2. 青藏高原动物遗传资源保护与利用国家教育部重点实验室, 成都 610041;
3. 西南民族大学动物科学国家民委重点实验室, 成都 610041
2. Key Laboratory of Qinghai-Tibetan Plateau Animal Genetic Resource Reservation and Utilization of Ministry of Education, Chengdu 610041, China;
3. Key Laboratory of Animal Science, State Ethnic Affairs Commission, Southwest Minzu University, Chengdu 610041, China
牦牛(Bos grunniens)是分布在青藏高原及其毗邻高寒牧区的特有牛种资源,因其能适应高原恶劣环境,并提供优质畜产品,是当地牧民主要的经济来源之一。因高原的气候环境、饲养管理及牦牛自身发育迟缓等问题的影响,牦牛存在自然繁殖效率低下的生殖缺陷[1]。为此,在牦牛生产中广泛应用同期发情、人工授精、胚胎移植等现代繁殖生物技术,以加速牦牛的改良与繁殖。然而,雌性牦牛的发情相对隐蔽,难以准确把握授精和胚胎移植的时机。此外,牦牛卵母细胞的体外成熟体系优化不够,尤其是体外成熟时间,且易出现卵母细胞胞质松散、透明带硬化等,导致体外成熟和体外受精效率较低。这严重制约了现代繁殖生物技术的应用和阻碍了牦牛改良的进程。
SIRT1蛋白是乙酰化酶(Sirtuin)家族中首个被发现且研究最多的成员,其由747个氨基酸组成,具有高度的保守性,蛋白N端与C端分别包含一个柔性区域用以促进酶与某些底物结合,该蛋白定位于细胞核与细胞质[2]。SIRT1在哺乳动物卵巢、子宫等多个组织器官中存在表达。已有的研究结果表明,SIRT1在雌性哺乳动物生殖过程中发挥重要作用。在原始卵泡发育到卵母细胞减数分裂成熟的进程中,SIRT1参与介导卵巢与子宫的正常发育、卵泡发生、卵母细胞染色质浓缩、颗粒细胞增殖等[3],在胚胎时期敲除SIRT1基因会造成胚胎发育停滞[4]。近年研究发现,过表达SIRT1能够显著抵抗卵巢老化[5],已证实该现象与mTOR信号传导的减弱、卵巢SIRT1以及其底物NRF1和FOXO3a的水平增加有关[6-7]。通过在细胞培养液中添加Sirtuins泛激动剂可以改善细胞的质量[8-9],诱导Sirtuins家族基因表达的增加,且与线粒体生物合成及细胞降解有关,能够改善细胞的线粒体功能和发育能力[10]。因此,本试验在牦牛卵母细胞体外成熟液中添加SIRT1特异性的激动剂(SRT2104)与抑制剂(Inauhzin),通过观察不同培养时间(24和36 h)内卵母细胞成熟及后续发育潜能,探究SIRT1对卵母细胞发育与老化的影响,同时探索改善牦牛卵母细胞发育的方法,加速牦牛的现代繁殖生物技术进程。
1 材料与方法 1.1 材料与试剂SRT2104和Inauhzin(Med Chem Express公司);透明质酸酶、DMSO和H2DCFDA(Sigma公司);青霉素和链霉素(Biosharp公司);胎牛血清(FBS)和M-199培养液(Gibco公司);FSH、LH和17β-E2(宁波第二激素厂);Single Cell-to-CTTM试剂盒(Invitrogen公司);PrimeScriptTM RT Reagent Kit反转录试剂盒和SYBR® Premix Ex TaqTM Ⅱ试剂盒(TaKaRa公司);定量引物均由南京金斯瑞生物科技有限公司合成;其余未特殊说明试剂均购于Sigma公司。
