山羊的等热区范围为13~27 ℃[1],耐热极限范围为35~40 ℃[2]。虽然山羊具有较宽的等热区和较高的耐热能力[3],被认为是最耐热的物种之一,但环境温度过高时仍会产生热应激[4]。我国大部分地区尤其是南方地区夏天会出现35~40 ℃的长期或短暂高温天气,容易造成山羊热应激,导致其生长性能和繁殖性能下降[5-6],严重时甚至导致其死亡[7]。因此,研究山羊热应激初期血液生化指标的变化规律和分子机制,对山羊热应激的早期监测和防治具有重要意义。山羊在热应激期间会通过生理生化、行为、分子等不同水平做出各种应答反应来抵抗热应激带来的不利影响[8-10]。但持续的热应激仍会导致山羊生理机能受损,抗氧化能力和免疫机能下降[11]。动物机体在受到应激时,组织细胞会快速产生一类高度保守的保护性热休克蛋白来抵抗应激对细胞的损伤[12-13]。根据相对蛋白质量大小和同源性程度,可把热休克蛋白分为HSP110、HSP90、HSP70、HSP60、小分子HSP及泛素等6大家族[14]。研究表明,HSP70是热休克蛋白家族中最保守,含量最丰富的一种非特异性的内源保护蛋白,具有提高细胞耐受力、抗细胞凋亡、参与机体免疫和分子伴侣等功能[15]。在高温环境刺激下,HSP70基因的表达量会显著升高[16]。Nagayach等[10]、Dangi等[17]研究认为,热休克蛋白家族中HSP70基因对温度最为敏感,可作为山羊对高温反应的生物标记物。Banerjee等[18]研究不同季节山羊HSP70家族成员基因(HSPA8、HSPA6、HSPA1A、HSPA1L和HSPA2)的表达,发现HSPA1A、HSPA6和HSPA8基因对温度变化最为敏感。
此前,有关山羊热应激的研究主要集中在慢性热应激方面,急性热应激是否会诱导山羊氧化应激,抑制免疫机能尚不清楚。此外,急性热应激条件下山羊血淋巴细胞HSP70家族基因mRNA的表达规律鲜有报道。本试验利用环控代谢舱精准控制温湿度,研究急性热应激条件下,山羊血淋巴细胞HSP70家族中HSPA1A、HSPA6和HSPA8基因mRNA表达规律和血清免疫和抗氧化指标的变化规律,旨在探究山羊热应激早期的响应机制,为山羊热应激的早期监测和防治提供理论依据。
1 材料与方法 1.1 试验动物和样品采集试验选取5只健康、体况接近的(12±0.5)月龄波尔山羊×关中奶山羊F1母羊,单只单笼饲养于同一环控舱中。试验羊在环控舱设置的舒适温湿度(温度20 ℃,相对湿度60%)条件下适应5 d,第6天早上08:00将环控代谢舱内温度从20 ℃迅速提高到38 ℃,相对湿度恒定为60%,对试验羊进行38 ℃急性热处理12 h。分别于热应激前(0 h)和热应激后2、4、8和12 h颈静脉采集每只试验羊非抗凝血10 mL和抗凝血10 mL。
1.2 试验动物饲养管理试验在西北农林科技大学(34.24′N,108.05′ E)智能环控代谢舱(7.4 m×4.2 m×2.7 m)内进行,温度和湿度(精度±0.5 ℃、±5%)按试验设计设置并控制保持稳定,每天光照时间为07:00-21:00。试验羊饲喂市售全价颗粒饲料,饲粮营养水平见表 1。每天07:30和20:30投料加水,试验期间羊自由采食、饮水。适应期,研究人员(固定)每天按后期试验设计的时间点进入环控舱,与试验羊接触和交流,使之熟识并适应研究者的介入,以降低非试验处理因素对测定指标的影响。
|
|
表 1 试验饲粮组成与营养水平(风干基础) Table 1 Composition and nutrient levels of the experimental diets (air-dry basis) |
每只羊各采样时间点的非抗凝血样品室温下静置,待血清析出后,在4 ℃、3 000 r·min-1条件下离心10 min,吸取上清液,分装置于1.5 mL离心管内,-20 ℃冷冻待测。血清总抗氧化能力(T-AOC)、超氧化物歧化酶(SOD)活性、谷胱甘肽过氧化物酶(GSH-Px)活性、丙二醛(MDA)含量采用生化法测定。白细胞介素-1β(IL-1β)、白细胞介素-2(IL-2)、白细胞介素-4(IL-4)、干扰素-γ(IFN-γ)、肿瘤坏死因子-α(TNF-α)、免疫球蛋白G(IgG)、免疫球蛋白M(IgM)、免疫球蛋白A(IgA)含量采用酶联免疫吸附测定法测定。试验所用试剂盒均购自北京华英生物技术研究所。
1.4 试验羊血淋巴细胞热休克蛋白70家族基因表达水平的检测 1.4.1 血淋巴细胞总RNA提取采集每只羊各采样时间点的抗凝血样品,参照羊外周血淋巴细胞分离液试剂盒(北京索莱宝科技有限公司)说明书分离出外周血淋巴细胞,加入1.5 mL RNA保存液,于-80 ℃冰箱中保存,用于总RNA提取。参照Eastp® Super总RNA试剂盒说明书提取羊外周血淋巴细胞总RNA,用Micro-Spectrophotometer核酸定量仪(K5800,上海DRAWELL科学仪器有限公司)测定总RNA的纯度和浓度,选出OD260nm/OD280nm为1.8~2.0的总RNA样品,用1%琼脂糖凝胶电泳检测总RNA的完整性。
1.4.2 cDNA合成cDNA第一链合成参照Thermo反转录试剂盒(ThermoScientific,USA)说明书对血淋巴细胞总RNA进行反转录。反转录体系20 μL:RNA 10 μL,Oligo(dT)181 μL,5×Reaction Buffer 4 μL,RiboLock RNase Inhibitor(20 U·μL-1) 1 μL,10 mmol·L-1 dNTP Mix 2 μL,RevertAid M-MuLV RT(200 U·μL-1) 1 μL,RNase Free ddH2O补至20 μL。反转录PCR反应程序:42 ℃,60 min;70 ℃,5 min;终止反应;cDNA产物于-20 ℃保存。
