牦牛和犏牛是我国青藏高原地区牧民的特有家畜,常年生活在寒冷及温差大的环境中,也是“世界屋脊”著名的景观牛种,而黄牛是中国原有的普通牛种,主要分布在平原,它们为生活在不同海拔和不同环境的农牧民提供了基本的乳品和肉品资源,也是当地畜牧业发展的重要保障[1]。
肝是机体最大的消化腺,对机体的物质代谢及免疫反应起着重要的作用。研究发现肝细胞的免疫及生长凋亡受到多种因子及多条信号通路的调控,如白细胞介素17(interleukin 17, IL-17)是免疫反应的中枢蛋白,既是免疫反应关键的炎性细胞因子,也是调控细胞凋亡的关键细胞因子。研究表明IL-17的高表达能促进T细胞介导的炎症反应及多种炎症因子的分泌[2],既能通过降低足细胞标记蛋白水平诱导足细胞发生凋亡,还与非酒精性脂肪肝(NAFLD)的脂肪变性及促炎反应有关,促进单纯脂肪变性向脂肪性肝炎的转变[3-4]。而IL-17的低表达能够减轻重症肺炎患者全身炎症和氧化应激反应,减轻心肌损伤[5],也能刺激口腔黏膜发生黏膜免疫,从而防止口腔发生炎症反应[6]。热休克蛋白(HSP)的最新研究发现,HSP能够改善肿瘤细胞生存环境,从而促进了肿瘤的生长和转移[7]。此外,小鼠肝细胞经过槲皮素预处理抑制HSP90,延缓小鼠肝再生的进程[8],而抑制HSP90可降低酒精诱导的脂肪变性和促炎细胞因子,并能抑制酒精性肝硬化带来的组织损伤[9-10]。
本实验室已经对HSP做了大量研究,发现HSP家族成员对牦牛睾丸和毛囊的发育、精子的成熟及乳腺泌乳都有调控作用[11-13]。而最新研究表明,IL-17和HSP90能够协同参与免疫及细胞凋亡。如机体细胞过度分泌HSP90和IL-17能够促使银屑病等自身免疫性疾病的发生,导致皮肤细胞发生大量炎症性死亡[14]。此外,当鸡胸腺损伤时,IL-17和HSPs能协同调控胸腺细胞的凋亡而起到保护作用[15]。当前,国内外学者研究证实,IL-17和HSP90对人和小鼠肝组织的免疫功能及肝细胞的生长与凋亡都起着重要的调控作用[16-19],但在牦牛、犏牛及黄牛肝细胞中阳性反应和特异性表达研究尚未见报道。因此,作者选取牦牛、犏牛及黄牛肝为实验样品,检测了IL-17和HSP90在肝组织中表达的差异性及阳性分布规律,为研究牛肝的免疫反应及细胞凋亡提供重要的实验数据。
1 材料与方法 1.1 样品及材料 1.1.1 样品成年(3~5岁)健康牦牛、犏牛及黄牛肝样品分别采自甘肃省甘南藏族自治州、青海省西宁市和兰州市,各取肝右叶背侧样品5头份(靠近背部,大小1 cm3)。采取肝样品的牛经颈动脉放血致死后快速剖检采集,一部分样品固定于4%的多聚甲醛溶液中,另一部分样品储存在液氮中。
1.1.2 主要仪器和试剂全自动排气组织脱水机(TP1020,Leica德国);全自动包埋仪(EG 1160,Leica德国);精密轮转式切片机(RM2235,Leica德国);HSP90抗体(鼠单克隆抗体,ab13492, Abcam);IL-17b抗体(兔多克隆抗体,bs-2609R,Bioss)等。
1.2 试验方法 1.2.1 HE染色法将肝组织样置于4%的多聚甲醛溶液中固定(1个月),分别选不同牛肝样品各一份,取组织块约1 cm×1 cm×0.5 cm大小,按常规组织学方法制作石蜡切片,其厚度4 μm,分别进行苏木精-伊红(HE)染色。用Olympus DP73型光学显微镜观察,拍摄组织学图片。