1.2 牦牛卵母细胞收集及培养牦牛卵巢采自四川省广汉市清真牛肉供应基地,无菌采集年龄在3~6岁,健康状况良好的牦牛卵巢。选取卵巢表面直径为3~8 mm的卵泡,10 mL一次性注射器抽取卵泡液。在体式显微镜下收集卵丘-卵母细胞复合体(cumulus-oocyte complexes, COCs)。卵母细胞成熟液(OM):M-199 90 mL、FBS 10 mL、1 μg·mL-1 FSH、1 μg·mL-1 LH、1 μg·mL-1 17β-E2、0.03 μg·mL-1谷氨酰胺、2.20 μg·mL-1碳酸氢钠、0.22 μg·mL-1丙酮酸钠。选取卵丘颗粒细胞3层以上、胞质均匀且结构致密的COCs转入预平衡的OM液中洗涤2~3次,随后将COCs随机放置在盛有3 mL OM液(Control组)、添加SRT2104(3 μmol·L-1)的OM液(SRT组)和添加Inauhzin(3 μmol·L-1)的OM液(INZ组)的35 mm细胞培养皿中,每组约30枚COCs,在温度38.5 ℃,CO2浓度为5.5%,湿度饱和的CO2培养箱中培养。分别收集各试验组体外培养24和36 h的COCs,用于后续试验。
1.3 评估卵丘细胞的扩展及卵母细胞的成熟COCs在SRT、INZ和Control组的培养过程中,通过体视显微镜观察0、8、16、24 h卵丘细胞扩展情况,并通过图像比例尺计算出各组中卵丘细胞的扩展程度;各试验组COCs体外培养24 h后经0.2%透明质酸酶脱颗粒细胞后观察第一极体排出情况,统计卵母细胞成熟率。
1.4 免疫荧光检测卵母细胞内ROS水平SRT、INZ和Control组分别培养24和36 h的COCs脱颗粒细胞后收集卵母细胞,在DPBS溶液中清洗2~3次后移入10 μmol·L-1 H2DCFDA溶液中,37 ℃恒温避光孵育20~30 min。然后将卵母细胞置于DPBS液滴中洗涤2~3次,再移至载玻片,滴加适量抗淬灭剂,少量凡士林封片。通过倒置相差荧光显微镜,488 nm波长下观察细胞绿色荧光强度。
1.5 抗氧化及凋亡基因表达的检测SRT、INZ和Control组分别培养24和36 h的COCs脱颗粒细胞后收集卵母细胞,按照Single Cell-to-CTTM试剂盒使用说明书,以卵母细胞为模板提取RNA。通过PrimeScriptTM RT Reagent Kit反转录试剂盒进行反转录,获得卵母细胞cDNA。参考NCBI数据库中已公布普通牛(Bos taurus)的SIRT1、Bax、SOD2、FOXO3a以及GAPDH序列,通过Premier 5.0软件设计引物,具体引物信息见表 1。荧光定量采用SYBR® Premix Ex TaqTM Ⅱ试剂盒;反应体系:SYBR Premix Ex TaqTM Ⅱ7.5 μL,模板1 μL,上、下游引物各0.5 μL,ddH2O 5.5 μL;反应条件:95 ℃预变性5 min;95 ℃变性10 s;退火45 s,72 ℃延伸35 s,循环35次。每个样本进行3次重复,用2-△△Ct法对结果进行统计分析。
