2. 郑州市畜牧局, 郑州 450052
2. Zhengzhou Animal Husbandry Bureau, Zhengzhou 450052, China
经典的双组分信号转导系统(two-component signal transduction system, TCS)由感应子激酶(sensor histidine kinase, HK)和应答调节子(response regulator, RR)组成[1]。当受到外界信号刺激时,HK的组氨酸残基发生自身磷酸化,并将磷酸基团转移到RR的天冬氨酸残基上使RR发生磷酸化,磷酸化的RR进而与靶基因的启动子区域结合调控靶基因的表达水平[2]。CpxAR由跨膜的感应子激酶CpxA和胞内的应答调节子CpxR组成,是革兰阴性菌中普遍存在的双组分之一,与多种抗菌药物的耐药相关[3-4]。此外,细菌的外排泵对药物的外排作用是细菌对多种抗生素耐药的重要机制之一,外排泵活性的增加通常会增加细菌对药物的外排作用[5-6]。研究表明,RND(resistance nodulation-cell division)家族的AcrAB-TolC外排泵活性与大肠杆菌和沙门菌的多重耐药相关[7-9]。AcrAB-TolC由AcrA、AcrB和TolC组成,在大肠杆菌中呈构成性表达,并且是大肠杆菌和沙门菌药物外排的主要外排泵,acrB的缺失能够增加大肠杆菌和沙门菌的药物敏感性[8, 10-11]。
黏菌素属于阳离子多肽,对革兰阴性菌具有良好的抑制、杀灭作用,但因其具有肾毒性和神经毒性曾在人类医学临床上的使用受到限制[12-13]。但随着革兰阴性菌多重耐药现象的增加,黏菌素作为治疗革兰阴性菌感染的最后一道防线被重新用来治疗这些细菌的感染。随着黏菌素在临床上的应用逐渐增加,黏菌素的耐药菌株也日益增多,目前已经出现革兰阴性菌对该药产生耐药[14-15]。革兰阴性菌对黏菌素的耐药机制包括染色体介导和质粒介导(mcr基因)两种途径[12]。染色体介导的黏菌素耐药通常与双组分基因pmrAB和phoPQ的点突变或缺失相关。pmrAB和phoPQ产生突变或表达量改变时,可以通过一系列类似于CpxAR的信号传递过程,使与LPS修饰相关的基因表达量上调,最终使LPS的修饰增加或构像发生变化,进而减少黏菌素与细菌的结合而使细菌产生黏菌素耐药现象[16-17]。笔者实验室前期的研究表明,与沙门菌标准菌株(JS)相比,acrB和cpxR双缺失的鼠伤寒沙门菌JSΔacrBΔcpxR::kan(JSΔΔ)对黏菌素的敏感性没有显著变化,而acrB和cpxR双缺失后的cpxR回补株JSΔΔ/pR对黏菌素的敏感性显著上升,其MIC值较JS显著下降,并且JSΔΔ/pR中的黏菌素耐药相关基因pmrB、phoP和phoQ的表达水平显著下降,我们猜测CpxR可能通过对JSΔΔ/pR中的黏菌素耐药通路相关的某些基因(如pmrAB或phoPQ)产生调控作用,使其对黏菌素的敏感性显著上升[18]。因此,本研究通过构建pmrB和phoQ的ΔacrBΔcpxR::kan双缺失过表达株JSΔΔ/pB和JSΔΔ/pQ,以及cpxR和pmrB或phoQ共表达的ΔacrBΔcpxR::kan双缺失回补株JSΔΔ/pRB和JSΔΔ/pRQ,并对其MIC值、生长曲线和黏菌素的杀菌曲线进行检测,探讨cpxR在acrB缺失背景下对沙门菌的黏菌素耐药相关基因pmrB和phoQ的调控作用,为深入分析CpxR调控黏菌素的分子机制、寻找新的药物作用靶点提供理论依据。
1 材料与方法 1.1 菌株和质粒鼠伤寒沙门菌标准菌株(CVCC541,命名为JS)购自中国兽医药品监察所。沙门菌acrB和cpxR双基因缺失株JSΔΔ和cpxR回补株JSΔΔ/pR由本实验室制备并保存[4, 19-20]。pBAD-HisA质粒由本实验室保存。
1.2 主要试剂和药品LB肉汤、LB琼脂等培养基购自青岛海博生物技术有限公司;质粒小提试剂盒和DNA胶回收试剂盒购自美国OMEGA公司;Primer STAR高保真酶和荧光染料mix购自TaKaRa公司;反转录试剂盒购自Roche公司;PCR引物由上海生工公司合成;卡那霉素(Kanr)、氨苄青霉素(Ampr)和L-阿拉伯糖均购自Solarbio公司;DL2000、DL15000 DNA Marker均购自北京擎科新业生物技术有限公司。