1.4.3 引物设计与合成根据NCBI(http: // www.ncbi.nlm.nih.gov/)公布的山羊HSPA1A、HSPA6、HSPA8、β-actin基因的mRNA序列,使用软件Primer Premier 5.0设计特异性引物,委托北京奥科鼎盛生物技术有限公司合成,引物序列见表 2。
|
|
表 2 引物序列 Table 2 Primer sequences |
进行普通PCR扩增检测引物特异性,后续做实时荧光定量PCR。普通PCR反应体系总体积为10 μL:cDNA 0.5 μL,2×Es Taq MasterMix (Dye) 5 μL,上、下游引物各0.5 μL,RNase Free ddH2O 3.5 μL。PCR扩增条件:95 ℃预变性3 min;95 ℃变性30 s,60 ℃退火30 s,72 ℃延伸15 s,35个循环;72 ℃延伸5 min;4 ℃保存。1.5%琼脂糖凝胶电泳检测PCR产物大小。
实时荧光定量PCR反应体系12.5 μL:SYBR Premix Ex TaqTMⅡ(2×) 6.25 μL,cDNA 1 μL,上、下游引物各0.5 μL,RNase Free ddH2O 4.25 μL。PCR扩增条件:95 ℃预变性30 s;95 ℃变性5 s,60 ℃退火30 s,72 ℃延伸30 s,共40个循环。熔解曲线分析65~95 ℃:0.5 ℃/0.05 s,熔解曲线显示为单峰,引物特异性强。
1.5 数据分析荧光定量结果采用2-△△CT方法计算目的基因的相对表达量;不同热应激时间点试验羊抗氧化指标、免疫指标和基因相对表达量数据采用SPSS 21.0统计软件中GLM过程进行单因素重复测量方差分析。结果用“平均数±标准差”表示,以P < 0.05作为差异显著性判断标准。
2 结果 2.1 急性热应激对山羊血清抗氧化指标的影响急性热应激对试验羊血清抗氧化指标的影响见表 3。热应激时间对试验羊血清抗氧化指标有显著影响。其中与热应激前0 h相比,试验羊血清T-AOC(P < 0.05)、SOD(P < 0.05)和GSH-Px(P < 0.01)活性均在热应激8 h后显著下降,MDA含量在热应激4 h时显著增加(P < 0.05),在热应激8和12 h时极显著增加(P < 0.01)。
|
|
表 3 急性热应激对山羊血清抗氧化指标的影响 Table 3 Effects of acute heat stress on serum antioxidant indexes in goats |
急性热应激对试验羊血清免疫指标的影响见表 4。热应激时间对试验羊血清免疫指标有显著影响。与热应激前0 h相比,试验羊血清TNF-α、IL-1β、IFN-γ和IL-2含量分别在热应激4、8、8和4 h后显著增加(P < 0.05);IL-4(P < 0.01)、IgG(P < 0.01)、IgM(P < 0.01)和IgA(P < 0.05)含量分别在热应激12、4、4和4 h后显著下降。
|
|
表 4 急性热应激对山羊血清免疫指标的影响 Table 4 Effects of acute heat stress on serum immune indexes in goats |
试验羊血淋巴细胞总RNA质量通过1%琼脂糖凝胶电泳检测,结果见图 1。图 1显示,28S和18S条带清晰,无明显降解,表明RNA样品质量可靠。HSP70家族成员HSPA1A、HSPA6、HSPA8和β-actin基因扩增产物通过1.5%琼脂糖凝胶电泳检测,结果见图 2。图 2显示,扩增的DNA条带与预期目的条带大小相符,没有非特异性条带,说明扩增成功,引物特异性好。
|
图 1 总RNA质量检测 Fig. 1 Total RNA quality testing |

|
M. DNA相对分子质量标准;A、B、C、D. HSPA1A、HSPA6、HSPA8和β-actin基因的PCR扩增产物 M.DL2000 DNA marker; A, B, C, D. The PCR products of HSPA1A, HSPA6, HSPA8 and β-actin genes 图 2 PCR产物特异性检测 Fig. 2 Specific detection of PCR products |
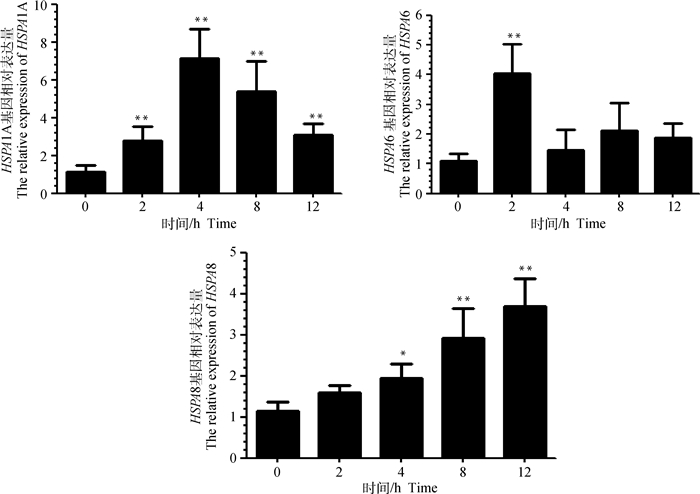
急性热应激条件下,试验羊血淋巴细胞HSPA1A、HSPA6、HSPA8 mRNA相对表达量变化见图 3。热应激时间对试验羊血淋巴细胞HSP70家族基因的表达量有显著影响。HSPA1A mRNA表达量呈先升高后下降的趋势,在热应激4 h时达到峰值,然后开始下降,各检测时间点均极显著高于应激前水平(P < 0.01);HSPA6 mRNA表达量在热应激2 h时极显著升高(P < 0.01),4 h后恢复到应激前水平(P>0.05);HSPA8 mRNA表达量在热应激4 h后显著升高(P < 0.05),热应激8和12 h均极显著高于应激前水平(P < 0.01)。
|