1.2.2 免疫组织化学检测组织样品固定好后常规制成石蜡切片,厚度为4 μm。免疫组织化学试验采用的一抗分别为IL-17b(兔多克隆抗体,bs-2609R,Bioss)和HSP90(鼠单克隆抗体,ab13492, Abcam),均按1:600稀释后使用。兔SP-0023和鼠SP-0024 SP免疫组织化学检测试剂盒(Bioss)。显色用的试剂为1:60稀释后的二氨基联苯胺溶液,用PBS(0.01 mol·L-1)代替一抗作阴性对照,苏木精复染,酒精梯度脱水,封片,用Olympus DP73型光学显微镜观察染色结果并拍照。
1.2.3 RT-PCR和qRT-PCR检测 1.2.3.1 引物设计从GenBank数据库中检索牛HSP90基因(NM-001012670.2)和IL-17基因(NC-037350.1)用Primer Premier 5.0软件各设计一对引物(表 1),用于qRT-PCR检测,β-actin作为内参检测引物。引物由上海华大基因科技有限公司合成。
|
|
表 1 牛目的基因和内参基因的引物序列 Table 1 Primers for bovine target and reference genes |
利用Trizol试剂盒(R1100,USA)分别提取健康成年不同牛肝的总RNA,再将总RNA反转录成cDNA,最后进行RT-PCR扩增cDNA(95 ℃预变性4 min;95 ℃变性25 s,56 ℃退火30 s,72 ℃延伸35 s,共42个循环;最后72 ℃延伸7 min),-20 ℃保存。
1.2.3.3 qRT-PCR检测IL-17和HSP90基因在牦牛、犏牛及黄牛肝中的表达采用qRT-PCR仪, 分别以引物IL-17 R/F、HSP90 R/F及内参β-actin-R/F对IL-17和HSP90基因在牦牛、犏牛及黄牛肝组织表达量进行检测。反应体系为20 μL(荧光定量酶10 μL,cDNA 1 μL,上、下游引物各1 μL,无菌去离子水7 μL)。95 ℃预变性3 min;95 ℃变性15 s;58 ℃退火15 s,72 ℃延伸20 s,共45个循环。
1.2.4 统计学分析采用Image-Pro Plus 6.0计算机图像分析软件,测定IL-17和HSP90阳性反应物的积分光密度值并进行分析;采用qRT-PCR检测牦牛、犏牛及黄牛不同肝组织的IL-17和HSP90基因相对表达量。以上各数据均采用SPSS19.0数据统计软件,对各试验组数据用单因子方差分析,数据用“x±sx”表示,P < 0.01时判为差异极显著, P < 0.05时判为差异显著。
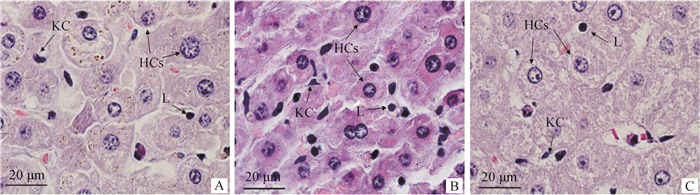
2 结果 2.1 牦牛、犏牛及黄牛肝细胞的HE染色组织学特点HE染色结果:观察到牦牛、犏牛及黄牛肝组织肝细胞呈索状排列,在肝索之间的肝血窦中有少量的淋巴细胞和枯否细胞(图 1)。
|
A.牦牛;B.犏牛;C.黄牛;KC.枯否细胞;HCs.肝细胞;L.淋巴细胞 A. Yak; B. Cattle-yak; C. Yellow cattle; KC. Kupffer cell; HCs; Hepatocytes; L. Lymphocyte 图 1 牦牛、犏牛及黄牛肝HE染色(1 000×) Fig. 1 Liver HE staining of Yak, Cattle-yak and Yellow cattle(1 000×) |