1.3 引物设计根据GenBank数据库公布的鼠伤寒沙门菌的全基因组序列(HG326213.1),利用Primer 5.0设计用于构建过表达株的特异引物。其中pmrB-F/pmrB-R和phoQ-F/phoQ-R用于PCR扩增pmrB和phoQ的全长ORF,cpxR-F/pmrB-oR/pmrB-oF/pmrB-R和cpxR-F/phoQ-oR/phoQ-oF/phoQ-R用于扩增cpxR-pmrB和cpxR-phoQ的共表达基因,其中cpxR-F位于cpxR基因的起始密码子ATG处,pmrB-oR/pmrB-oF和phoQ-oR/phoQ-oF分别由cpxR基因的末端序列和pmrB或phoQ基因的启动子(pmrB和phoQ基因的起始密码子ATG上游600 bp处)起始序列组成,pmrB-R和phoQ-R分别位于pmrB和phoQ基因的终止密码子处,引物序列见表 1。
|
|
表 1 pBAD-pmrB、pBAD-phoQ、pBAD-cpxR-pmrB和pBAD-cpxR-phoQ重组质粒的构建及鉴定引物 Table 1 Primers for construction and identification of pBAD-pmrB, pBAD-phoQ, pBAD-cpxR-pmrB and pBAD-cpxR-phoQ recombinant plasmids |
以JS标准菌株的基因组DNA为模板,用pmrB-F、pmrB-R和phoQ-F、phoQ-R引物分别扩增出pmrB和phoQ的完整ORF,与pBAD-HisA质粒连接,构成pBAD-pmrB、pBAD-phoQ重组质粒。以JS的基因组DNA为模板,用cpxR-F、pmrB-oR和pmrB-oF、pmrB-R两对引物分别扩增出cpxR-pmrB前端的cpxR片段和后端的pmrB片段,然后以cpxR片段和pmrB的PCR产物为模板,用cpxR-F、pmrB-R引物通过overlapping PCR扩增出共表达基因cpxR-pmrB,与pBAD-HisA质粒连接之后构成pBAD-cpxR-pmrB重组质粒。用cpxR-F、phoQ-oR和phoQ-oF、phoQ-R两对引物分别扩增出cpxR-phoQ的前端cpxR片段和后端phoQ片段,然后以cpxR片段和phoQ的PCR产物为模板,用cpxR-F、phoQ-R引物通过overlapping PCR扩增出共表达基因cpxR-phoQ,与pBAD-HisA质粒连接之后构成pBAD-cpxR-phoQ重组质粒。
1.4.2 过表达重组质粒的电转化挑取JSΔΔ单菌落接种于Kanr(50 mg·L-1)的LB肉汤培养基中过夜培养。过夜培养物按1:100的比例转接新鲜的LB肉汤培养基中,培养至OD600 nm= 0.4后制备电转感受态。用2.3 kV、2.5 mm进行电转化,涂布Ampr(100 mg·L-1)LB琼脂平板筛选阳性电转化子。
1.4.3 阳性转化子的鉴定挑取Ampr(100 mg·L-1)筛选平板上的单克隆接种于Ampr(100 mg·L-1)的LB肉汤培养基中,于37 ℃摇床中200 r·min-1振荡培养过夜,分别用pmrB-F/pmrB-R、phoQ-F/phoQ-R、cpxR-F/pmrB-R和cpxR-F/phoQ-R进行菌液PCR鉴定,PCR产物送上海生工公司测序验证。阳性转化子分别命名为JSΔΔ/pB、JSΔΔ/pQ、JSΔΔ/pRB、JSΔΔ/pRQ。
1.5 MIC值的测定用微量肉汤稀释法[21]测定JS、JSΔΔ、JSΔΔ/pR、JSΔΔ/pB、JSΔΔ/pQ、JSΔΔ/pRB和JSΔΔ/pRQ对黏菌素的MIC,回补和过表达菌株培养基中加入0.2%的L-阿拉伯糖。
1.6 生长曲线的绘制挑取JSΔΔ、JSΔΔ/pR、JSΔΔ/pB、JSΔΔ/pQ、JSΔΔ/pRB和JSΔΔ/pRQ的单菌落于LB肉汤中过夜振荡培养后,转接入新鲜LB肉汤,振荡培养至OD600 nm到达0.40~0.42之间时,将培养物按照1:1 000比例接种到LB肉汤中37 ℃ 200 r·min-1振荡培养12 h。分别在1、2、4、6、8、12和13 h取样,用紫外分光光度计测OD600 nm值,重复三次取平均值并记录数据,以OD600 nm值为纵坐标,时间为横坐标绘制细菌生长曲线。