图 3 山羊血淋巴细胞HSPA1A、HSPA6、HSPA8基因表达量变化 Fig. 3 Expression changes of HSPA1A, HSPA6 and HSPA8 genes in blood lymphocytes of goats |
T-AOC是机体防御体系的抗氧化能力,MDA是在脂质过氧化过程中产生的脂质过氧化产物,二者是衡量机体抗氧化系统功能状况的综合性指标[19]。SOD和GSH-Px可以清除超氧阴离子自由基,对机体的氧化与抗氧化平衡起关键作用[20]。张灿等[5]、许啸[21]研究发现,慢性热应激会使山羊血清T-AOC、GSH-Px、SOD等抗氧化酶活性下降,MDA含量增加。王立志[22]将环控舱温度从25 ℃提高到35 ℃对萨能奶山羊进行急性热应激处理24 h,发现热应激4 h后血浆SOD和GSH-Px活性开始显著下降,16 h后开始回升但仍低于应激前水平。不同程度的热应激会使抗氧化酶活性不同,而动物不同的耐热能力也会使抗氧化酶活性存在差异[23]。Yang等[24]对肉鸡进行35 ℃急性热应激处理3 h发现,急性热应激会抑制肉鸡肝线粒体呼吸链活动,使细胞内活性氧(ROS)含量显著升高,抗氧化酶活性下降,导致脂质过氧化反应和氧化应激。但有研究表明,GSH-Px和SOD等抗氧化酶活性在热应激初期会升高,热应激导致抗氧化酶活性升高的原因,一般认为是机体产生的过多氧自由基诱导了抗氧化酶基因的表达,酶活性也随之升高,这是机体的一种代偿保护作用[25]。本试验发现,山羊在急性热应激4 h后,MDA含量显著增加,8 h后T-AOC、SOD和GSH-Px活性有显著的下降,与王立志[22]、Yang等[24]结果类似,说明急性热应激下山羊抗氧化能力下降,细胞和组织受到了一定的氧化损伤,抗氧化酶活性没有出现升高,可能与热应激程度及时间有关,因此可设置不同温度和时间梯度,做进一步研究。
3.2 急性热应激对山羊血清免疫功能的影响TNF-α、IL-1β等促炎细胞因子的少量分泌可以减少应激对机体造成的损伤,但分泌过多会造成组织损伤,损害机体的免疫功能[26-27]。于静等[28]研究发现,小鼠在39 ℃急性热应激2 h后肺TNF-α含量显著增加。熊嫣[29]研究发现,肉鸡在32 ℃急性热应激12 h后血清IL-1β含量显著增加。马燕芬等[30]研究发现,热应激能显著增加奶山羊血清中TNF-α、IL-1β含量。本研究也发现,山羊血清TNF-α、IL-1β含量分别在急性热应激4、8 h后显著增加,表明机体可能出现了炎症反应。
IL-2又称T细胞生长因子,在启动炎症反应中起着重要作用,其水平的高低是机体细胞免疫水平的重要标志[31]。韩爱云等[32]研究发现,肉鸡在35 ℃急性热应激3 h后血清中IL-2含量显著增加。本试验也发现,山羊血清IL-2含量在急性热应激4 h后显著增加,与胡艳欣等[33]研究一致,表明机体在热应激初期提高细胞免疫水平有利于抵抗不利环境。有研究表明,慢性热应激下山羊血清IL-2含量有下降的趋势[5],这可能与热应激的时间和强度有关。IL-4又称B细胞生长因子-1,主要是诱导B细胞成熟并刺激其产生免疫球蛋白,对于体液免疫与获得性免疫具有重要调节作用[34]。IFN-γ主要由活化的T细胞产生,能促进Th 1细胞分泌IL-2,抑制Th 1细胞分化为Th 2细胞进而分泌IL-4,其上调会导致炎症反应[35]。前人关于动物热应激后IL-4含量的变化报道不一,刘晓曦等[36]研究发现,小鼠在40 ℃急性热应激2 h后回肠IL-4 mRNA表达量显著降低。Peli等[37]研究发现,肉牛慢性热应激后血清IL-4含量显著升高,胡煜等[38]研究发现,肉牛慢性热应激后血清IL-4含量没有明显变化。本研究发现,山羊在急性热应激12 h后血清IL-4含量会显著下降,表明热应激初期会抑制山羊体液免疫机能,没有出现升高的趋势可能与热应激程度和时间有关。于静等[28]研究发现,小鼠在39 ℃急性热应激1 h后肺IFN-γ含量显著增加。马燕芬等[30]研究发现,热应激能增加奶山羊血清IFN-γ含量。本研究也发现,山羊在急性热应激8 h后血清IFN-γ含量会显著增加,表明机体出现了炎症反应。IFN-γ与IL-4相互颉颃,二者都可以调节T细胞辅助细胞的分化,其中一种细胞因子分泌量的上升就会导致另一种细胞因子分泌量的减少[39],本试验也得到类似结果。
IgG、IgM、IgA主要由浆细胞分泌,在机体特异性免疫中发挥重要作用[40]。翟杰[41]研究发现,雏鸡在热应激72 h内血清IgA和IgM含量总体呈先下降后短暂上升再下降趋势,IgG呈先上升后持续下降趋势,表明持续高温对雏鸡体液免疫的损害明显,应激初期机体的免疫功能受到抑制。张灿等[5]研究发现,慢性热应激会显著降低山羊血清IgG、IgM、IgA浓度。本试验也发现,山羊在急性热应激4 h后血清IgG、IgM、IgA含量会显著降低,表明急性热应激抑制了肉羊的体液免疫功能,之后没有进一步的降低,但也没有明显上升的趋势,可能与热应激时间有关。Wu等[42]提出,免疫球蛋白含量的变化可能取决于应激类型、强度、持续时间以及动物种类、健康和生理阶段,因此可适当延长急性热应激时间,做进一步深入研究。