|
|
表 1 定量PCR基因的引物信息 Table 1 The information of primers for RT-qPCR |
SRT、INZ与Control组培养24和36 h的COCs转移至预平衡的G-IVF清洗微滴中,清洗2~3次,然后转移至预平衡的G-IVF受精微滴中培养,每个受精微滴放置20~30枚COCs。采用上游法精子获能,将精液移入盛有预平衡的精子获能液的底部,放入CO2恒温培养箱中获能40~50 min。获能后取适量上清转移至新离心管中,1 500 g离心5 min后,弃上清,吹打混匀底部剩余50 μL精液。将适量精液加入放置有COCs的G-IVF受精微滴中,精液浓度为1×106个·mL-1,随后转移至CO2恒温培养箱中。共孵育20 h后,捡出受精的卵母细胞,转移至预平衡的G-1微滴中培养。每个微滴放入10~20枚卵母细胞,放置于38 ℃、CO2浓度为5.5%,湿度饱和的CO2培养箱中培养,72 h后更换一次液体。培养24与168 h后分别观察并统计卵裂率和囊胚形成率。
1.7 数据分析所有组别的试验至少重复3次,卵裂率、囊胚率等均使用SPSS 19进行ANOVA检验分析。荧光定量结果用“平均值±标准误(Mean±SEM)”表示,应用t检验作统计分析。P < 0.05表示差异显著,P>0.05表示差异不显著。
2 结果 2.1 SIRT1对牦牛卵母细胞成熟的影响COCs在SRT、INZ与Control组培养24 h过程中,动态观察COCs卵丘细胞扩展情况,3组卵丘细胞扩展的动态变化见图 1,卵丘细胞的扩展程度见表 2,结果显示,在3组COCs培养成熟过程中,卵丘细胞扩展程度均较0 h显著增加(P < 0.05);培养24 h,3组间卵丘扩展面积差异显著(P < 0.05),其中SRT组扩展程度最大(497.46 μm2)。体外培养24 h后,在体视显微镜下观察卵母细胞第一极体排出情况,统计卵母细胞第一极体排出率(表 3),结果显示,SRT组卵母细胞第一极体排出率较Control组有所上升,INZ组卵母细胞成熟率显著低于SRT和Control组(P < 0.05)。
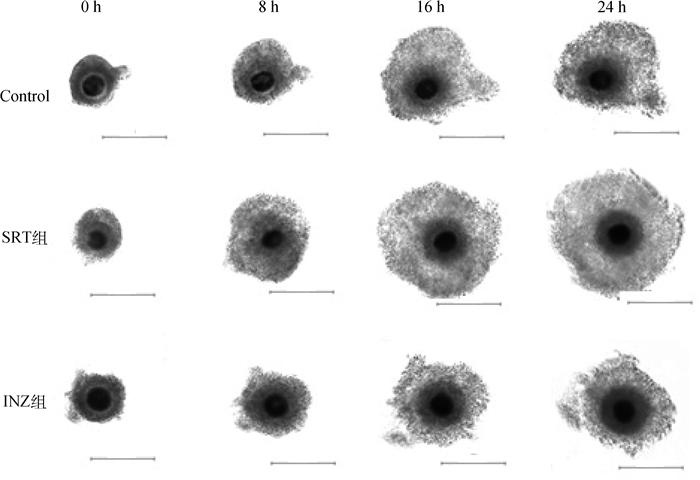
|
图中比例尺代表100 μm The scale represents 100 μm 图 1 不同培养时间卵丘细胞的扩展情况 Fig. 1 Expansion of cumulus cells at different culture times |
|
|
表 2 不同添加剂对牦牛卵丘细胞扩展的影响 Table 2 Effects of different additives on the cumulus expansion maturation |
|
|
表 3 不同添加剂对牦牛卵母细胞成熟率的影响 Table 3 Effects of different additives on the maturation rate of yak oocytes |
