2. 中国农业大学动物医学院, 北京 100193
2. Laboratory of Anatomy of Domestic Animals, College of Animal Medicine, China Agricultural University, Beijing 100193, China
褪黑激素(melatonin,MT)由美国皮肤学家Lerner于1958年从肉牛松果体中首次分离出,属于吲哚类激素。MT主要是由松果体合成,少量由视网膜、眼眶腔的副泪腺、唾液腺、肠的嗜铬细胞及红细胞生成[1]。光信号通过MT转化为动物体内的化学信号,从而调控动物的多种生理过程[2]。MT首先通过结合其G蛋白偶联膜受体MT1(Mel1a)、MT2(Mel1b)[3-4]或MT3醌还原酶结合[5-7]实现其功能。
MT受体广泛分布于身体各处,其中包括十分重要的免疫系统[8-9]、内分泌系统[10]、生殖系统[11-13]和心血管系统等,参与调节皮肤色素沉着、毛发生长、癌发生和老化等多个生理过程。MT1在睾丸、卵巢、皮肤、肾、肾上腺皮质、乳房、视网膜、胰腺、脾[14-18]以及脑部的下丘脑、小脑、海马等组织器官中均有表达[16]。MT2被认为是MT1的突变体,与MT1具有60%的同源性[19]。MT2主要在免疫系统、脑部、视网膜、垂体、血管、睾丸、肾、乳房、皮肤等组织器官中表达[17, 20-22]。
处于不同年龄段、不同季节以及不同组织器官,MT受体的表达存在差异,这与MT调节机体多种生理功能的特点相吻合。大鼠心、肝、脾、肾MT1和MT2的表达随着年龄的增长而逐渐下降,而胸腺的MT1和MT2则随年龄增长显著增加[23],Guo等[24]也发现脾中MT受体的表达存在时间差异性和区域特异性。MT1的表达量及与MT的亲和力在繁殖季节和非繁殖季节存在显著的差异。绵羊在繁殖季节,其垂体的MT1表达量升高,且与MT的亲和力升高[25],而Masana和Dubocovich[26]在大鼠的研究试验中观察到了相同的现象。因此推测褪黑激素能调节机体多个器官的功能,并能随着时间的改变产生不同的影响,可能是通过其受体时空表达的差异实现的。但是受体在母兔性腺轴中的表达变化尚是空白。
本研究拟通过给予母兔不同光照时间,研究不同光周期下褪黑激素受体在性腺轴中的分布和表达模式,进一步探究褪黑激素在光周期调控母兔发情中的作用机制。
1 材料与方法 1.1 实验动物实验动物的选取、分组、饲养方式和免疫方法等均采用张玉仙等[27]处理方法。
1.2 光源与光照制度本研究采用的光源、光照制度、饲养方法、光源强度、光源设备规格和型号等均与张玉仙等[27]相关内容一致。
1.3 qPCR法检测下丘脑、垂体、卵巢组织中褪黑激素受体亚型mRNA表达于转换光照后第7天,将兔处死后取头部,打开颅腔取下丘脑、垂体,打开腹腔取一侧卵巢,将这些组织放入锡箔纸袋,液氮内保存,放于-80 ℃冰箱中备用。应用TRIzol法提取母兔下丘脑、垂体、卵巢组织中的mRNA,超微量紫外可见分光光度计(NanophotometerTM Peal,Implen,德国)测mRNA浓度,并将mRNA浓度调整至500~1 200 pg·μL-1。反转录为cDNA,应用荧光定量PCR仪(LightCycler® 480,Roche Applied Science,德国)进行GAPDH,GnRH SYBR实时荧光定量(退火温度为60 ℃,循环数40)。上述所需要的引物均由上海英骏生物技术有限公司合成。PCR的引物序列见表 1。
|
|
表 1 PCR的引物序列 Table 1 Sequences of primers used for RT-PCR |