3.3 急性热应激对山羊血淋巴细胞HSP70家族基因表达的影响HSP70具有保护变性蛋白折叠、展开和复性的伴侣活性[43],其赋予细胞或生物体从应激状态中恢复的能力,并保护它们免受应激的损害,是对环境和代谢应激综合反映的代表[44]。有研究发现,结构型热休克蛋白70(HSPA8)在所有应激源刺激下均表现为相同的表达,而诱导型热休克蛋白70(HSPA1A)则极易被应激源诱导而表达量升高[45]。HSPA6(HSP70B’)是一种诱导型热休克蛋白,呈现独特的热特异性,在绝大多数细胞中不表达[46],仅在少数热应激后的细胞中被发现[47],说明HSPA6可能专一地参与热应激反应[48]。Sonna等[49]研究发现,热应激会增加人血液中HSPA1A和HSPA6 mRNA的表达量。白丹丹等[50]研究发现,热应激会增加肉牛血淋巴细胞HSPA1A、HSPA1B和HSPA8 mRNA的表达量。Hooper等[51]研究发现,山羊白细胞体外40 ℃热应激3 h会显著增加细胞内HSP70 mRNA表达量。本研究也发现,山羊热应激后血淋巴细胞HSPA1A、HSPA1B和HSPA8 mRNA表达量都有不同程度的升高,说明HSP70家族基因对热应激较为敏感。
HSP70不仅作为分子伴侣发挥作用,而且在炎症反应和免疫调节中起重要作用[52]。有研究表明,HSP70的高表达可以增加机体SOD活性[53]。Gaubin等[54]研究发现,HSP70的过表达可以使人肺A549细胞抵抗氧化应激的损伤,但并没有增加细胞内SOD和GSH-Px活性,本试验也得到类似结果,推测HSP70的表达与抗氧化酶活性的变化并没有直接关系。有研究表明,细胞内HSP70能与核因子抑制蛋白α结合,最终抑制NF-kB信号通路的激活,从而抑制TNF-α、IL-1β等促炎症因子的转录[55],提高细胞对炎症因子的耐受力;而外源性HSP70能刺激单核/巨噬细胞、血管内皮细胞、树突状细胞(DC)等合成和释放TNF-α和IL-1β等促炎症因子[56],促进DC成熟和T淋巴细胞增生,激发T细胞介导的适应性免疫反应[57]。本研究发现,急性热应激能增加山羊血清TNF-α、IL-1β等促炎细胞因子含量,可能与HSP70家族基因(HSPA1A、HSPA6和HSPA8)介导了热应激导致山羊炎症反应的免疫调控过程有关,具体机制有待进一步研究。
Dangi等[17]研究发现,山羊暴露在41和45 ℃下各3 h,血淋巴细胞HSP70 mRNA的表达量都在1 h时到达峰值,总体呈先增加后下降的趋势,可能原因是HSP70 mRNA的表达量先升高可以维持蛋白质的空间构象、保护细胞免受损伤,在增加细胞耐受力后其表达量又下降。本试验也发现,山羊急性热应激后血淋巴细胞HSPA1A和HSPA6 mRNA的表达量呈先增加后下降的趋势,分别在热应激4和2 h到达峰值,与Dangi等[17]结果类似,到达峰值时间和表达倍数不同,可能与羊的品种、热应激程度及时间有关。Rout等[58]研究发现,热应激条件下山羊不同热休克蛋白表达水平和表达规律不同。本研究也发现,HSPA1A、HSPA6和HSPA8 mRNA表达水平和表达规律不一致,可能与不同的热休克蛋白对热应激程度和时间的敏感性不同有关。热休克蛋白的表达具有时间性,与HSPA6、HSPA8相比,HSPA1A对温度更敏感,宜作为热应激早期的分子标志物。
4 结论在本试验条件下,38 ℃急性热应激能够抑制山羊的免疫和抗氧化功能;提高血淋巴细胞HSPA1A、HSPA6和HSPA8基因的表达量,其中HSPA1A对热应激温度和时间更敏感,可作为山羊热应激早期的分子标志物。
| [1] | GUPTA M, DANGI S S, MAURYA D, et al. Expression profile of cold shock protein genes in goats (Capra hircus) during different seasons[J]. Iran J Vet Res, 2014, 15(1): 7–12. |
| [2] | APPLEMAN R D, DELOUCHE J C. Behavioral, physiological and biochemical responses of goats to temperature, 0 to 40℃[J]. J Anim Sci, 1958, 17(2): 326–335. DOI: 10.2527/jas1958.172326x |
| [3] | SEJIAN V, SRIVASTAVA R S. Effects of melatonin on adrenal cortical functions of Indian goats under thermal stress[J]. Vet Med Int, 2010, 2010: 348919. |
| [4] | SALAMA A A K, CAJA G, HAMZAOUI S, et al. Different levels of response to heat stress in dairy goats[J]. Small Rumin Res, 2014, 121(1): 73–79. DOI: 10.1016/j.smallrumres.2013.11.021 |
| [5] |
张灿, 王之盛, 彭全辉, 等. 湿热应激对藏绵羊和山羊生长性能、抗氧化能力以及免疫功能的影响[J]. 动物营养学报, 2017, 29(6): 2179–2187.