收集培养24与36 h的COCs,脱颗粒细胞后,经H2DCFDA染色,检测各组培养24与36 h卵母细胞内的ROS水平(图 2)。结果显示,培养36 h后3个试验组卵母细胞中荧光强度较24 h均显著增强,表明随着体外培养时间延长,卵母细胞中ROS水平显著增加(P < 0.05);24 h时,SRT组荧光信号最弱,而INZ组信号最强。与Control和INZ组相比,SRT组卵母细胞中ROS水平最低(P < 0.05);36 h时,SRT组细胞内荧光信号强度显著低于Control与INZ组(P < 0.05),表明36 h时SRT组细胞内ROS水平最低,INZ组的水平最高。
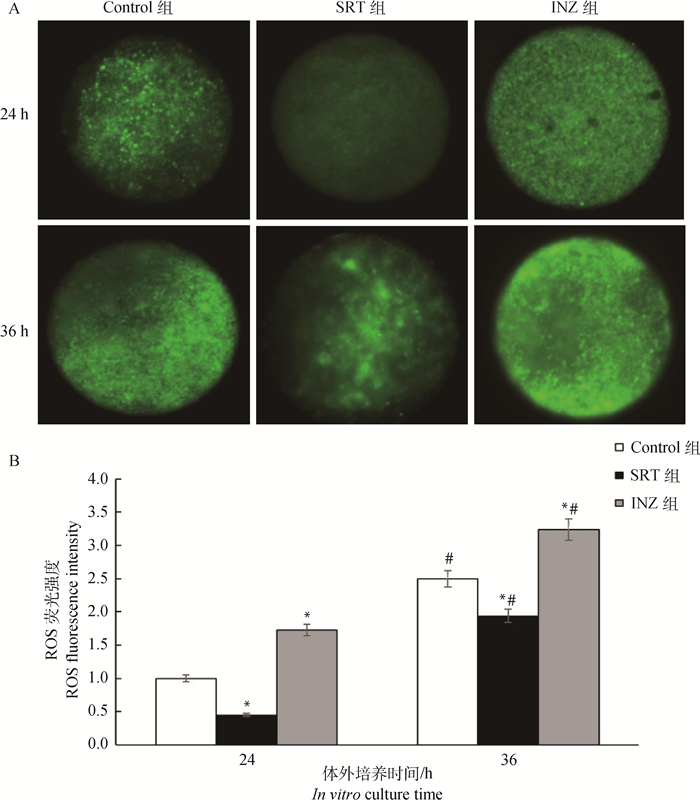
|
A. Control、SRT和INZ组体外培养24与36 h后获得的卵母细胞,使用H2DCFDA荧光染色检测卵母细胞内ROS水平;B.不同处理与体外培养时间对牦牛卵母细胞内ROS水平的影响。*.同一时间不同处理与Control组相比差异显著(P < 0.05);#.同一组培养36与24 h相比差异显著(P < 0.05),下同 A. After 24 and 36 h in vitro maturation, oocytes were stained with H2DCFDA to detect ROS level in oocytes; B. The effects of different treatments and in vitro culture times on ROS level in yak oocytes. *. Indicate the significant difference (P < 0.05) compared with the control group at the same time; #. Indicate the significant differences (P < 0.05) compared with 24 h culture in the same group, the same as below 图 2 不同处理及体外培养时间对卵母细胞中ROS水平的影响 Fig. 2 Effects of different treatments and in vitro culture times on ROS levels in oocytes |
为了进一步评估牦牛卵母细胞的质量,以SRT、INZ与Control组培养24和36 h的卵母细胞为模板,GAPDH基因作为内参基因,检测SIRT1基因、参与细胞凋亡的叉头转录因子(FOXO3a)基因、与抗氧化能力相关的超氧化物歧化酶(SOD2)基因以及促凋亡因子(Bax)基因的表达。结果见图 3,随着卵母细胞体外培养时间的增加,培养36 h时卵母细胞中SIRT1、FOXO3a以及SOD2 mRNA表达均较24 h时卵母细胞显著下降(P < 0.05);添加SRT2104能提高SIRT1、FOXO3a与SOD2 mRNA的表达,但随体外培养时间的增加(36 h),其作用效果不显著(P>0.05);然而,随培养时间的增加,促凋亡因子Bax mRNA的表达显著增加(P < 0.05),添加SRT2104能够显著抑制Bax mRNA的表达(P < 0.05)。
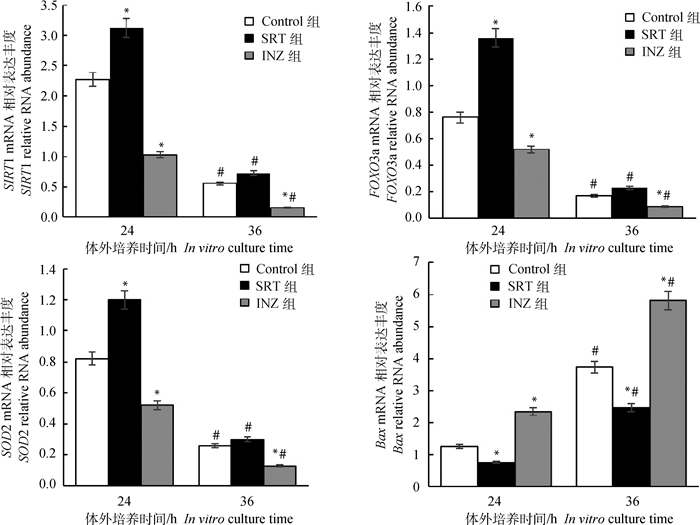
|
图 3 不同处理对卵母细胞中发育与凋亡相关基因表达的影响 Fig. 3 Effects of different treatments on the development and apoptosis related gene expressions in oocytes |