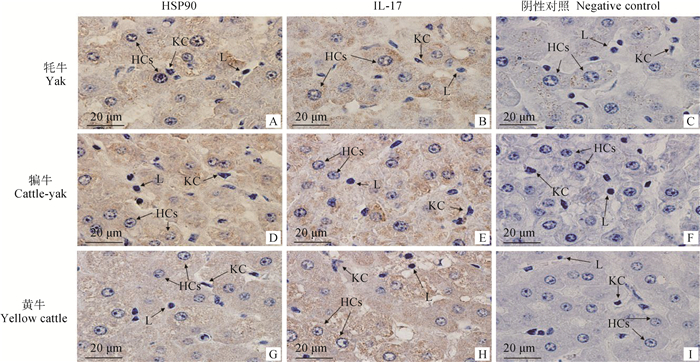
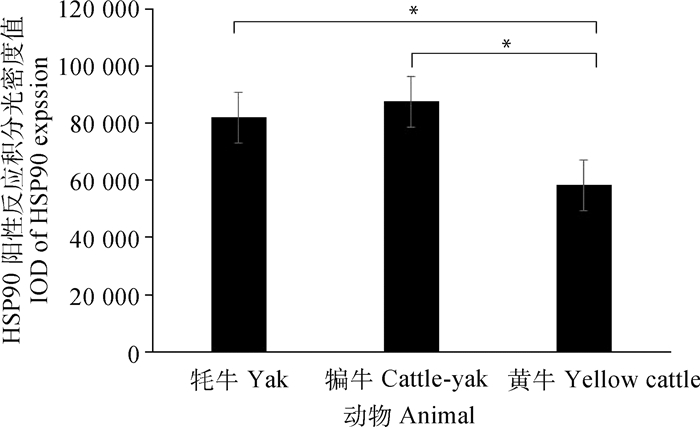
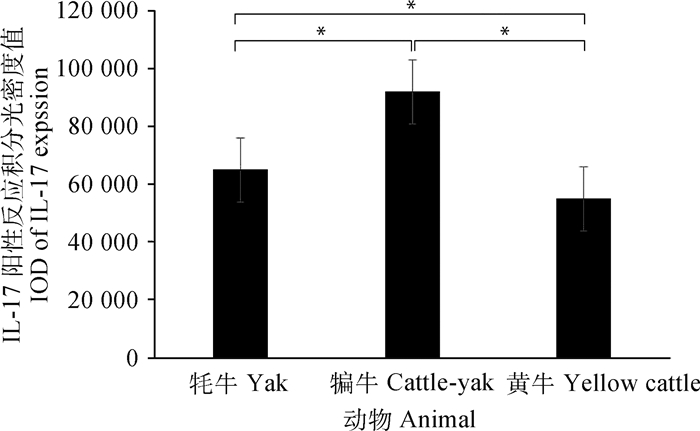
免疫组化结果显示,在牦牛、犏牛及黄牛肝组织中,HSP90和IL-17主要表达在肝细胞的细胞质中,呈棕黄色,其中在淋巴细胞和枯否细胞周围的肝细胞细胞质中呈强阳性表达,其他部位的肝细胞表达较弱(图 2)。积分光密度值分析发现,HSP90在犏牛肝的阳性表达最强、牦牛次之、黄牛最低;且牦牛和犏牛与黄牛之间呈显著性差异(P < 0.05)(图 3)。此外,IL-17在犏牛肝阳性表达最强、牦牛次之、黄牛最低,且相互之间呈显著性差异(P < 0.05)(图 4)。
|
用PBS(0.01 mol·L-1)代替一抗作阴性对照;KC.枯否细胞;HCs.肝细胞;L.淋巴细胞 Negative control was conducted by replacing first antibody of with 0.01 mol·L-1 PBS; KC. Kupffer cell; HCs. Hepatocytes; L. Lymphocyte 图 2 HSP90和IL-17在成年牦牛、犏牛及黄牛肝组织的分布(免疫组化学法,1 000×) Fig. 2 Immunohistochemistry of HSP90 and IL-17 in liver tissues of Yak, Cattle-yak and Yellow cattle (immunohistochemical method, 1 000×) |
|
*.差异显著(P < 0.05).**.差异极显著(P < 0.01) *. Significant difference (P < 0.05); **. The difference is extremely significant (P < 0.01) 图 3 HSP90在成年牦牛、犏牛及黄牛肝免疫组化阳性反应的积分光密度值分析 Fig. 3 Analysis of HSP90 immunohistochemical integral optical density in adult Yak, Cattle-yak and Yellow cattle liver |
|
*.差异显著(P < 0.05).**.差异极显著(P < 0.01) *. Significant difference (P < 0.05); **. The difference is extremely significant (P < 0.01) 图 4 IL-17在成年牦牛、犏牛及黄牛肝免疫组化阳性反应的积分光密度值分析 Fig. 4 Analysis of IL-17 immunohistochemical integral optical density in adult Yak, Cattle-yak and Yellow cattle liver |
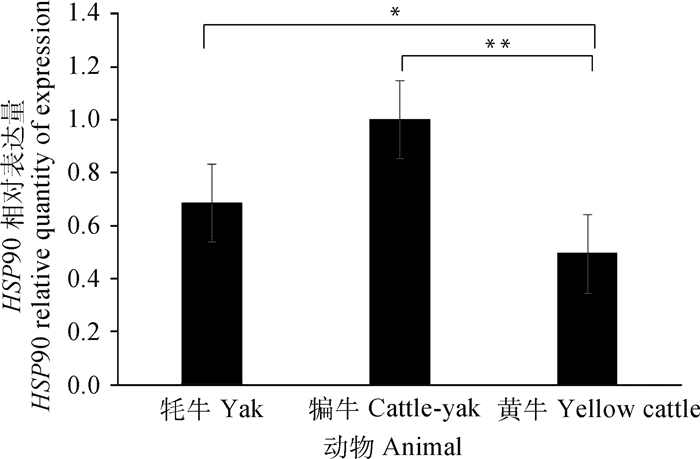
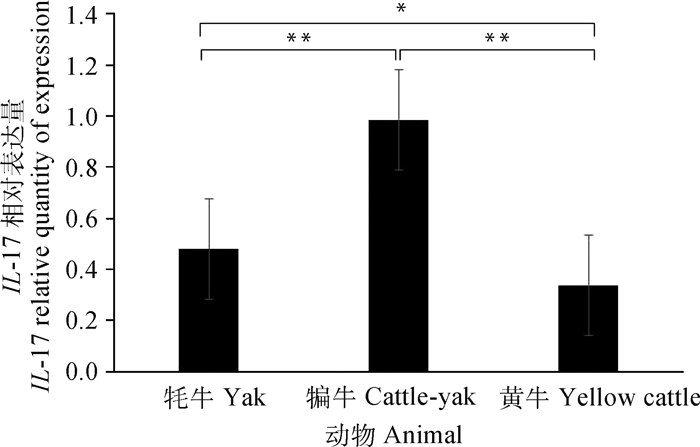