1.7 杀菌曲线的制作挑取抗性筛选平板上的JSΔΔ、JSΔΔ/pR、JSΔΔ/pB、JSΔΔ/pQ、JSΔΔ/pRB和JSΔΔ/pRQ于LB肉汤中过夜培养后,转接入新鲜LB肉汤,振荡培养至OD600 nm到达0.40~0.50。用不同浓度(0、0.1、0.8和12.8 mg·L-1)的黏菌素孵育12 h,分别在1、4、8和12 h取样,作10倍稀释后涂布无抗LB琼脂平板,37 ℃培养12 h后记录各平板的菌落数。各菌落数与相应时间点的空白对照(0 mg·L-1)菌落数的比值即为存活率。
2 结果 2.1 重组质粒的构建将pmrB、phoQ的完整ORF和cpxR-pmrB、cpxR-phoQ的共表达基因经PCR扩增后连接到pBAD-HisA中,挑取Ampr(100 mg·L-1)筛选平板上的单菌落于相同抗性的LB肉汤培养基中振荡培养过夜,小提质粒后进行双酶切鉴定并测序验证。如图 1所示,重组质粒双酶切之后在1 071(pmrB)、1 464(phoQ)、2 370(cpxR-pmrB)、2 763 bp(cpxR-phoQ)处出现相应的阳性片段,测序显示完全正确,表明重组质粒构建成功。
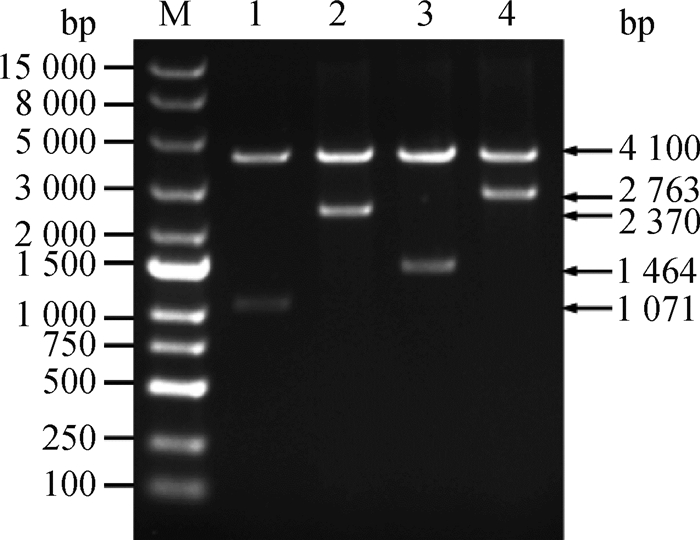
|
M. DL15000 DNA相对分子质量标准;1. pBAD-HisA-pmrB;2. pBAD-HisA-cpxR-pmrB;3. pBAD-HisA-phoQ;4. pBAD-HisA-cpxR-phoQ M. DL15000 DNA marker; 1. pBAD-HisA-pmrB; 2. pBAD-HisA-cpxR-pmrB; 3. pBAD-HisA-phoQ; 4. pBAD-HisA-cpxR-phoQ 图 1 pmrB、phoQ过表达质粒和cpxR-pmrB、cpxR-phoQ共表达质粒的酶切鉴定 Fig. 1 Restriction endonuclease analysis of pmrB, phoQ, cpxR-pmrB and cpxR-phoQ overexpression plasmids |
将“2.1”中构建的阳性重组质粒电转入JSΔΔ感受态中,挑取Ampr(100 mg·L-1)筛选平板上的单菌落于相同抗性的LB培养基中振荡培养过夜,菌液PCR鉴定阳性转化子并测序验证。如图 2所示,在1 071(pmrB)、1 464(phoQ)、2 370(cpxR-pmrB)、2 763 bp(cpxR-phoQ)处均出现相应的阳性片段且测序完全正确,表明过表达菌株构建成功。
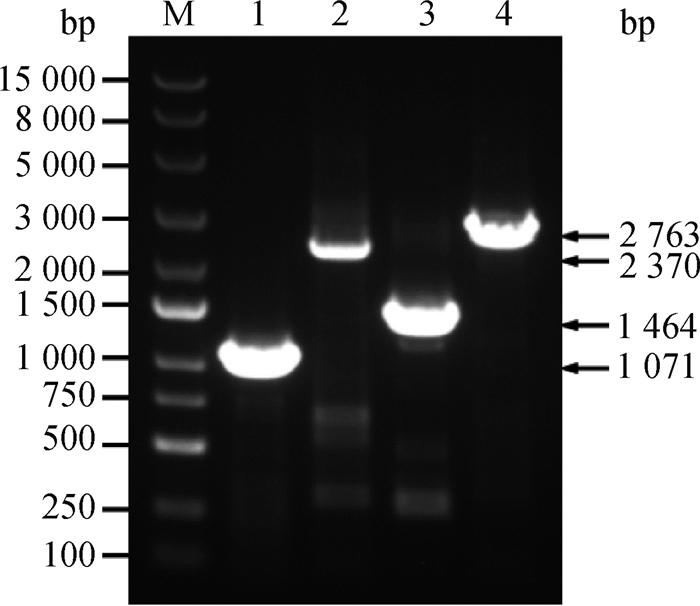
|
M. DL15000 DNA相对分子质量标准;1.用pmrB-F/pmrB-R引物扩增JSΔΔ/pB中的pmrB;2.用cpxR-F/pmrB-R扩增JSΔΔ/pRB中的cpxR-pmrB;3.用phoQ-F/phoQ-R引物扩增JSΔΔ/pQ中的phoQ;4.用cpxR-F/phoQ-R扩增JSΔΔ/pRQ中的cpxR-phoQ M. DL15000 DNA marker; 1.pmrB amplification from JSΔΔ/pB by primers pmrB-F/pmrB-R; 2. cpxR-pmrB amplification from JSΔΔ/pRB by primers cpxR-F/pmrB-R; 3. phoQ amplification from JSΔΔ/pQ by primers phoQ-F/phoQ-R; 4. cpxR-phoQ amplification from JSΔΔ/pRQ by primers cpxR-F/phoQ-R 图 2 pmrB、phoQ过表达质粒和cpxR-pmrB、cpxR-phoQ共表达质粒的阳性电转化子的PCR鉴定 Fig. 2 Identification of the positive transformants of pmrB, phoQ, cpxR-pmrB and cpxR-phoQ overexpression plasmids by PCR |