耳缘静脉注射10 mL空气处死兔,取头部,打开颅腔迅速取下丘脑、垂体;打开腹腔取卵巢,称重,取60 mg置于研钵中在液氮中研磨成粉末,置于一支洁净EP管中,并按质量:体积=1:10的比例加入RIPA裂解液和蛋白酶抑制剂(100:1),混匀,冰上静置20 min;14 000 r·min-1 4 ℃离心,取上清液。依照BCA蛋白定量试剂盒说明书操作,测定总蛋白浓度,将所有蛋白样品的浓度统一调整至1.5 mg·mL-1。混合等体积2×SDS样品缓冲液,加热变性后进行SDS-PAGE凝胶电泳,在4 ℃条件下220 mA恒流湿转70 min;用0.01 mol·L-1TBST配制5%脱脂奶,PVDF膜经TBST漂洗后转入脱脂奶,水平摇床上室温封闭2 h,PVDF膜经TBST漂洗;移入1:4 000倍稀释抗β-actin鼠单克隆抗体(CW0096,康为世纪,中国)或1:100倍稀释多克隆山羊抗MT1(sc-13186,Santa Cru,美国)、1:100倍稀释MT2(sc-13177,Santa Cru,美国)抗体中,水平摇床上孵育1 h,4 ℃过夜,经TBST洗涤3次,每次10 min;浸入1:5 000倍稀释HRP标记驴抗鼠IgG(CW0102,康为世纪,中国)或1:5 000倍稀释驴抗山羊IgG(CW0211,康为世纪,中国),水平摇床上室温孵育2 h,经TBST洗涤3次,每次10 min;Easy See Western blot Kit试剂盒暗室内化学发光;UVP凝胶成像系统拍照检测。
1.5 免疫组织化法检测下丘脑室周核和室旁核MT1阳性细胞兔脑在4%多聚甲醛固定液中固定48 h后取出,用蒸馏水冲洗表面的固定液,在4 ℃中经10%、20%、30%三级的蔗糖磷酸缓冲液浸泡脱水,用OTC包埋剂包埋后置于-20 ℃中冷冻。2 h后采用冰冻切片机(CM1950型,Leica,德国)切取40 nm厚的切片,小心置于0.01 mol·L-1PBS缓冲液中,4 ℃保存备用。
冰冻切片采用SABC法进行免疫组化检测,其操作步骤按SABC试剂盒的说明书进行。阴性对照组用PBS代替首抗。染色后的切片在光学显微镜(Olympus光学显微镜,D72,日本)下观察,每个核团取3张切片,统计40倍镜视野中单位面积阳性细胞数,MT1阳性细胞均为黄色细胞质着色。试剂盒由中国康为世纪提供。
1.6 数据分析试验结果表示为“x±sx”。试验数据采用SPSS 22(SPSS Inc, Chicago, IL, USA)单因素方差分析(ANOVA)进行比较,P < 0.05表示不同周期差异显著。
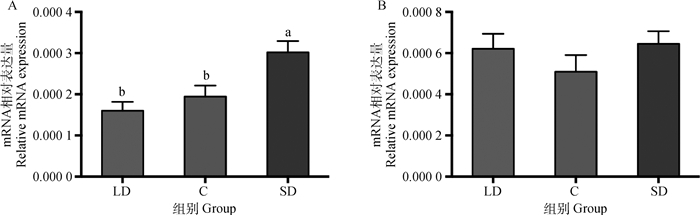
2 结果 2.1 光照周期对母兔下丘脑褪黑激素受体亚型表达的影响 2.1.1 不同光照周期褪黑激素受体亚型mRNA的表达如图 1所示,饲养在不同光照周期下的母兔褪黑激素受体亚型的mRNA相对表达量在下丘脑中有明显的差异。短光照组的MT1 mRNA相对表达量最高,其次是对照组,长光照组最低。短光照组的MT1 mRNA相对表达量比长光照组显著高88.8%(P < 0.05),比对照组显著高54.9%(P < 0.05),长光照组与对照组差异不显著(P>0.05),见图 1A。而MT2 mRNA相对表达量在不同光照周期下表达量差异不显著(P>0.05),短光照组最高,其次是长光照组,对照组的相对表达量最低,分别比长光照组低17.9%、比短光照组低20.9%(图 1B)。
|
LD.长光照组;C.对照组;SD.短光照组。不同字母表示不同光照周期间差异显著(P<0.05),下同 LD. Long photoperiod group; C. Control; SD. Short photoperiod group. Different letters mean significant differences among the groups (P < 0.05), the same as below 图 1 不同光照周期下母兔下丘脑褪黑激素受体MT1(A)和MT2(B)的mRNA表达(n=16) Fig. 1 Effects of different photoperiod on the MT1 (A) and MT2 (B) mRNA level of hypothalamus (n=16) |
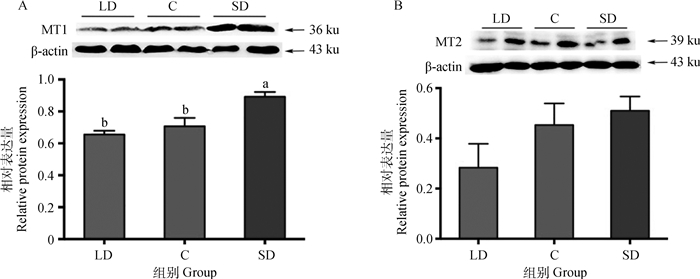