通过SPSS 19.0软件对qRT-PCR检测的结果统计分析,HSP90和IL-17基因在牦牛、犏牛及黄牛肝组织中均有表达。其中HSP90在犏牛肝组织中表达最高,牦牛次之、黄牛最低;牦牛与黄牛之间差异显著(P < 0.05),而犏牛和黄牛之间差异极显著(P < 0.01)(图 5)。IL-17在犏牛肝组织表达最高、牦牛次之、黄牛最低;犏牛与牦牛和黄牛之间呈极显著差异(P < 0.01),而牦牛和黄牛之间为显著差异(P < 0.05)(图 6)。
|
*.差异显著(P < 0.05).**.差异极显著(P < 0.01) *. Significant difference (P < 0.05); **. The difference is extremely significant (P < 0.01) 图 5 HSP90在不同牛肝实时荧光定量分析 Fig. 5 Real-time fluorescence quantitative analysis of HSP90 in different bovine livers |
|
*.差异显著(P < 0.05).**.差异极显著(P < 0.01) *. Significant difference (P < 0.05); **. The difference is extremely significant (P < 0.01) 图 6 IL-17在不同牛肝实时荧光定量分析 Fig. 6 Real-time fluorescence quantitative analysis of IL-17 in different bovine livers |
作者发现,HE染色观察到牦牛、犏牛及黄牛肝组织中肝细胞呈索状排列,肝索之间的肝血窦中有少量的淋巴细胞和枯否细胞。已有研究证实这些免疫细胞具有吞噬细菌和病毒的作用,也与细胞因子的分泌有关,并参与免疫调节、炎症反应及组织修复等功能,还能清除衰老、变性的血细胞和肿瘤细胞[20-21]。
IL-17由淋巴细胞、枯否细胞等细胞分泌,是一种多效性细胞因子[22]。它不仅作用于多种细胞增强炎性分子的表达,而且在异常表达时参与自身免疫性疾病及肿瘤致病过程[23-24]。作者研究发现,牦牛、犏牛及黄牛肝组织中都存在少量的淋巴细胞和枯否细胞,正常生理条件下也能分泌一定量的IL-17等免疫因子。而研究已证实IL-17的表达随着肝纤维化程度的增加而增加,其抗体能减轻肝肉芽肿和纤维化的炎症[25-26]。笔者进一步从蛋白和基因水平检测IL-17,发现犏牛表达最高,牦牛次之,黄牛最少,且它们之间呈现出不同差异性,这种差异性表达可能与物种有关。研究还发现IL-17不仅对地塞米松诱导细胞凋亡有明显的抑制作用,还可能通过降低足细胞标记蛋白水平诱导足细胞凋亡[3, 27]。而且作者发现,IL-17表达在犏牛和牦牛肝组织表达都高于黄牛。由此可见,IL-17可能参与调控犏牛和牦牛肝细胞的生理功能,从而更好地适应外界低温刺激。
HSP90是分子伴侣的重要成员,参与器官的发育、神经退行性疾病和DNA损伤修复,还能协同免疫因子参与免疫反应[28]。近年来有大量学者研究发现,HSP90对调控细胞凋亡和炎症反应也发挥重要功能,抗氧化系统和热休克蛋白能够保护细胞免受氨诱导的氧化应激和细胞凋亡[29]。本研究的检测结果显示,HSP90在犏牛表达最高,牦牛次之,黄牛最低,且犏牛和牦牛与黄牛之间表达呈现不同的差异性。前人研究发现,HSP90是细胞生长周期调控的关键信号分子,调节细胞的生长、存活、分化和凋亡等多种细胞生理过程[30]。因此,HSP90在牦牛和犏牛肝相对于黄牛肝是高表达,这与它们生活环境息息相关,也可能与牦牛和犏牛肝细胞存活和凋亡存在一定的关联性。