如表 2所示,黏菌素对JSΔΔ/pR的MIC值较JS和JSΔΔ下降93.75%,与前期研究结果一致[18]。此外,本研究发现与JS相比,黏菌素对pmrB和phoQ的过表达菌株JSΔΔ/pB和JSΔΔ/pQ的MIC值显著升高,为JS的4倍,而黏菌素对cpxR-pmrB和cpxR-phoQ过表达的菌株JSΔΔ/pRB和JSΔΔ/pRQ的MIC值则分别下降, 为JS的1/2和1/4。
|
|
表 2 各菌株对黏菌素的敏感性检测(MICs) Table 2 Susceptibility of Salmonella Typhimurium to colistin |
为测定pmrB和phoQ过表达对沙门菌生长特性的影响,本研究制作了JSΔΔ/pHisA、JSΔΔ/pR、JSΔΔ/pB、JSΔΔ/pQ、JSΔΔ/pRB和JSΔΔ/pRQ在LB肉汤中的生长曲线。如图 3所示,与JSΔΔ/pHisA相比,JSΔΔ/pR的生长活性最差,JSΔΔ/pB、JSΔΔ/pQ、JSΔΔ/pRB和JSΔΔ/pRQ的生长活性低于JSΔΔ/pHisA,但高于JSΔΔ/pR。
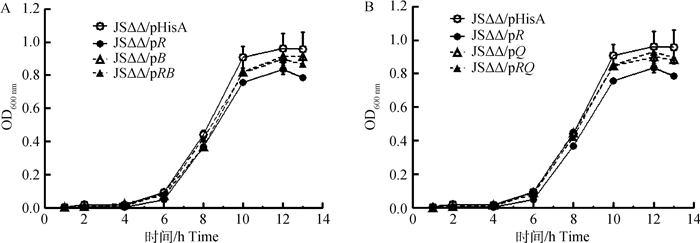
|
图 3 JSΔΔ/pHisA、JSΔΔ/pR和JSΔΔ/pB、JSΔΔ/pRB (A)或JSΔΔ/pQ, JSΔΔ/pRQ (B)在LB肉汤培养基中的生长曲线 Fig. 3 Growth curves of JSΔΔ/pHisA, JSΔΔ/pR, and JSΔΔ/pB, JSΔΔ/pRB (A) or JSΔΔ/pQ, JSΔΔ/pRQ (B) in LB broth |
如图 4所示,随着黏菌素浓度上升各菌株的存活率均逐渐减少,但JSΔΔ/pB和JSΔΔ/pQ在不同浓度的黏菌素中(0.1、0.8、12.8 mg·L-1)的存活率显著高于黏菌素较敏感的JSΔΔ/pRB和JSΔΔ/pRQ。
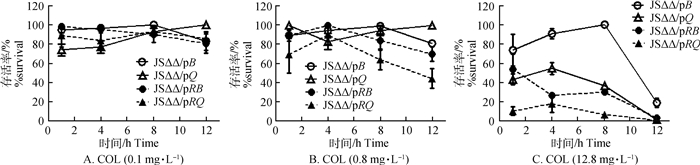
|
图 4 不同浓度的黏菌素对JSΔΔ/pB、JSΔΔ/pQ、JSΔΔ/pRB和JSΔΔ/pRQ的杀菌曲线 Fig. 4 Colistin time-kill assay of JSΔΔ/pB, JSΔΔ/pQ, JSΔΔ/pRB and JSΔΔ/pRQ |
CpxAR是目前研究较多的双组分系统之一,研究发现CpxAR与细菌耐药相关,过表达的CpxR与细菌的新生霉素、β-内酰胺类药物和脱氧胆酸盐的耐药相关[22-23]。当CpxA被激活时,磷酸化的CpxR可以降低细菌对氨基糖苷类抗菌药物和羟基脲的敏感性[24-25]。外排泵的药物外排作用是细菌对抗菌药物及环境中的有毒物质产生耐受的重要机制[26]。外排泵AcrAB在细菌的药物外排作用中发挥主要作用,与β-内酰胺类、氟喹诺酮类、四环素类等药物的耐药相关,acrAB过表达通常与细菌的临床耐药相关[6]。
笔者实验室的前期研究表明,acrB单缺失及cpxR超生理表达(JSΔacrB、JSΔacrB/pcpxR或JSΔcpxR/pcpxR)仅能使菌株对氨基糖苷类、β-内酰胺类等抗菌药物的敏感性发生改变,但并不影响菌株的黏菌素敏感性[4]。只有在acrB缺失且cpxR超生理表达同时存在时(JSΔΔ/pcpxR)才能引起cpxR超临界表达,使菌株对黏菌素敏感性显著上升(16倍),在JSΔΔ/pcpxR中pmrB、phoQ的表达量显著下降,说明在JSΔΔ背景下cpxR对pmrB和phoQ具有负调控作用[18]。因此,为了进一步阐明cpxR对pmrB和phoQ的调控作用及其对沙门菌黏菌素敏感性的调控机制,本试验构建了pmrB、phoQ的单独过表达株JSΔΔ/pB、JSΔΔ/pQ,并用overlapping PCR的方法获得cpxR和带有自身启动子的pmrB、phoQ基因的cpxR-pmrB、cpxR-phoQ共表达菌株JSΔΔ/pRD、JSΔΔ/pRQ。