ZHANG C, WANG Z S, PENG Q H, et al. Effects of moist-heat stress on growth performance, oxidation resistance and immunity of Tibetan sheep and goats[J]. Chinese Journal of Animal Nutrition, 2017, 29(6): 2179–2187. DOI: 10.3969/j.issn.1006-267x.2017.06.041 (in Chinese) |
| [6] | MAHJOUBI E, YAZDI M H, AGHAZIARATI N, et al. The effect of cyclical and severe heat stress on growth performance and metabolism in Afshari lambs[J]. J Anim Sci, 2015, 93(4): 1632–1640. DOI: 10.2527/jas.2014-8641 |
| [7] | HAMZAOUI S, SALAMA A A K, ALBANELL E, et al. Physiological responses and lactational performances of late-lactation dairy goats under heat stress conditions[J]. J Dairy Sci, 2013, 96(10): 6355–6365. DOI: 10.3168/jds.2013-6665 |
| [8] | POPOOLA M A, SAKA A A, OLANIYI T A, et al. Influence of temperature humidity index on skin temperature of West African dwarf goats raised in Nigeria[J]. Agric Adv, 2013, 2(12): 303–307. |
| [9] | DARCAN N, CEDDEN F, CANKAYA S, et al. Spraying effects on some physiological and behavioural traits of goats in a subtropical climate[J]. Italian J Anim Res, 2008, 7(1): 77–85. DOI: 10.4081/ijas.2008.77 |
| [10] | NAGAYACH R, GUPTA U D, PRAKASH A. Expression profiling of hsp70 gene during different seasons in goats (Capra hircus), under sub-tropical humid climatic conditions[J]. Small Rumin Res, 2017, 147: 41–47. DOI: 10.1016/j.smallrumres.2016.11.016 |
| [11] |
蔡丽媛.集约化羊舍的环境控制及热应激对山羊瘤胃发酵的影响[D].武汉: 华中农业大学, 2015.
CAI L Y.Environmental control of intensive goat buildings and the effects of heat stress on rumen fermentation of goats[D].Wuhan: Huazhong Agricultural University, 2015.(in Chinese) http://cdmd.cnki.com.cn/Article/CDMD-10504-1016156133.htm |
| [12] | BAKTHISARAN R, TANGIRALA R, RAO C M. Small heat shock proteins:role in cellular functions and pathology[J]. Biochim Biophys Acta, 2015, 1854(4): 291–319. DOI: 10.1016/j.bbapap.2014.12.019 |
| [13] | DEANE C A S, BROWN I R. Knockdown of heat shock proteins HSPA6(Hsp70B') and HSPA1A (Hsp70-1) sensitizes differentiated human neuronal cells to cellular stress[J]. Neurochem Res, 2018, 43(2): 340–350. DOI: 10.1007/s11064-017-2429-z |
| [14] | DANGI S S, DANGI S K, CHOUHAN V S, et al. Modulatory effect of betaine on expression dynamics of HSPs during heat stress acclimation ingoat (Capra hircus)[J]. Gene, 2016, 575(2): 543–550. DOI: 10.1016/j.gene.2015.09.031 |
| [15] |
唐雪峰, 李建柱, 赵存真, 等. 热休克蛋白70生物学功能及在家禽组织中的表达[J]. 畜牧与兽医, 2018, 50(4): 144–148.