如图 2所示,饲养在不同光照周期下的母兔,其褪黑激素受体亚型在下丘脑中的蛋白表达有明显的差异,短光照组MT1蛋白相对表达量最高,其次为对照组,长光照组MT1蛋白相对表达量最低。短光照组的MT1蛋白相对表达量比长光照组显著高36.2%(P < 0.05),比对照组显著高26.2%(P < 0.05),长光照组与对照组差异不显著(P>0.05), 见图 2A;短光照组MT2蛋白相对表达量最高,对照组其次,长光照组最低,分别比短光照组低44.5%(P>0.05)、比对照组低37.5%(P>0.05),但三个组之间的蛋白相对表达量均差异不显著(P>0.05),见图 2B。
|
图 2 不同光照周期下母兔下丘脑褪黑激素受体MT1(A)和MT2(B)蛋白的表达(n=16) Fig. 2 Effects of different photoperiod on the MT1(A) and MT2(B) proteins level of hypothalamus (n=16) |
如图 3所示,饲养在不同光照周期下的母兔垂体中褪黑激素受体亚型的mRNA相对表达量有明显的差异,短光照组最高,其次是对照组,长光照组最低。短光照组的MT1 mRNA相对表达量最高,比长光照组显著高164%(P < 0.05),比对照组显著高49.5%(P < 0.05),同时长光照组比对照组显著低43.5%(P < 0.05),见图 3A。MT2 mRNA相对表达量在不同光照周期下表达量没有显著差异(P>0.05),长光照组的相对表达量最低,比短光照组低63.2%,比对照组低40.1%(图 3B)。

|
图 3 不同光照周期下母兔垂体中褪黑激素受体MT1(A)和MT2(B)的mRNA表达(n=16) Fig. 3 Effects of different photoperiod on the MT1 (A) and MT2 (B) mRNA level of the pituitary(n=16) |
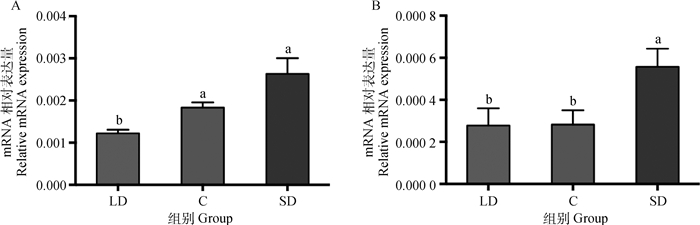
不同光照周期下卵巢褪黑激素受体亚型的mRNA表达如图 4所示,MT1、MT2的mRNA相对表达量均是在短光照组最高,其次是对照组,长光照组最低。MT1的mRNA相对表达量在长光照、对照和短光照组三组中的相对表达量分别为(122.0±8.8)×10-5、(183.0±12.5)×10-5、(263.0±37.3)×10-5,长光照组比对照组显著低33.3%(P < 0.05)、比短光照组显著低53.6%(P < 0.05),短光照组与对照组差异不显著(P>0.05),见图 4A。MT2的mRNA相对表达量在长光照、对照和短光照三组中的相对表达量分别为(27.8±8.2)×10-5、(28.2±6.8)×10-5、(55.7±8.6)×10-5,短光照组比对照组显著高90.0%(P < 0.05),比长光照组显著高100%(P < 0.05),长光照组与对照组差异不显著(P>0.05),见图 4B。
|
图 4 不同光照周期下母兔卵巢中褪黑激素受体MT1(A)和MT2(B)的mRNA表达(n=16) Fig. 4 Effects of different photoperiod on the MT1 (A) and MT2 (B) mRNA level of the ovary (n=16) |
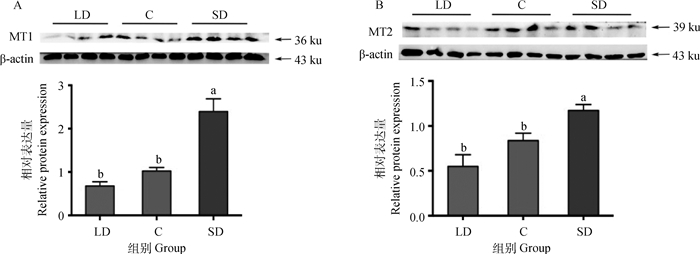
不同光照周期下卵巢褪黑激素受体亚型的蛋白表达如图 5所示,MT1、MT2的蛋白相对表达量均是在短光照组最高,其次是对照组,长光照组最低。MT1的蛋白相对表达量在长光照、对照和短光照组三组中的相对表达量分别为0.679±0.096、1.020±0.081、2.390±0.292,短光照组比对照组显著高134%(P < 0.05)、比长光照组显著高252%(P < 0.05),长光照组与对照组差异不显著(P>0.05),见图 5A。MT2的蛋白相对表达量在长光照、对照和短光照组三组中的相对表达量分别为0.549±0.131、0.837±0.083、1.170±0.066,长光照组比对照组降低34.4%(P>0.05)、比短光照组显著低53.1%(P < 0.05),对照组比短光照组显著低28.5%(P < 0.05),见图 5B。