业已证实,当机体受到热应激刺激时,热应激因子HSP90和免疫应答蛋白IL-17的基因表达水平增加,调节相关组织器官产生抗应激反应及细胞免疫反应。蛋白半定量检测发现,HSP90和IL-17在犏牛和牦牛肝中表达较强,说明犏牛和牦牛肝对温差变化的刺激具有更好的抗应激反应。此外,学者研究还发现HSP90抑制剂能诱导IL-17促使肺黏膜上皮损伤的杯状细胞进行修复,使细胞形态和功能得到恢复[31]。HSP90的低表达能够导致IL-17信号传导缺陷,使胃肠道中的微生物菌群出现异常,产生组织炎症反应,不利于肠道黏膜对细菌的免疫作用[32]。免疫组化结果显示,HSP90和IL-17蛋白阳性反应主要呈现于肝细胞的细胞质中。因此,推测由淋巴细胞和枯否细胞分泌的IL-17和HSP90转移到肝细胞的细胞质中,可能参与了肝细胞对外源物质的免疫排斥反应和出现损伤时的炎症反应,来更好地适应外界环境变化。
4 结论IL-17和HSP90基因在牦牛、犏牛及黄牛肝中表达的差异说明具有种属特异性;IL-17和HSP90蛋白半定量及阳性反应,说明犏牛和牦牛肝与黄牛肝相比,对温差变化刺激具有更好的适应性。
| [1] | WIENR G, JIANLIN H, RUIJUN L. The yak[M]//ULLREY D E, BAER C K, POND W G. Encyclopedia of Animal Science. Boca Raton, FL: CRC Press, 2010. |
| [2] | HIROTA K, DUARTE J H, VELDHOEN M, et al. Fate mapping of IL-17-producing T cells in inflammatory responses[J]. Nat Immunol, 2011, 12(3): 255–263. DOI: 10.1038/ni.1993 |
| [3] | WANG L, LI Q, WANG L, et al. The role of Th17/IL-17 in the pathogenesis of primary nephrotic syndrome in children[J]. Kidney Blood Press Res, 2013, 37(4-5): 332–345. DOI: 10.1159/000350161 |
| [4] | TANG Y, BIAN Z, ZHAO L, et al. Interleukin-17 exacerbates hepatic steatosis and inflammation in non-alcoholic fatty liver disease[J]. Clin Exp Immunol, 2011, 166(2): 281–290. DOI: 10.1111/j.1365-2249.2011.04471.x |
| [5] |
叶艳萍, 宋雷英. 谷氨酰胺肠内营养+低分子肝素对重症肺炎患者全身炎症反应程度的影响[J]. 海南医学院学报, 2018, 24(15): 1406–1409.