TANG X F, LI J Z, ZHAO C Z, et al. Biological function and expression of heat shock protein 70 in poultry tissues[J]. Animal Husbandry & Veterinary Medicine, 2018, 50(4): 144–148. (in Chinese) |
| [16] | PARMAR M S, MADAN A K, HUOZHA R, et al. Heat shock protein70(HSP70) gene expression pattern in peripheral blood mononuclear cells (PBMCs) during different seasons in sahiwal cows (Bos Indicus)[J]. J Anim Res, 2015, 5(1): 109–113. DOI: 10.5958/2277-940X.2015.00018.2 |
| [17] | DANGI S S, GUPTA M, NAGAR V, et al. Impact of short-term heat stress on physiological responses and expression profile of HSPs in Barbari goats[J]. Int J Biometeorol, 2014, 58(10): 2085–2093. DOI: 10.1007/s00484-014-0809-5 |
| [18] | BANERJEE D, UPADHYAY R C, CHAUDHARY U B, et al. Seasonal variation in expression pattern of genes under HSP70:seasonal variation in expression pattern of genes under HSP70 family in heat- and cold-adapted goats (Capra hircus)[J]. Cell Stress Chaperones, 2014, 19(3): 401–408. DOI: 10.1007/s12192-013-0469-0 |
| [19] | GUPTA M, KUMAR S, DANGI S S, et al. Physiological, biochemical and molecular responses to thermal stress in goats[J]. Int J Livest Res, 2013, 3(2): 27–38. DOI: 10.5455/ijlr. |
| [20] | CONG X, ZHANG Q, LI H T, et al. Puerarin ameliorates heat stress-induced oxidative damage and apoptosis in bovine Sertoli cells by suppressing ROS production and upregulating Hsp72 expression[J]. Theriogenology, 2017, 88: 215–227. DOI: 10.1016/j.theriogenology.2016.09.033 |
| [21] |
许啸.奶山羊高温预警指标的筛选及有机铬调控作用研究[D].武汉: 华中农业大学, 2013.
XU X.Sifting for the high temperature early warning indice in dairy goats and the regulating effect of organicchromium[D].Wuhan: Huazhong Agricultural University, 2013.(in Chinese) http://cdmd.cnki.com.cn/Article/CDMD-10504-1013336440.htm |
| [22] |
王立志.热应激对奶牛、奶山羊体内内毒素含量的影响及缓解热应激的营养技术研究[D].雅安: 四川农业大学, 2010.
WANG L Z.Effects of heat stress on endotoxin content in dairy cows and dairy goats and nutritional techniques for alleviating heat stress[D].Ya'an: Sichuan Agricultural University, 2010.(in Chinese) http://cdmd.cnki.com.cn/Article/CDMD-10626-1011222527.htm |
| [23] |
张轶凤, 齐智利. 热应激条件下机体发生氧化应激的机制[J]. 动物营养学报, 2017, 29(9): 3051–3058.
ZHANG Y F, QI Z L. Mechanism of oxidative stress in body under heat stress[J]. Chinese Journal of Animal Nutrition, 2017, 29(9): 3051–3058. DOI: 10.3969/j.issn.1006-267x.2017.09.005 (in Chinese) |
| [24] | YANG L, TAN G Y, FU Y Q, et al. Effects of acute heat stress and subsequent stress removal on function of hepatic mitochondrial respiration, ROS production and lipid peroxidation in broiler chickens[J]. Comp Biochem Physiol C Toxicol Pharmacol, 2010, 151(2): 204–208. DOI: 10.1016/j.cbpc.2009.10.010 |
| [25] | PAMOK S, AENGWANICH W, KOMUTRIN T. Adaptation to oxidative stress and impact of chronic oxidative stress on immunity in heat-stressed broilers[J]. J Therm Biol, 2009, 34(7): 353–357. DOI: 10.1016/j.jtherbio.2009.06.003 |
| [26] |
魏凤仙, 胡骁飞, 张敏红, 等. 相对湿度和氨气应激对肉仔鸡血氨水平及细胞因子含量的影响[J]. 动物营养学报, 2013, 25(10): 2246–2253.
WEI F X, HU X F, ZHANG M H, et al. Effects of relative humidity and ammonia stress on plasma ammonia level and cytokine contents of broilers[J]. Chinese Journal of Animal Nutrition, 2013, 25(10): 2246–2253. DOI: 10.3969/j.issn.1006-267x.2013.10.008 (in Chinese) |
| [27] | YANG Z J, LIU C, ZHENG W J, et al. The functions of antioxidants and heat shock proteins are altered in the immune organs of selenium-deficient broiler chickens[J]. Biol Trace Elem Res, 2016, 169(2): 341–351. DOI: 10.1007/s12011-015-0407-3 |
| [28] |
于静, 薛佳, 胡艳欣, 等. 急性热应激对小鼠肺组织中热应激蛋白和细胞因子基因表达的影响[J]. 中国兽医杂志, 2015, 51(10): 20–23.
YU J, XUE J, HU Y X, et al. The effects of acute heat stress on gene expression of several HSPs and cytokines in lung tissue of mice[J]. Chinese Journal of Veterinary Medicine, 2015, 51(10): 20–23. DOI: 10.3969/j.issn.0529-6005.2015.10.006 (in Chinese) |
| [29] |
熊嫣.温热环境因子及氨气对肉鸡健康的影响机制研究[D].北京: 中国农业科学院, 2017.