革兰阴性菌的PmrAB和PhoPQ双组分系统发生突变可使其下游的pmrC、pmrH等LPS修饰相关基因的表达量上升,进而使细菌的类脂A发生4-氨基-4-脱氧-L-阿拉伯糖(Ara4N)或磷酸乙醇胺(pENT)修饰,LPS修饰使细菌表面的负电荷减少,降低细菌对阳离子多肽黏菌素的吸附而产生黏菌素耐药(图 5)[27]。本研究中黏菌素对cpxR回补株JSΔΔ/pR的MIC值较JS下降93.75%,与前期研究结果一致[18]。而黏菌素对单基因表达株JSΔΔ/pB、JSΔΔ/pQ的MIC值较JS上升3倍,黏菌素对cpxR共表达株JSΔΔ/pRB、JSΔΔ/pRQ的MIC值较JS分别下降50%和75%,各菌株的黏菌素杀菌曲线进一步证明菌株的敏感性由高到低依次为JSΔΔ/pR > JSΔΔ/pRQ > JSΔΔ/pRB>JS、JSΔΔ>JSΔΔ/pB、JSΔΔ/pQ。以上结果表明:在JSΔΔ/pB和JSΔΔ/pQ中,当cpxR缺失时其对pmrB和phoQ的负调控作用丧失,此时JSΔΔ中pmrB和phoQ表达量上升(图 5),cpxR缺失与JSΔΔ/pB和JSΔΔ/pQ中过表达的pmrB、phoQ发挥协同调控作用,PmrAB和PhoPQ双组分系统被激活使下游LPS修饰相关基因的表达量上升,从而使LPS发生Ara4N或pENT修饰[16, 27],细菌对黏菌素的敏感性降低,因此JSΔΔ/pB、JSΔΔ/pQ的MIC值较JS上升。而在JSΔΔ/pRB和JSΔΔ/pRQ中,当cpxR与含有自身启动子的pmrB或phoQ共表达时,cpxR对pmrB、phoQ的启动子活性具有负性调控作用,下调共表达的pmrB、phoQ基因的表达,从而最终使JSΔΔ/pRB和JSΔΔ/pRQ对黏菌素的敏感性显著上升。本研究表明cpxR能够负性调控黏菌素耐药基因pmrB和phoQ的表达,增加鼠伤寒沙门菌对黏菌素的敏感性,但沙门菌对黏菌素的敏感性可能受到多方面的复杂调控[28],因此过表达的CpxR对PmrAB和PhoPQ双组分系统的调控机制仍然需要进一步研究。

|
“—”表示负调控;“+”表示正调控 "—"represents a negative regulation; "+" represents a positive regulation 图 5 CpxR对黏菌素耐药相关基因pmrB和phoQ的调控通路图 Fig. 5 A pathway for the regulation of CpxR on colistin resistance-related genes pmrB and phoQ |
成功构建了鼠伤寒沙门菌的黏菌素耐药相关基因pmrB和phoQ的单基因表达株JSΔΔ/pB、JSΔΔ/pQ及cpxR-pmrB、cpxR-phoQ共表达株JSΔΔ/pRB、JSΔΔ/pRQ;cpxR缺失时,pmrB和phoQ过表达使沙门菌的黏菌素敏感性下降,而当cpxR与pmrB和phoQ共表达后沙门菌对黏菌素的敏感性显著上升。cpxR能够负性调控鼠伤寒沙门菌的黏菌素耐药相关基因pmrB和phoQ的表达,增加沙门菌对黏菌素的敏感性。
| [1] | ZSCHIEDRICH C P, KEIDEL V, SZURMANT H. Molecular mechanisms of two-component signal transduction[J]. J Mol Biol, 2016, 428(19): 3752–3775. DOI: 10.1016/j.jmb.2016.08.003 |
| [2] | KAWADA-MATSUO M, KOMATSUZAWA H. Role of Streptococcus mutans two-component systems in antimicrobial peptide resistance in the oral cavity[J]. Jpn Dent Sci Rev, 2017, 53(3): 86–94. DOI: 10.1016/j.jdsr.2016.12.002 |
| [3] | HU W S, CHEN H W, ZHANG R Y, et al. The expression levels of outer membrane proteins STM1530 and OmpD, which are influenced by the CpxAR and BaeSR two-component systems, play important roles in the ceftriaxone resistance of Salmonella enterica serovar Typhimurium[J]. Antimicrob Agents Chemother, 2011, 55(8): 3829–3837. DOI: 10.1128/AAC.00216-11 |