|
图 5 不同光照周期下母兔卵巢中褪黑激素受体MT1(A)和MT2(B)的蛋白表达(n=16) Fig. 5 Effects of different photoperiod on the MT1(A) and MT2(B) proteins level of the ovary(n=16) |
免疫组织化学定位发现,MT1阳性细胞主要位于下丘脑室周核和室旁核。呈棕黄色,形态不一。
2.4.1 不同光照周期下母兔下丘脑室周核MT1的表达图 6显示了分布在母兔下丘脑第三脑室周围的室周核中MT阳性细胞。室周核的MT1阳性细胞形态呈现出多种特点,其中包括多边形、水滴形(图 6D)、椭圆形(图 6E)的阳性神经元及少量梭形(图 6F)的阳性神经元。

|
A、D为长光照组,B、E为对照组,C、F为短光照组。箭头所指为MT阳性细胞。A、B、C比例尺=50 μm,D、E、F比例尺=25 μm A and D from the long light group, B and E from the control group, C and F from the short light group. Arrows indicate MT positive cells. A, B, C scale bars=50 μm, D, E, F scale bars=25 μm 图 6 不同光照周期下雌性新西兰白兔下丘脑室周核MT1免疫组织化学结果 Fig. 6 MT1 positive cells of the rabbit hypothalamus PerVN at various photoperiod |
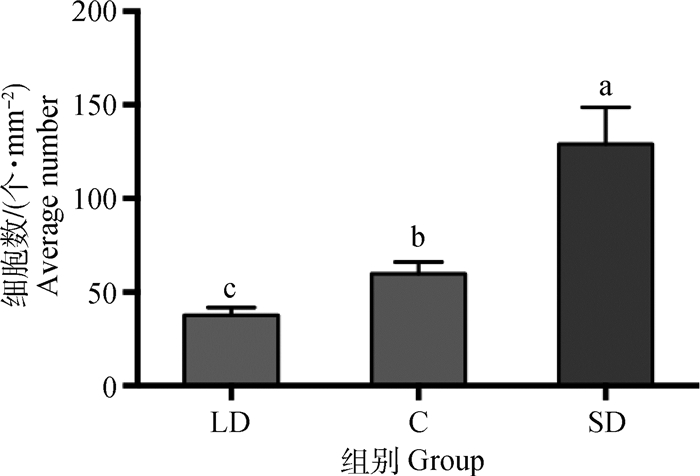
图 7显示不同光照周期下饲养的新西兰母兔下丘脑室周核的MT1阳性细胞数有显著的差异,长光照组的MT1阳性细胞数比短光照组显著少69.4%(P < 0.05),比对照组显著少37.3%(P < 0.05)。短光照组比对照组显著多104.8%(P < 0.05)。
|
图 7 40倍镜视野中不同光照周期下母兔下丘脑室周核单位面积的MT1阳性神经元数(n=16) Fig. 7 Average number of MT1 positive cells under the 40× field of microscope in PerVN(n=16) |
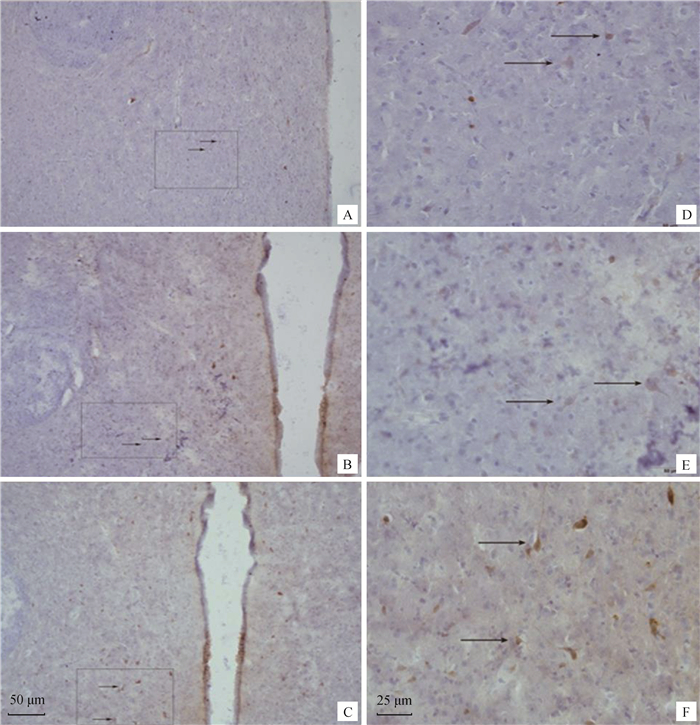
图 8显示了分布在母兔下丘脑室旁核中的MT1阳性细胞。室旁核MT1阳性细胞的形态多呈现水滴形、梭形和椭圆形,同时存在少部分多边形的阳性神经元。
|
A、D为长光照组,B、E为对照组,C、F为短光照组。箭头所指为MT阳性细胞。A、B、C比例尺=50 μm,D、E、F比例尺=25 μm A and D from the long light group, B and E from the control group, C and F from the short light group. Arrows indicate MT positive cells. A, B, C scale bars=50 μm, D, E, F scale bars=25 μm 图 8 不同光照周期下雌性新西兰白兔下丘脑室旁核MT1免疫组织化学结果 Fig. 8 MT1 positive cells of the rabbit hypothalamus PVN at various photoperiod |
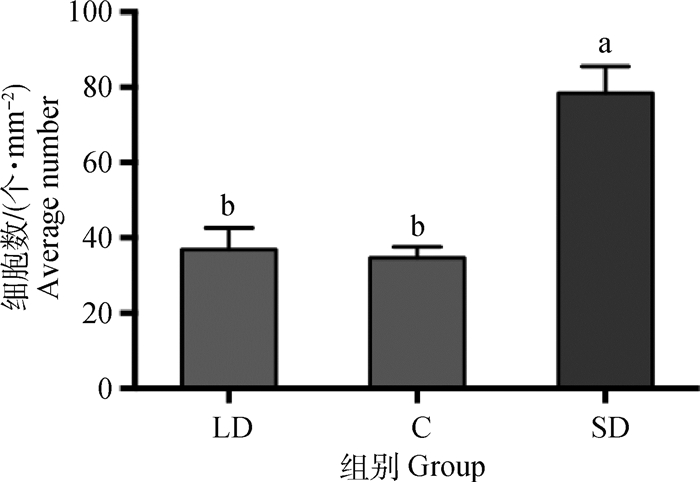
图 9显示短光照组的新西兰母兔下丘脑室旁核的MT1阳性细胞数显著多于另外两组(P < 0.05),而长光照组与对照组差异不显著(P>0.05),平均数也基本一致。长光照组的MT1阳性细胞数比短光照组显著少52.9%(P < 0.05),对照组比短光照组显著少55.8%(P < 0.05)。
|
