| [59] | ROUX M, NIZOU A, FORESTIER L, et al. Characterization of the bovine PRKAG3 gene:structure, polymorphism, and alternative transcripts[J]. Mamm Genome, 2006, 17(1): 83–92. |
| [60] | ZHANG M, LI B, WANG J, et al. lnc9141-a and -b play a different role in bovine myoblast proliferation, apoptosis, and differentiation[J]. Mol Ther Nucleic Acids, 2019, 18: 554–566. |
| [61] | WU J Y, LI A M, CAI H F, et al. Intron retention as an alternative splice variant of the cattle ANGPTL6 gene[J]. Gene, 2019, 709: 17–24. |
| [62] | LI Z X, XU Y, LIN Y Q. Transcriptome analyses reveal genes of alternative splicing associated with muscle development in chickens[J]. Gene, 2018, 676: 146–155. |
| [63] | SHIN S, SONG Y, AHN J, et al. A novel mechanism of myostatin regulation by its alternative splicing variant during myogenesis in avian species[J]. Am J Physiol Cell Physiol, 2015, 309(10): C650–C659. |
| [64] | JU X H, XU H J, YONG Y H, et al. Heat stress upregulates the expression of TLR4 and its alternative splicing variant in Bama miniature pigs[J]. J Integr Agric, 2014, 13(11): 2479–2487. |
| [65] | ZHONG D L, YU W Y, LIU Y H, et al. Molecular cloning and expression of two chicken invariant chain isoforms produced by alternative splicing[J]. Immunogenetics, 2004, 56(9): 650–656. |
| [66] | YANG F, WANG N, LIU Y J, et al. Identification and functional analysis of porcine basic helix-loop-helix transcriptional factor 3(TCF3) and its alternative splicing isoforms[J]. Res Vet Sci, 2016, 105: 1–4. |
| [67] | YANG X Q, JING X Y, ZHANG C X, et al. Isolation and characterization of porcine PILRB gene and its alternative splicing variants[J]. Gene, 2018, 672: 8–15. |
| [68] | ZHANG Y, XUE L Y, XU H, et al. Global analysis of alternative splicing difference in peripheral immune organs between Tongcheng pigs and Large White pigs artificially infected with PRRSV in vivo[J]. BioMed Res Int, 2020, 2020: 4045204. |
| [69] | SHI P D, SU Y X, LI Y, et al. The alternatively spliced porcine FcγRI regulated PRRSV-ADE infection and proinflammatory cytokine production[J]. Dev Comp Immunol, 2019, 90: 186–198. |
| [70] | WANG X G, HUANG J M, ZHAO L H, et al. The exon 29 c.3535A>T in the alpha-2-macroglobulin gene causing aberrant splice variants is associated with mastitis in dairy cattle[J]. Immunogenetics, 2012, 64(11): 807–816. |
| [71] | HUANG J M, WANG Z Y, JU Z H, et al. Two splice variants of the bovine lactoferrin gene identified in Staphylococcus aureus isolated from mastitis in dairy cattle[J]. Genet Mol Res, 2011, 10(4): 3199–3203. |
| [72] | HUANG J M, WANG X G, JIANG Q, et al. Identification of CD14 transcript in blood polymorphonuclear neutrophil leukocytes and functional variation in Holsteins[J]. Genet Mol Res, 2016, 15(2). |
| [73] | YANG L, GUO R Q, JU Z H, et al. Production of an aberrant splice variant of CCL5 is not caused by genetic mutation in the mammary glands of mastitis-infected Holstein cows[J]. Mol Med Rep, 2019, 19(5): 4159–4166. |
| [74] | JU Z H, JIANG Q, WANG J P, et al. Genome-wide methylation and transcriptome of blood neutrophils reveal the roles of DNA methylation in affecting transcription of protein-coding genes and miRNAs in E.coli-infected mastitis cows[J]. BMC Genomics, 2020, 21(1): 102. |
| [75] | ZHANG Y, WANG X G, JIANG Q, et al. DNA methylation rather than single nucleotide polymorphisms regulates the production of an aberrant splice variant of IL6R in mastitic cows[J]. Cell Stress Chaperones, 2018, 23(4): 617–628. |
| [76] | PATEL A K, BHATT V D, TRIPATHI A K, et al. Identification of novel splice variants in horn cancer by RNA-Seq analysis in Zebu cattle[J]. Genomics, 2013, 101(1): 57–63. |
| [77] | YU Y, ZHANG H M, BYERLY M S, et al. Alternative splicing variants and DNA methylation status of BDNF in inbred chicken lines[J]. Brain Res, 2009, 1269: 1–10. |
| [78] | GAO C, ZHAI J, DANG S Y, et al. Analysis of alternative splicing in chicken embryo fibroblasts in response to reticuloendotheliosis virus infection[J]. Avian Pathol, 2018, 47(6): 585–594. |
| [79] | WEI X Q, QIAN W, SIZHU S L, et al. Negative regulation of interferon-β production by alternative splicing of tumor necrosis factor receptor-associated factor 3 in ducks[J]. Front Immunol, 2018, 9: 409. |
| [80] | FANG A, BI Z W, YE H L, et al. SRSF10 inhibits the polymerase activity and replication of avian influenza virus by regulating the alternative splicing of chicken ANP32A[J]. Virus Res, 2020, 286: 198063. |
| [81] | DOUAUD M, FEVE K, PITUELLO F, et al. Epilepsy caused by an abnormal alternative splicing with dosage effect of the SV2A gene in a chicken model[J]. PLoS One, 2011, 6(10): e26932. |
| [82] |
宋艳芳, 张彩霞, 杜芳芳, 等. 猪hnRNPUL1基因克隆及变异剪接体鉴定[J]. 畜牧兽医学报, 2020, 51(3): 443–451.
SONG Y F, ZHANG C X, DU F F, et al. Cloning and identification of splice variants of the porcine hnRNPUL1 gene[J]. Acta Veterinaria et Zootechnica Sinica, 2020, 51(3): 443–451. (in Chinese) |
| [83] | YANG J, LIU X, ZHANG Q, et al. Identification and quantitative mRNA analysis of a novel splice variant of GPIHBP1 in dairy cattle[J]. J Anim Sci Biotechnol, 2014, 5(1): 50. |
| [84] | YU Y B, ZHEN Z, QI H, et al. U2AF65 enhances milk synthesis and growth of bovine mammary epithelial cells by positively regulating the mTOR-SREBP-1c signalling pathway[J]. Cell Biochem Funct, 2019, 37(2): 93–101. |
| [85] | ZHANG X Y, LI M X, WU X F, et al. Novel splice isoforms of dairy goat DBC1 and their diverse mRNA expression profiles[J]. Small Rumin Res, 2015, 130: 15–26. |
| [86] | ZHANG Y J, WANG L L, LI Z, et al. Transcriptome profiling reveals transcriptional and alternative splicing regulation in the early embryonic development of hair follicles in the cashmere goat[J]. Sci Rep, 2019, 9(1): 17735. |
| [87] | WANG Y F, LIU Y Y, NIE H Y, et al. Alternative splicing of inner-ear-expressed genes[J]. Front Med, 2016, 10(3): 250–257. |
| [88] | YU S, WANG G, LIAO J, et al. Five alternative splicing variants of the TYR gene and their different roles in melanogenesis in the Muchuan black-boned chicken[J]. Br Poult Sci, 2019, 60(1): 8–14. |