XIONG Y.Effects of thermal environmental factors and ammonia on the broiler health[D].Beijing: Chinese Academy of Agricultural Sciences, 2017.(in Chinese) http://cdmd.cnki.com.cn/Article/CDMD-82101-1017262302.htm |
| [30] |
马燕芬, 陈琦, 杜瑞平, 等. 热应激对奶山羊瘤胃上皮细胞屏障通透性的影响[J]. 中国农业科学, 2013, 46(21): 4478–4485.
MA Y F, CHEN Q, DU R P, et al. Effect of heat stress on rumen epithelial cell barrier permeability in dairy goat[J]. Scientia Agricultura Sinica, 2013, 46(21): 4478–4485. DOI: 10.3864/j.issn.0578-1752.2013.21.010 (in Chinese) |
| [31] | CHEN J, ZHOU Y X, JIN X D, et al. Expression of interleukin-2 in Candidal balanoposthitis and its clinical significance[J]. Chin Med J, 2011, 124(17): 2776–2778. |
| [32] |
韩爱云, 左晓磊, 姚清国, 等. 急性高温对肉鸡生理状态及细胞免疫的影响[J]. 江苏农业科学, 2012, 40(4): 215–217.
HAN A Y, ZUO X L, YAO Q G, et al. Effects of acute high temperature on physiological status and cellular immunity in Broilers[J]. Jiangsu Agricultural Sciences, 2012, 40(4): 215–217. DOI: 10.3969/j.issn.1002-1302.2012.04.079 (in Chinese) |
| [33] |
胡艳欣, 佘锐萍, 张洪玉, 等. 热应激后猪血清中IL-2、IFN-γ及TNF-α水平的动态变化[J]. 畜牧兽医学报, 2006, 37(5): 496–499.
HU Y X, SHE R P, ZHANG H Y, et al. Studies on the dynamic changes of the level of IL-2, IFN-γ and TNF-α in porcine serum after heat stress[J]. Acta Veterinaria et Zootechnica Sinica, 2006, 37(5): 496–499. DOI: 10.3321/j.issn:0366-6964.2006.05.015 (in Chinese) |
| [34] |
周瑞进, 郭景茹, 宿甲子, 等. 急性冷暴露对仔猪PBMC中HSP70和血浆抗炎性细胞因子IL-4、IL-10的影响[J]. 中国兽医学报, 2010, 30(5): 677–680.
ZHOU R J, GUO H R, XU J Z, et al. Effect of acute cold exposure on the HSP70 expression of PBMC and the level of IL-4, IL-10 in plasma of piglets[J]. Chinese Journal of Veterinary Science, 2010, 30(5): 677–680. (in Chinese) |
| [35] | OKAMURA M, LILLEHOJ H S, RAYBOURNE R B, et al. Cell-mediated immune responses to a killed Salmonella enteritidis vaccine:lymphocyte proliferation, T-cell changes and interleukin-6(IL-6), IL-1, IL-2, and IFN-γ production[J]. Comp Immunol Microbiol Infect Dis, 2004, 27(4): 255–272. DOI: 10.1016/j.cimid.2003.12.001 |
| [36] |
刘晓曦, 刘凤华, 刘明江, 等.热应激大鼠肠道黏膜免疫及抗原递呈功能的变化[C]//中国畜牧兽医学会2013年学术年会论文集.北京: 中国畜牧兽医学会, 2013.
LIU X X, LIU F H, LIU M J, et al.Changes of heat stress on intestinal mucosal immunity and antigen presentation function in rats[C]//Proceedings of the 2013 Annual Academic Meeting of Chinese Association of Animal Science and Veterinary Medicine.Beijing: Chinese Association of Animal Science and Veterinary Medicine, CAAV, 2013.(in Chinese) |
| [37] | PELI A, SCAGLIARINI L, BERGAMINI P F, et al. Influence of heat stress on the immunity in growing beef cattle[J]. Large Anim Rev, 2013, 19(5): 215–218. |
| [38] |
胡煜, 蔡明成, 王玲, 等. 热应激状态下牛血清生化指标、miRNA表达变化及其相关性分析[J]. 畜牧兽医学报, 2016, 47(9): 1840–1847.
HU Y, CAI M C, WANG L, et al. The serum biochemical indexes and miRNA expression in cattle under heat stress and their correlation analysis[J]. Acta Veterinaria et Zootechnica Sinica, 2016, 47(9): 1840–1847. (in Chinese) |
| [39] | MORINOBU A, KUMAGAI S. Cytokine measurement at a single-cell level to analyze human Th1 and Th2 cells[J]. Rinsho Byori Jpn Clin Pathol, 1998, 46(9): 908–1014. |
| [40] |
刁华杰, 冯京海, 王雪洁, 等. 日循环高温对蛋鸡抗氧化能力及免疫机能的影响[J]. 畜牧兽医学报, 2017, 48(6): 1044–1053.
DIAO H J, FENG J H, WANG X J, et al. Effect of cyclic high temperature on antioxidant capacity and immune function of laying hens[J]. Acta Veterinaria et Zootechnica Sinica, 2017, 48(6): 1044–1053. (in Chinese) |
| [41] |
翟杰.热应激对雏鸡外周血液免疫细胞功能及相关因子的影响[D].哈尔滨: 东北农业大学, 2015.