各试验组培养24与36 h的COCs进行体外受精,检测SIRT1对卵母细胞受精率及后续胚胎发育的影响,结果见表 4。随着体外培养时间的增加,卵裂率和囊胚率显著下降(P < 0.05);卵母细胞体外培养24 h时,3个处理组的卵裂率分别为59.73%、71.67%和44.70%,添加SIRT1激动剂显著增加卵裂率(P < 0.05)和囊胚形成率(P < 0.05),但SIRT1抑制剂组的卵裂率却显著下降(P < 0.05)。卵母细胞体外培养36 h时,INZ组的卵裂率和囊胚形成率显著低于其他组(P < 0.05)。
|
|
表 4 SIRT1对卵母细胞体外受精的影响 Table 4 Effects of SIRT1 on in vitro fertilization of oocytes |
SIRT1是Sirtuins家族成员之一,其参与调节细胞增殖分化、DNA损伤修复和细胞老化凋亡等生理功能[11],在哺乳动物生殖过程中介导卵母细胞染色质浓缩、颗粒细胞增殖等[12]。因此,研究SIRT1在卵母细胞成熟中的作用对探索改善卵母细胞体外成熟具有重要指导意义。
卵丘细胞通过影响卵母细胞的物质交换与信号传导进而调节卵母细胞成熟[13],卵丘细胞扩展程度与卵母细胞第一极体排出情况常作为检测卵母细胞成熟的特征。本研究通过在体外成熟液中添加SIRT1激动剂(SRT2104)和SIRT1抑制剂(Inauhzin),比较分析卵丘细胞扩展与第一极体排出情况,检测SIRT1对牦牛卵母细胞体外成熟的影响。结果表明,SRT组能显著促进牦牛体外成熟过程中卵丘细胞的扩展及卵母细胞第一极体排出,INZ组抑制了卵丘细胞扩展及第一极体排出。与本试验结果相同,Lee等[14]研究发现,猪COCs体外培养液中添加适量Sirtuins泛激动剂白藜芦醇能够显著增强卵丘细胞的扩展。在牛卵母细胞体外培养液中添加白藜芦醇也能提高第一极体排出率[15]。然而,在小鼠卵母细胞体外成熟培养基中添加Sirtuins泛抑制剂NAM与Sirtinol后,卵母细胞中出现多极纺锤体、染色体排布异常以及染色体滞后分裂等表型[16]。研究发现,猪卵丘细胞的扩展受前列腺素内过氧化物合酶1(PTGS1)和透明质烷合酶2(HAS2)的影响,在肿瘤转移相关研究中证实,提高SIRT1表达能够增加HAS2酶活性,促进透明质酸的分泌[14],且SIRT1能通过影响烟酰胺磷酸核糖转移酶(Nampt)介导PTGS1 mRNA的表达[17]。此外,有研究证实,Sirtuins家族蛋白影响卵母细胞减数分裂的组织结构[16]。因此推测,SIRT1通过介导卵丘细胞扩展相关途径与改善卵母细胞相关组织结构进而影响牦牛卵母细胞的体外成熟,具体的调控机制有待进一步研究。
ROS积累是降低卵母细胞质量的关键因素之一[18],体细胞相关研究证明,SIRT1可作为细胞氧化还原状态的传感器以及起到防止氧化应激和老化的保护作用,白藜芦醇调节SIRT1/PGC-1α轴能够减少足细胞氧化应激和凋亡[19-20]。在NAD+/NADH比率增加的驱动下,SIRT1激活能介导线粒体生物合成,进而使ATP产量显着增加,降低了线粒体的损伤消耗以抵抗ROS造成的损害[21-22]。ROS会降低SIRT1的表达,而SIRT1下调会进一步增加ROS水平,从而导致恶性循环[23]。本试验通过免疫荧光检测不同试验组培养24与36 h后卵母细胞内的ROS水平,结果表明,随着培养时间的增加,卵母细胞逐渐老化,卵母细胞中ROS水平显著升高,SIRT1激动剂处理组卵母细胞内ROS水平得到有效的抑制。证实SIRT1表达随着ROS积累而降低,SIRT1在排卵后卵母细胞老化过程中对ROS的适应性保护起重要作用。