图 9 40倍镜视野中不同光照周期下母兔下丘脑室旁核单位面积的MT1阳性神经元数(n=16) Fig. 9 Average number of MT1 positive cells under the 40× field of microscope in PVN(n=16) |
在生产中,常采用光照刺激哺乳期母兔同期发情,但是其作用机制不清楚。褪黑激素对牛、鼠、禽类的生殖系统具有抑制作用,但是对于羊具有促进作用。对于兔的研究也逐渐增多,长光照褪黑激素浓度较低,短光照褪黑激素浓度升高[28-30]。褪黑激素受体激动剂能降低眼压[31]。本研究表明,人工光照对母兔下丘脑褪黑激素受体MT1的mRNA及蛋白表达的影响基本一致,短光照组的相对表达量显著高于其他两组,但长光照组与对照组的相对表达量没有显著差异。与MT1不同,人工光照对下丘脑的MT2 mRNA及蛋白表达几乎没有显著的影响,可能褪黑激素主要通过MT1型受体调控雌兔下丘脑的功能,这与王忻[32]的试验结果相似,蒙古羊MT1在下丘脑中的表达同样受到光照的调节,与对照组相比差异显著。另有报道在具有明显季节性繁殖特质的哺乳动物下丘脑中未能检测到MT2受体,这可能是由于不同物种存在差异[33];本研究试验结果发现膜受体MT2并未随光照周期的改变而发生变化,提示MT2表达不受光照时间长短的影响。多个研究结果也表明,MT1在调节繁殖中起着核心作用,Howard和Lutterschmidt[34]发现MT1参与了雄性树蛙的季节性求偶行为的调控,而绵羊的性腺轴仅发现MT1型受体[30]。因此本文试验主要研究褪黑激素受体MT1在下丘脑中的表达及分布,而且其表达部位主要存在于室旁核和室周核,短光照周期显著提高了下丘脑室周核和室旁核中MT1阳性神经元数量。
垂体中褪黑激素受体的表达情况与下丘脑的情况相似,MT1的表达显著受到光照周期的影响,但MT2的表达在不同光照周期下没有显著差异。光周期能够显著影响大鼠垂体MT1基因的表达,但对蒙古羊的MT1基因表达没有显著影响研究[18],这表明光照周期对不同哺乳动物垂体的褪黑激素受体表达不同,对于不同的物种存在不同的调控机制。有研究认为成年动物的垂体仅在结节部大量表达MT1,但是垂体促性腺内激素细胞存在于垂体前叶[35]。Lincoln等[36]发现,只有垂体结节部的褪黑激素能够影响由光周期诱导的FSH和LH的分泌,因而褪黑激素是如何通过垂体作用于性腺轴的仍然有待进一步的研究。
处于不同年龄段、不同季节以及不同组织器官,褪黑激素受体的表达存在差异,这与褪黑激素调节机体多种生理功能的特点吻合。MT1的表达量及与褪黑激素的亲和力在繁殖季节和非繁殖季节存在显著的差异。绵羊在繁殖季节,其垂体的MT1表达量升高,且与褪黑激素的亲和力变高[25],而Masana和Dubocovich[26]在大鼠的研究试验中观察到了相同的现象。褪黑激素能调节机体多个器官的功能,并能随着时间的改变产生不同的影响,这可能是通过其受体时空表达的差异实现的。
卵巢中褪黑激素受体的表达情况与下丘脑、垂体的结果存在差异,MT1、MT2的基因及蛋白的表达均显著受到光照周期的调控,短光照组的MT1、MT2的基因及蛋白质的表达均显著高于长光照组。我们发现,在母兔的性腺轴中也存在上述现象。已有研究表明褪黑激素能直接作用于卵巢。例如El-Raey等[37]发现MT1基因在牛的COCs中有表达,而MT2仅在卵母细胞上有表达。浓度为10和50 ng·mL-1的褪黑激素能明显促进体外培养的牛卵母细胞核的成熟、卵丘细胞的增殖,并且能够明显改变线粒体的分布状况,但对卵母细胞线粒体的活性和COCs分泌性激素没有影响。而Kang等[38]的分析显示MT1基因在猪的卵丘细胞和颗粒层细胞有表达,但在卵母细胞上不表达,并且发现外源褪黑激素能促进IVM卵母细胞核和细胞质的成熟。这表明褪黑激素可能直接作用于卵巢,褪黑激素受体在不同动物卵巢中的分布不同,显示褪黑激素可能对不同动物卵巢的调控存在不同的调控机制。
因此,不同光照周期、不同组织器官的不同褪黑激素受体亚型的表达存在差异。光照周期能够显著影响下丘脑、垂体MT1型受体及卵巢MT1型受体、MT2型受体的表达,但对下丘脑、垂体MT2型受体没有显著影响。