ZHAI J.The effect of heat stress on the function of immunologic cell and cytocines in peripheral blood of chicks[D].Harbin: Northeast Agricultural University, 2015.(in Chinese) http://cdmd.cnki.com.cn/Article/CDMD-10224-1016017740.htm |
| [42] | WU Y N, YAN F F, HU J Y, et al. The effect of chronic ammonia exposure on acute-phase proteins, immunoglobulin, and cytokines in laying hens[J]. Poult Sci, 2017, 96(6): 1524–1530. DOI: 10.3382/ps/pew454 |
| [43] | ARCHANA P R, ALEENA J, PRAGNA P, et al. Role of heat shock proteins in livestock adaptation to heat stress[J]. J Dairy Vet Anim Res, 2017, 5(1): 00127. |
| [44] | LIEW P K, ZULKIFLI I, HAIR-BEJO M, et al. Effects of early age feed restriction and heat conditioning on heat shock protein 70 expression, resistance to infectious bursal disease, and growth in male broiler chickens subjected to heat stress[J]. Poult Sci, 2003, 82(12): 1879–1885. DOI: 10.1093/ps/82.12.1879 |
| [45] | COLLIER R J, COLLIER J L, RHOADS R P, et al. Invited review:genes involved in the bovine heat stress response[J]. J Dairy Sci, 2008, 91(2): 445–454. DOI: 10.3168/jds.2007-0540 |
| [46] | NOONAN E J, PLACE R F, GIARDINA C, et al. Hsp70B' regulation and function[J]. Cell Stress Chaperones, 2007, 12(3): 219–229. DOI: 10.1379/CSC-278.1 |
| [47] | LEUNG T K, RAJENDRAN M Y, MONFRIES C, et al. The human heat-shock protein family.Expression of a novel heat-inducible HSP70(HSP70B') and isolation of its cDNA and genomic DNA[J]. Biochem J, 1990, 267(1): 125–132. DOI: 10.1042/bj2670125 |
| [48] | ROHDE M, DAUGAARD M, JENSEN M H, et al. Members of the heat-shock protein 70 family promote cancer cell growth by distinct mechanisms[J]. Genes Dev, 2005, 19(5): 570–582. DOI: 10.1101/gad.305405 |
| [49] | SONNA L A, WENGER C B, FLINN S, et al. Exertional heat injury and gene expression changes:a DNA microarray analysis study[J]. J Appl Physiol, 2004, 96(5): 1943–1953. DOI: 10.1152/japplphysiol.00886.2003 |
| [50] |
白丹丹, 敖日格乐, 王纯洁, 等. 慢性冷热应激对三河牛血液生化指标及相关基因表达的影响[J]. 中国农业大学学报, 2017, 22(8): 50–56.
BAI D D, AORI GELE, WANG C J, et al. Influences of chronic cold and heat stress on blood biochemical parameters and related gene expression in Sanhe cattle[J]. Journal of China Agricultural University, 2017, 22(8): 50–56. (in Chinese) |
| [51] | HOOPER H B, DOS SANTOS SILVA P, DE OLIVEIRA S A, et al. Acute heat stress induces changes in physiological and cellular responses in Saanen goats[J]. Int J Biometeorol, 2018, 62(12): 2257–2265. DOI: 10.1007/s00484-018-1630-3 |
| [52] |
高雅君.炎症条件下草鱼HSP70的表达及HSF1对其调控的初步研究[D].成都: 电子科技大学, 2018.
GAO Y J.Preliminary study on the expression of HSP70 and its regulation by HSF1 under inflammatory conditions in grass carp[D].Chengdu: University of Electronic Science and Technology of China, 2018.(in Chinese) |
| [53] |
肖定福, 张彬, 胡雄贵. 热应激蛋白对畜禽抗应激机理的研究进展[J]. 中国畜牧兽医, 2009, 36(4): 58–62.
XIAO D F, ZHANG B, HU X G. Research progress on anti-stress mechanism of HSPs to livestock and poultry[J]. China Animal Husbandry & Veterinary Medicine, 2009, 36(4): 58–62. (in Chinese) |
| [54] | GAUBIN Y, VAISSADE F, CROUTE F, et al. Implication of free radicals and glutathione in the mechanism of cadmium-induced expression of stress proteins in the A549 human lung cell-line[J]. Biochim Biophys Acta, 2000, 1495(1): 4–13. DOI: 10.1016/S0167-4889(99)00149-4 |
| [55] | SHIMIZU M, TAMAMORI-ADACHI M, ARAI H, et al. Lipopolysaccharide pretreatment attenuates myocardial infarct size:a possible mechanism involving heat shock protein 70-inhibitory κBα complex and attenuation of nuclear factor κB[J]. J Thorac Cardiovasc Surg, 2002, 124(5): 933–941. DOI: 10.1067/mtc.2002.122305 |
| [56] | ASEA A, KRAEFT S K, KURT-JONES E A, et al. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine[J]. Nat Med, 2000, 6(4): 435–442. DOI: 10.1038/74697 |
| [57] | MILLAR D G, GARZA K M, ODERMATT B, et al. Hsp70 promotes antigen-presenting cell function and converts T-cell tolerance to autoimmunity in vivo[J]. Nat Med, 2003, 9(12): 1469–1476. DOI: 10.1038/nm962 |
| [58] | ROUT P K, KAUSHIK R, RAMACHANDRAN N. Differential expression pattern of heat shock protein 70 gene in tissues and heat stress phenotypes in goats during peak heat stress period[J]. Cell Stress Chaperones, 2016, 21(4): 645–651. DOI: 10.1007/s12192-016-0689-1 |