本试验通过实时荧光定量检测不同试验组培养24与36 h的卵母细胞内SIRT1基因、抗氧化(SOD2)及凋亡相关基因(FOXO3a、Bax)的表达。结果表明,SRT组能显著改善培养24 h卵母细胞中FOXO3a、SOD2 mRNA丰度,而INZ组显著上调了卵母细胞中Bax mRNA的丰度,推测卵母细胞内SIRT1的含量能够影响卵母细胞的抗氧能力及质量。前期研究发现,在氧化应激作用下SIRT1通过对FOXO3a的去乙酰化作用影响细胞的质量,同时FOXO3a是激活SOD2表达的关键转录因子[23]。氧化应激前后小鼠卵母细胞内FOXO3a的分布发生显著的变化,氧化应激初期FOXO3a蛋白质在细胞核中大量聚集,随后表现为均匀的核质分布,这种分布特点与SIRT1分布相似[22]。根据FOXO3a在抗氧化反应中的作用,以及FOXO3a和SOD2 mRNA与SIRT1转录本相同的动力学变化,表明3种基因间存在一定的内在联系[24]。此外研究证实,卵母细胞内ROS累积加深老化程度,细胞接受促凋亡信号后线粒体通透性转换孔开放使Ca2+内流、上调Bax的表达,同时激活Caspase,最终导致细胞凋亡[25]。结合本研究结果,推测SIRT1通过调节SIRT1-FOXO3a-SOD2途径从而使SOD2上调,抑制ROS积累,进而保护卵母细胞,减少氧化应激损伤。
不同试验组培养24与36 h的卵母细胞进行体外受精,卵母细胞体外培养36 h的受精率显著低于24 h组,体外培养24 h添加SIRT1激动剂能显著改善卵母细胞体外受精效率,而添加SIRT1抑制剂对卵母细胞体外受精产生消极意义。由此表明,改变卵母细胞中SIRT1的表达会对卵母细胞后续受精及胚胎发育产生影响。老化卵母细胞体外受精后卵裂率与囊胚形成率均显著低于未老化卵母细胞,这是多种卵母细胞氧化应激的负面效应叠加的结果,如透明带硬化、质膜脂质过氧化膜流动性降低导致受精困难[26];皮质颗粒异常胞吐机制导致多精受精[27];受精后的异常Ca2+波动以及老化卵母细胞因纺锤体分布与组装异常导致受精后细胞启动凋亡机制等[28-29]。本试验发现,提高SIRT1 mRNA表达后,卵母细胞内ROS水平降低,抗氧化相关基因表达升高,减弱了氧化应激对卵母细胞质量的影响,进而提高了卵母细胞体外受精后的发育潜能。
4 结论在牦牛卵母细胞成熟液中适当提高SIRT1基因的表达,能够促进卵母细胞体外成熟及其后续胚胎发育;SIRT1参与调节牦牛卵母细胞抗氧化能力,通过抑制ROS水平和调控基因(FOXO3a、SOD2等)的表达缓解牦牛卵母细胞的老化进程并改善细胞质量,具体作用机制有待进一步验证。
| [1] |
兰道亮, 熊显荣, 位艳丽, 等. 基于RNA-Seq高通量测序技术的牦牛卵巢转录组研究:进一步完善牦牛基因结构及挖掘与繁殖相关新基因[J]. 中国科学:生命科学, 2014, 44(3): 307–317.