YE Y P, SONG L Y. Effect of glutamine enteral nutrition + low molecular weight heparin on systemic inflammatory response in patients with severe pneumonia[J]. Journal of Hainan Medical University, 2018, 24(15): 1406–1409. (in Chinese) |
| [6] | ABUSLEME L, MOUTSOPOULOS N M. IL-17: overview and role in oral immunity and microbiome[J]. Oral Dis, 2017, 23(7): 854–865. DOI: 10.1111/odi.12598 |
| [7] | LINDQUIST S, CRAIG E A. The heat-shock proteins[J]. Ann Rev Genet, 1988, 22: 631–677. DOI: 10.1146/annurev.ge.22.120188.003215 |
| [8] | SHI Q, DONG Z J, WEI H M. The involvement of heat shock proteins in murine liver regeneration[J]. Cell Mol Immunol, 2007, 4(1): 53–57. |
| [9] | WEI T, GAO Y H, WANG R X, et al. A heat shock protein 90 β isoform involved in immune response to bacteria challenge and heat shock from Miichthys miiuy[J]. Fish Shellfish Immunol, 2013, 35(2): 429–437. DOI: 10.1016/j.fsi.2013.04.045 |
| [10] | AMBADE A, CATALANO D, LIM A, et al. Inhibition of heat shock protein 90 alleviates steatosis and macrophage activation in murine alcoholic liver injury[J]. J Hepatol, 2014, 61(4): 903–911. DOI: 10.1016/j.jhep.2014.05.024 |
| [11] | LIU P G, YU S J, CUI Y, et al. Cloning of HSP90, expression and localization of HSP70/90 in different tissues including lactating/non-lactating yak (Bos grunniens) breast tissue[J]. PLoS One, 2017, 12(7). |
| [12] | YANG X, CUI Y, YUE J, et al. The histological characteristics, age-related thickness change of skin, and expression of the HSPs in the skin during hair cycle in yak (Bos grunniens)[J]. PLoS One, 2017, 12(5). |
| [13] | LIU P G, YU S J, CUI Y, et al. Regulation by HSP27/P53 in testis development and sperm apoptosis of male cattle (cattle-yak and yak)[J]. J Cell Physiol, 2019, 234(1): 650–660. DOI: 10.1002/jcp.26822 |
| [14] | WU L, WANG C H, BOISSON B, et al. The differential regulation of human ACT1 isoforms by HSP90 in IL-17 signaling[J]. J Immunol, 2014, 193(4): 1590–1599. DOI: 10.4049/jimmunol.1400715 |
| [15] | LIU J J, ZHAO H J, WANG Y, et al. Impacts of simultaneous exposure to arsenic (Ⅲ) and copper (Ⅱ) on inflammatory response, immune homeostasis, and heat shock response in chicken thymus[J]. Int Immunopharmacol, 2018, 64: 60–68. DOI: 10.1016/j.intimp.2018.08.021 |
| [16] | HAMMERICH L, HEYMANN F, TACKE F. Role of IL-17 and Th17 cells in liver diseases[J]. Clin Dev Immunol, 2011, 2011: 345803. |
| [17] | WANG L Y, CHEN S J, XU K S. IL-17 expression is correlated with hepatitis B-related liver diseases and fibrosis[J]. Int J Mol Med, 2011, 27(3): 385–392. |
| [18] | NAKAGAWA S I, UMEHARA T, MATSUDA C, et al. HSP90 inhibitors suppress HCV replication in replicon cells and humanized liver mice[J]. Biochem Biophys Res Commun, 2007, 353(4): 882–888. DOI: 10.1016/j.bbrc.2006.12.117 |