4 结论短光照周期显著提高了下丘脑、垂体和卵巢中褪黑激素受体亚型MT1表达,而不是MT2受体亚型。光照周期调控母兔发情的作用机制可能是褪黑激素主要通过MT1受体途径实现的。
| [1] | SHIU S Y W, LI L, XU J N, et al. Melatonin-induced inhibition of proliferation and G1/S cell cycle transition delay of human choriocarcinoma JAr cells: possible involvement of MT2(MEL1B) receptor[J]. J Pineal Res, 1999, 27(3): 183–192. DOI: 10.1111/j.1600-079X.1999.tb00614.x |
| [2] | GOLDMAN B D. Mammalian photoperiodic system: formal properties and neuroendocrine mechanisms of photoperiodic time measurement[J]. J Biol Rhythms, 2001, 16(4): 283–301. DOI: 10.1177/074873001129001980 |
| [3] | DUBOCOVICH M L, RIVERA-BERMUDEZ M A, GERDIN M J, et al. Molecular pharmacology, regulation and function of mammalian melatonin receptors[J]. Front Biosci, 2003, 8: d1093–d1108. DOI: 10.2741/1089 |
| [4] | WITT-ENDERBY P A, BENNETT J, JARZYNKA M J, et al. Melatonin receptors and their regulation: biochemical and structural mechanisms[J]. Life Sci, 2003, 72(20): 2183–2198. DOI: 10.1016/S0024-3205(03)00098-5 |
| [5] | NOSJEAN O, FERRO M, COGÉ F, et al. Identification of the melatonin-binding site MT3 as the quinone reductase 2[J]. J Biol Chem, 2000, 275(40): 31311–31317. DOI: 10.1074/jbc.M005141200 |
| [6] | VINCENT L, COHEN W, DELAGRANGE P, et al. Molecular and cellular pharmacological properties of 5-methoxycarbonylamino-N-acetyltryptamine (MCA-NAT): a nonspecific MT3 ligand[J]. J Pineal Res, 2010, 48(3): 222–229. DOI: 10.1111/j.1600-079X.2010.00746.x |
| [7] | MARKOWSKA M, MROZKOWIAK A, SKWARLO-SONTA K. Influence of melatonin on chicken lymphocytes in vitro:involvement of membrane receptors[J]. Neuro Endocrinol Lett, 2002, 23(Suppl 1): 67–72. |
| [8] |
周瑞祥, 魏建恩, 刘卉, 等. 松果体及其褪黑素影响胸腺T细胞发育周期[J]. 解剖学报, 2005, 36(1): 70–75.