| [4] | HUANG H, SUN Y W, YUAN L, et al. Regulation of the two-component regulator CpxR on aminoglycosides and β-lactams resistance in Salmonella enterica serovar Typhimurium[J]. Front Microbiol, 2016, 7: 604. |
| [5] | WANG-KAN X, BLAIR J M A, CHIRULLO B, et al. Lack of AcrB efflux function confers loss of virulence on Salmonella enterica serovar Typhimurium[J]. mBio, 2017, 8(4): e00968–17. |
| [6] | SCHUSTER S, VAVRA M, SCHWEIGGER T M, et al. Contribution of AcrAB-TolC to multidrug resistance in an Escherichia coli sequence type 131 isolate[J]. Int J Antimicrob Agents, 2017, 50(3): 477–481. DOI: 10.1016/j.ijantimicag.2017.03.023 |
| [7] | BAUCHERON S, TYLER S, BOYD D, et al. AcrAB-TolC directs efflux-mediated multidrug resistance in Salmonella enterica serovar Typhimurium DT104[J]. Antimicrob Agents Chemother, 2004, 48(10): 3729–3735. DOI: 10.1128/AAC.48.10.3729-3735.2004 |
| [8] | EAVES D J, RICCI V, PIDDOCK L J V. Expression of acrB, acrF, acrD, marA, and soxS in Salmonella enterica serovar Typhimurium: role in multiple antibiotic resistance[J]. Antimicrob Agents Chemother, 2004, 48(4): 1145–1150. DOI: 10.1128/AAC.48.4.1145-1150.2004 |
| [9] | GIRAUD E, CLOECKAERT A, KERBOEUF D, et al. Evidence for active efflux as the primary mechanism of resistance to ciprofloxacin in Salmonella enterica serovar Typhimurium[J]. Antimicrob Agents Chemother, 2000, 44(5): 1223–1228. DOI: 10.1128/AAC.44.5.1223-1228.2000 |
| [10] | HORIYAMA T, NISHINO K. AcrB, AcrD, and MdtABC multidrug efflux systems are involved in enterobactin export in Escherichia coli[J]. PLoS One, 2014, 9(9): e108642. DOI: 10.1371/journal.pone.0108642 |
| [11] | NISHINO K, YAMADA J, HIRAKAWA H, et al. Roles of TolC-dependent multidrug transporters of Escherichia coli in resistance to β-lactams[J]. Antimicrob Agents Chemother, 2003, 47(9): 3030–3033. DOI: 10.1128/AAC.47.9.3030-3033.2003 |