LAN D L, XIONG X R, WEI Y L, et al. RNA-Seq analysis of yak ovary:improving yak gene structure information and mining reproduction-related genes[J]. Science China: Life Sciences, 2014, 44(3): 307–317. (in Chinese) |
| [2] | BEI W L, ZHANG X H. Nucleus or cytoplasm? The mysterious case of SIRT1's subcellular localization[J]. Cell Cycle, 2016, 15(24): 3337–3338. DOI: 10.1080/15384101.2016.1237170 |
| [3] | TATONE C, DI EMIDIO G, VITTI M, et al. Sirtuin functions in female fertility:possible role in oxidative stress and aging[J]. Oxid Med Cell Longev, 2015, 2015: 659687. |
| [4] | ARUL NAMBI RAJAN K, KHATER M, SONCIN F, et al. Sirtuin1 is required for proper trophoblast differentiation and placental development in mice[J]. Placenta, 2018, 62: 1–8. DOI: 10.1016/j.placenta.2017.12.002 |
| [5] | KOJIMA Y A, ASSYLBEKOVA B, ZHAO B H, et al. Morphoproteomics identifies the EZH2 and SIRT1 pathways as potential blocks to differentiation in yolk sac tumor of the ovary and provides therapeutic options:a case study[J]. Ann Clin Lab Sci, 2017, 47(1): 88–91. |
| [6] | DOU X W, SUN Y, LI J Z, et al. Short-term rapamycin treatment increases ovarian lifespan in young and middle-aged female mice[J]. Aging Cell, 2017, 16(4): 825–836. DOI: 10.1111/acel.12617 |
| [7] | ZHANG J J, FANG L, LU Z Y, et al. Are sirtuins markers of ovarian aging?[J]. Gene, 2016, 575: 680–686. DOI: 10.1016/j.gene.2015.09.043 |
| [8] | LIU M Y, YIN Y, YE X Y, et al. Resveratrol protects against age-associated infertility in mice[J]. Hum Reprod, 2013, 28(3): 707–717. DOI: 10.1093/humrep/des437 |
| [9] | KHAN I, KIM S W, LEE K L, et al. Polydatin improves the developmental competence of bovine embryos in vitro via induction of sirtuin 1 (Sirt1)[J]. Reprod Fert Develop, 2017, 29(10): 2011–2020. DOI: 10.1071/RD16302 |
| [10] | SATO D, ITAMI N, TASAKI H, et al. Relationship between mitochondrial DNA copy number and SIRT1 expression in porcine oocytes[J]. PLoS One, 2014, 9(4): e94488. DOI: 10.1371/journal.pone.0094488 |
| [11] | SANTOS L, ESCANDE C, ESCANDE A. Potential modulation of sirtuins by oxidative stress[J]. Oxid Med Cell Longev, 2016, 2016: 9831825. |
| [12] |
田长永, 杨景晁, 马泽芳, 等. 卵丘细胞对辽宁绒山羊卵母细胞体外成熟与孤雌发育的影响[J]. 畜牧兽医学报, 2010, 41(5): 543–548.
TIAN C Y, YANG J C, MA Z F, et al. Effects of cumulus cells on in vitro maturation and parthenogenetic development of Liaoning cashmere goats oocytes[J]. Acta Veterinaria et Zootechnica Sinica, 2010, 41(5): 543–548. (in Chinese) |
| [13] | FERNANDES H, CASTRO F C, SCHEFER L, et al. Effect of melatonin and its receptors on bovine oocyte maturation and cumulus cell gene expression after heat shock in vitro:preliminary results[J]. Reprod Fert Develop, 2018, 31(1): 206–207. |
| [14] | LEE S, JIN J X, TAWEECHAIPAISANKUL A, et al. Sonic hedgehog signaling mediates resveratrol to improve maturation of pig oocytes in vitro and subsequent preimplantation embryo development[J]. J Cell Physiol, 2018, 233(6): 5023–5033. DOI: 10.1002/jcp.26367 |
| [15] | WANG F, TIAN X Z, ZHANG L, et al. Beneficial effect of resveratrol on bovine oocyte maturation and subsequent embryonic development after in vitro fertilization[J]. Fertil Steril, 2014, 101(2): 577–586. DOI: 10.1016/j.fertnstert.2013.10.041 |