ZHOU R X, WEI J E, LIU H, et al. Pineal gland and melatonin influence the cell cycle of thymocyte[J]. Acta Anatomica Sinica, 2005, 36(1): 70–75. DOI: 10.3321/j.issn:0529-1356.2005.01.016 (in Chinese) |
| [9] | CHEN F J, REHEMAN A, CAO J, et al. Effect of melatonin on monochromatic light-induced T-lymphocyte proliferation in the thymus of chickens[J]. J Photochem Photobiol B:Biol, 2016, 161: 9–16. DOI: 10.1016/j.jphotobiol.2016.05.001 |
| [10] |
裴小英, 郭定宗, 郭锐. 松果体和褪黑素的生物学作用研究进展[J]. 中国畜牧兽医, 2007, 34(7): 60–63.
PEI X Y, GUO D Z, GUO R. Advances in the biological effects of pineal gland and melatonin[J]. China Animal Husbandry & Veterinary Medicine, 2007, 34(7): 60–63. DOI: 10.3969/j.issn.1671-7236.2007.07.016 (in Chinese) |
| [11] |
王淑娟, 刘文举, 王立克, 等. 褪黑激素对雌性动物生殖系统调节作用的研究进展[J]. 江苏农业科学, 2016, 44(6): 15–20.
WANG S J, LIU W J, WANG L K, et al. Research progress on the regulation of melatonin on female reproductive system[J]. Jiangsu Agricultural Sciences, 2016, 44(6): 15–20. (in Chinese) |
| [12] | BALÍK A, KRETSCHMANNOVÁ K, MAZNA P, et al. Melatonin action in neonatal gonadotrophs[J]. Physiol Res, 2004, 53(S1): S153–S166. |
| [13] | TAMURA H, NAKAMURA Y, TERRON M P, et al. Melatonin and pregnancy in the human[J]. Reprod Toxicol, 2008, 25(3): 291–303. DOI: 10.1016/j.reprotox.2008.03.005 |
| [14] | DUBOCOVICH M L, MARKOWSKA M. Functional MT1 and MT2 melatonin receptors in mammals[J]. Endocrine, 2005, 27(2): 101–110. DOI: 10.1385/ENDO:27:2:101 |
| [15] | FISCHER T W, SLOMINSKI A, ZMIJEWSKI M A, et al. Melatonin as a major skin protectant:from free radical scavenging to DNA damage repair[J]. Exp Dermatol, 2008, 17(9): 713–730. DOI: 10.1111/j.1600-0625.2008.00767.x |
| [16] | PANDI-PERUMAL S R, TRAKHT I, SRINIVASAN V, et al. Physiological effects of melatonin:role of melatonin receptors and signal transduction pathways[J]. Prog Neurobiol, 2008, 85(3): 335–353. DOI: 10.1016/j.pneurobio.2008.04.001 |
| [17] | SLOMINSKI A, FISCHER T W, ZMIJEWSKI M A, et al. On the role of melatonin in skin physiology and pathology[J]. Endocrine, 2005, 27(2): 137–147. DOI: 10.1385/ENDO:27:2:137 |
| [18] | SLOMINSKI A, TOBIN D J, ZMIJEWSKI M A, et al. Melatonin in the skin: synthesis, metabolism and functions[J]. Trends Endocrinol Metab, 2008, 19(1): 17–24. DOI: 10.1016/j.tem.2007.10.007 |
| [19] | REPPERT S M, GODSON C, MAHLE C D, et al. Molecular characterization of a second melatonin receptor expressed in human retina and brain: the Mel1b melatonin receptor[J]. Proc Natl Acad Sci U S A, 1995, 92(19): 8734–8738. DOI: 10.1073/pnas.92.19.8734 |
| [20] | REPPERT S M, WEAVER D R, EBISAWA T, et al. Cloning of a melatonin-related receptor from human pituitary[J]. FEBS Lett, 1996, 386(2-3): 219–224. DOI: 10.1016/0014-5793(96)00437-1 |
| [21] | ROCA A L, GODSON C, WEAVER D R, et al. Structure, characterization, and expression of the gene encoding the mouse Mel1a melatonin receptor[J]. Endocrinology, 1996, 137(8): 3469–3477. DOI: 10.1210/endo.137.8.8754776 |
| [22] | DUBOCOVICH M L, HUDSON R L, SUMAYA I C, et al. Effect of MT1 melatonin receptor deletion on melatonin-mediated phase shift of circadian rhythms in the C57BL/6 mouse[J]. J Pineal Res, 2005, 39(2): 113–120. DOI: 10.1111/j.1600-079X.2005.00230.x |
| [23] | SÁNCHEZ-HIDALGO M, GUERRERO MONTÁV-EZ J M, CARRASCOSA-SALMORAL M D P, et al. Decreased MT1 and MT2 melatonin receptor expression in extrapineal tissues of the rat during physiological aging[J]. J Pineal Res, 2009, 46(1): 29–35. DOI: 10.1111/j.1600-079X.2008.00604.x |
| [24] | GUO Q Y, DONG Y L, CAO J, et al. Developmental changes of melatonin receptor expression in the spleen of the chicken, Gallus domesticus[J]. Acta Histochem, 2015, 117(6): 559–565. DOI: 10.1016/j.acthis.2015.05.002 |
| [25] | MONTGOMERY G W, MARTIN G B, PELLETIER J. Changes in pulsatile LH secretion after ovariectomy in Ile-de-France ewes in two seasons[J]. J Reprod Fertil, 1985, 73(1): 173–183. DOI: 10.1530/jrf.0.0730173 |
| [26] | MASANA M I, DUBOCOVICH M L. Melatonin receptor signaling:finding the path through the dark[J]. Sci STKE, 2001, 2001(107): pe39. |
| [27] |
张玉仙, 王文利, 原展航, 等. 不同光照周期对雌兔卵泡发育和下丘脑-垂体-卵巢轴的影响[J]. 畜牧兽医学报, 2019, 50(8): 1694–1701.