| [12] | LIU Y Y, WANG Y, WALSH T R, et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study[J]. Lancet Infect Dis, 2016, 16(2): 161–168. DOI: 10.1016/S1473-3099(15)00424-7 |
| [13] | ANJUM M F, DUGGETT N A, ABUOUN M, et al. Colistin resistance in Salmonella and Escherichia coli isolates from a pig farm in great Britain[J]. J Antimicrob Chemother, 2016, 71(8): 2306–2313. DOI: 10.1093/jac/dkw149 |
| [14] | QUESADA A, UGARTE-RUIZ M, IGLESIAS M R, et al. Detection of plasmid mediated colistin resistance (MCR-1) in Escherichia coli and Salmonella enterica isolated from poultry and swine in Spain[J]. Res Vet Sci, 2016, 105: 134–135. DOI: 10.1016/j.rvsc.2016.02.003 |
| [15] | LI X P, FANG L X, SONG J Q, et al. Clonal spread of mcr-1 in PMQR-carrying ST34 Salmonella isolates from animals in China[J]. Sci Rep, 2016, 6: 38511. DOI: 10.1038/srep38511 |
| [16] | CATRY B, CAVALERI M, BAPTISTE K, et al. Use of colistin-containing products within the European Union and European Economic Area (EU/EEA):development of resistance in animals and possible impact on human and animal health[J]. Int J Antimicrob Agents, 2015, 46(3): 297–306. DOI: 10.1016/j.ijantimicag.2015.06.005 |
| [17] | LEE J Y, KO K S. Mutations and expression of PmrAB and PhoPQ related with colistin resistance in Pseudomonas aeruginosa clinical isolates[J]. Diagn Microbiol Infect Dis, 2014, 78(3): 271–276. DOI: 10.1016/j.diagmicrobio.2013.11.027 |
| [18] | ZHAI Y J, HUANG H, LIU J H, et al. CpxR overexpression increases the susceptibility of acrB and cpxR double-deleted Salmonella enterica serovar Typhimurium to colistin[J]. J Antimicrob Chemother, 2018, 73(11): 3016–3024. DOI: 10.1093/jac/dky320 |
| [19] |
黄慧, 陈丽鹏, 孙亚伟, 等. 亚MIC庆大霉素下CpxR对鼠伤寒沙门菌生长特性和acrD基因转录的影响[J]. 中国兽医学报, 2016, 36(6): 995–1000.