| [19] | HE W, HU H X. BIIB021, an HSP90 inhibitor:a promising therapeutic strategy for blood malignancies[J]. Oncol Rep, 2018, 40(1): 3–15. |
| [20] | MOVITA D, VAN DE GARDE M D B, BIESTA P, et al. Inflammatory monocytes recruited to the liver within 24 hours after virus-induced inflammation resemble Kupffer cells but are functionally distinct[J]. J Virol, 2015, 89(9): 4809–4817. DOI: 10.1128/JVI.03733-14 |
| [21] | ARMENDARIZ-BORUNDA J, SEYER J M, POSTLETHWAITE A E, et al. Kupffer cells from carbon tetrachloride-injured rat livers produce chemotactic factors for fibroblasts and monocytes:the role of tumor necrosis factor-α[J]. Hepatology, 1991, 14(5): 895–900. DOI: 10.1002/hep.1840140523 |
| [22] | MONTEIRO M, ALMEIDA C F, AGUA-DOCE A, et al. Induced IL-17-producing invariant NKT cells require activation in presence of TGF-β and IL-1β[J]. J Immunol, 2013, 190(12): 5909–5910. |
| [23] | BARIN J G, BALDEVIANO G C, TALOR M V, et al. Macrophages participate in IL-17-mediated inflammation[J]. Eur J Immunol, 2012, 42(3): 726–736. DOI: 10.1002/eji.201141737 |
| [24] | CYPOWYJ S, PICARD C, MARÍDI L, et al. Immunity to infection in IL-17-deficient mice and humans[J]. Eur J Immunol, 2012, 42(9): 2246–2254. DOI: 10.1002/eji.201242605 |
| [25] | CHEN D H, LUO X P, XIE H Y, et al. Characteristics of IL-17 induction by Schistosoma japonicum infection in C57BL/6 mouse liver[J]. Immunology, 2013, 139(4): 523–532. DOI: 10.1111/imm.12105 |
| [26] | DU W J, ZHEN J H, ZENG Z Q, et al. Expression of Interleukin-17 associated with disease progression and liver fibrosis with hepatitis B virus infection:IL-17 in HBV infection[J]. Diagn Pathol, 2013, 8: 40. DOI: 10.1186/1746-1596-8-S1-S40 |
| [27] | VAZQUEZ-TELLO A, HALWANI R, HAMID Q, et al. Glucocorticoid receptor-beta up-regulation and steroid resistance induction by IL-17 and IL-23 cytokine stimulation in peripheral mononuclear cells[J]. J Clin Immunol, 2013, 33(2): 466–478. DOI: 10.1007/s10875-012-9828-3 |
| [28] | RITOSSA F. Discovery of the heat shock response[J]. Cell Stress Chaperones, 1996, 1(2): 97–98. DOI: 10.1379/1466-1268(1996)001<0097:DOTHSR>2.3.CO;2 |
| [29] | CHENG C H, YANG F F, LING R Z, et al. Effects of ammonia exposure on apoptosis, oxidative stress and immune response in pufferfish (Takifugu obscurus)[J]. Aquat Toxicol, 2015, 164: 61–71. DOI: 10.1016/j.aquatox.2015.04.004 |
| [30] | TUKAJ S, WEGRZYN G. Anti-HSP90 therapy in autoimmune and inflammatory diseases:a review of preclinical studies[J]. Cell Stress Chaperones, 2016, 21(2): 213–218. DOI: 10.1007/s12192-016-0670-z |
| [31] | PEZZULO A A, TUDAS R A, STEWART C G, et al. HSP90 inhibitor geldanamycin reverts IL-13 and IL-17-induced airway goblet cell metaplasia[J]. J Clin Invest, 2019, 129(2): 744–758. DOI: 10.1172/JCI123524 |
| [32] | KUMAR P, KOLLS J K. Act1-hsp90 heats up TH17 inflammation[J]. Nat Immunol, 2013, 14(1): 16–17. |