ZHANG Y X, WANG W L, YUAN Z H, et al. The effect of different photoperiods on follicular development and hypothalamus-pituitary-ovary axis in female rabbits[J]. Acta Veterinaria et Zootechnica Sinica, 2019, 50(8): 1694–1701. (in Chinese) |
| [28] | AGUILAR-ROBLERO R, GONZÁLEZ-MARISCAL G. Behavioral, neuroendocrine and physiological indicators of the circadian biology of male and female rabbits[J]. Eur J Neurosci, 2018. DOI: 10.1111/ejn.14265 |
| [29] | BASURTO E, HOFFMAN K, LEMUS A C, et al. Electrolytic lesions to the anterior hypothalamus-preoptic area disrupt maternal nest-building in intact and ovariectomized, steroid-treated rabbits[J]. Horm Behav, 2018, 102: 48–54. DOI: 10.1016/j.yhbeh.2018.04.012 |
| [30] | KENNEDY G A, HUDSON R. Phase response curve to 1 h light pulses for the European rabbit (Oryctolagus cuniculus)[J]. Chronobiol Int, 2016, 33(8): 1120–1128. DOI: 10.1080/07420528.2016.1191506 |
| [31] | SPADONI G, BEDINI A, FURIASSI L, et al. Identification of bivalent ligands with melatonin receptor agonist and fatty acid amide hydrolase (FAAH) inhibitory activity that exhibit ocular hypotensive effect in the rabbit[J]. J Med Chem, 2018, 16(17): 7902–7916. |
| [32] |
王忻.非繁殖季节光照控制对蒙古羊发情排卵和褪黑激素受体表达的影响[D].兰州: 甘肃农业大学, 2009.
WANG Q. Effects of light control on oestrus and ovulation and MTNR1a expression of Mongolia ewe in non-breeding season[D]. Lanzhou: Gansu Agricultural University, 2009. (in Chinese) http://cdmd.cnki.com.cn/Article/CDMD-10733-2009253198.htm |
| [33] | REPPERT S M, WEAVER D R, EBISAWA T. Cloning and characterization of a mammalian melatonin receptor that mediates reproductive and circadian responses[J]. Neuron, 1994, 13(5): 1177–1185. DOI: 10.1016/0896-6273(94)90055-8 |
| [34] | HOWARD C M, LUTTERSCHMIDT D I. The effects of melatonin on brain arginine vasotocin:relationship with sex and seasonal differences in melatonin receptor type 1 in green treefrogs (Hyla cinerea)[J]. J Neuroendocrinol, 2015, 27(8): 670–679. DOI: 10.1111/jne.12292 |
| [35] | GAUER F, MASSON-PÉVET M, PÉVET P. Melatonin receptor density is regulated in rat pars tuberalis and suprachiasmatic nuclei by melatonin itself[J]. Brain Res, 1993, 602(1): 153–156. DOI: 10.1016/0006-8993(93)90256-M |
| [36] | LINCOLN G A, ALMEIDA O F X, KLANDORF H, et al. Hourly fluctuations in the blood levels of melatonin, prolactin, luteinizing hormone, follicle-stimulating hormone, testosterone, tri-iodothyronine, thyroxine and cortisol in rams under artificial photoperiods, and the effects of cranial sympathectomy[J]. J Endocrinol, 1982, 92(2): 237–250. DOI: 10.1677/joe.0.0920237 |
| [37] | EL-RAEY M, GESHI M, SOMFAI T, et al. Evidence of melatonin synthesis in the cumulus oocyte complexes and its role in enhancing oocyte maturation in vitro in cattle[J]. Mol Reprod Dev, 2011, 78(4): 250–262. DOI: 10.1002/mrd.21295 |
| [38] | KANG J T, KOO O J, KWON D K, et al. Effects of melatonin on in vitro maturation of porcine oocyte and expression of melatonin receptor RNA in cumulus and granulosa cells[J]. J Pineal Res, 2009, 46(1): 22–28. DOI: 10.1111/j.1600-079X.2008.00602.x |