HUANG H, CHEN L P, SUN Y W, et al. Influence of CpxR on growth characteristics and AcrD gene transcription of Salmonella enterica serovar typhimurium at the subinhibitory concentrations of gentamicin[J]. Chinese Journal of Veterinary Science, 2016, 36(6): 995–1000. (in Chinese) |
| [20] |
黄慧, 刘保光, 孙亚伟, 等. 鼠伤寒沙门菌cpxR和acrB双基因缺失菌株的构建及其对抗菌药物敏感性分析[J]. 畜牧兽医学报, 2016, 47(3): 595–602.
HUANG H, LIU B G, SUN Y W, et al. Construction of cpxR and acrB double gene deletion strain of Salmonella enterica serovar Typhimurium and analysis of its susceptibility to antibacterial agents[J]. Acta Veterinaria et Zootechnica Sinica, 2016, 47(3): 595–602. (in Chinese) |
| [21] | Clinical and Laboratory Standards Institute. M100-S26 Performance standards for antimicrobial susceptibility testing; Twenty-sixth informational supplement[S]. Wayne, PA: CLSI, 2016. |
| [22] | HIRAKAWA H, NISHINO K, HIRATA T, et al. Comprehensive studies of drug resistance mediated by overexpression of response regulators of two-component signal transduction systems in Escherichia coli[J]. J Bacteriol, 2003, 185(6): 1851–1856. DOI: 10.1128/JB.185.6.1851-1856.2003 |
| [23] | HIRAKAWA H, NISHINO K, YAMADA J, et al. β-Lactam resistance modulated by the overexpression of response regulators of two-component signal transduction systems in Escherichia coli[J]. J Antimicrob Chemother, 2003, 52(4): 576–582. DOI: 10.1093/jac/dkg406 |
| [24] | MAHONEY T F, SILHAVY T J. The Cpx stress response confers resistance to some, but not all, bactericidal antibiotics[J]. J Bacteriol, 2013, 195(9): 1869–1874. DOI: 10.1128/JB.02197-12 |
| [25] | BIALEK-DAVENET S, LAVIGNE J P, GUYOT K, et al. Differential contribution of AcrAB and OqxAB efflux pumps to multidrug resistance and virulence in Klebsiella pneumoniae[J]. J Antimicrob Chemother, 2015, 70(1): 81–88. DOI: 10.1093/jac/dku340 |
| [26] | RAJAMOHAN G, SRINIVASAN V B, GEBREYES W A. Molecular and functional characterization of a novel efflux pump, AmvA, mediating antimicrobial and disinfectant resistance in Acinetobacter baumannii[J]. J Antimicrob Chemother, 2010, 65(9): 1919–1925. DOI: 10.1093/jac/dkq195 |
| [27] | HJORT K, NICOLOFF H, ANDERSSON D I. Unstable tandem gene amplification generates heteroresistance (variation in resistance within a population) to colistin in Salmonella enterica[J]. Mol Microbiol, 2016, 102(2): 274–289. DOI: 10.1111/mmi.13459 |
| [28] | BARON S, HADJADJ L, ROLAIN J M, et al. Molecular mechanisms of polymyxin resistance: knowns and unknowns[J]. Int J Antimicrob Agents, 2016, 48(6): 583–591. DOI: 10.1016/j.ijantimicag.2016.06.023 |