| [16] | RIEPSAMEN A, WU L, LAU L, et al. Nicotinamide impairs entry into and exit from meiosis I in mouse oocytes[J]. PLoS One, 2015, 10(5): e0126194. DOI: 10.1371/journal.pone.0126194 |
| [17] | BORRADAILE N M, PICKERING J G. Polyploidy impairs human aortic endothelial cell function and is prevented by nicotinamide phosphoribosyltransferase[J]. Am J Physiol Cell Physiol, 2010, 298(1): C66–C74. DOI: 10.1152/ajpcell.00357.2009 |
| [18] | THAKER R, MISHRA V, GOR M, et al. The role of stimulation protocol, number of oocytes retrieved with respect to follicular fluid oxidative stress and IVF outcome[J]. Hum Fert, 2019. DOI: 10.1080/14647273.2018.1551630 |
| [19] | HORI Y S, KUNO A, HOSODA R, et al. Regulation of FOXOs and p53 by SIRT1 modulators under oxidative stress[J]. PLoS One, 2013, 8(9). |
| [20] | ZHANG T, CHI Y Q, REN Y Z, et al. Resveratrol reduces oxidative stress and apoptosis in podocytes via sir2-related enzymes, Sirtuins1 (SIRT1) / peroxisome proliferator-activated receptor γ co-activator 1α (PGC-1α) axis[J]. Med Sci Monit, 2019, 25: 1220–1231. DOI: 10.12659/MSM.911714 |
| [21] | SONG S B, HWANG E S. A rise in ATP, ROS, and mitochondrial content upon glucose withdrawal correlates with a dysregulated?mitochondria turnover mediated by the activation of the protein deacetylase SIRT1[J]. Cells, 2019, 8(1): 11. |
| [22] | DI EMIDIO G, FALONE S, VITTI M, et al. SIRT1 signalling protects mouse oocytes against oxidative stress and is deregulated during aging[J]. Hum Reprod, 2014, 29(9): 2006–2017. DOI: 10.1093/humrep/deu160 |
| [23] | CAITO S, RAJENDRASOZHAN S, COOK S, et al. SIRT1 is a redox-sensitive deacetylase that is post-translationally modified by oxidants and carbonyl stress[J]. FASEB J, 2010, 24(9): 3145–3159. DOI: 10.1096/fj.09-151308 |
| [24] | PECK B, CHEN C Y, HO K K, et al. SIRT inhibitors induce cell death and p53 acetylation through targeting both SIRT1 and SIRT2[J]. Mol Cancer Ther, 2010, 9(4): 844–855. DOI: 10.1158/1535-7163.MCT-09-0971 |
| [25] | LORD T, MARTIN J H, AITKEN R J. Accumulation of electrophilic aldehydes during postovulatory aging of mouse oocytes causes reduced fertility, oxidative stress, and apoptosis[J]. Biol Reprod, 2015, 92(2): 1–13. |
| [26] | SUN S C, GAO W W, XU Y N, et al. Degradation of actin nucleators affects cortical polarity of aged mouse oocytes[J]. Fertil Steril, 2012, 97(4): 984–990. DOI: 10.1016/j.fertnstert.2012.01.101 |
| [27] | ZHENG J, YIN X Q, GE W, et al. Post-ovulatory aging of mouse oocytes in vivo and in vitro:effects of caffeine on exocytosis and translocation of cortical granules[J]. Anim Sci J, 2016, 87(11): 1340–1346. DOI: 10.1111/asj.12611 |
| [28] | ZHAO S, LIU Z X, AO Z J, et al. Age-associated potency decline in bovine oocytes is delayed by blocking extracellular Ca2+ influx[J]. Theriogenology, 2015, 83(9): 1493–1501. DOI: 10.1016/j.theriogenology.2015.01.034 |
| [29] | DEMYDA-PEYRÁS S, DORADO J, HIDALGO M, et al. Effects of oocyte quality, incubation time and maturation environment on the number of chromosomal abnormalities in IVF-derived early bovine embryos[J]. Reprod Fertil Dev, 2013, 25(7): 1077–1084. DOI: 10.1071/RD12140 |



