2. 南京农业大学羊业科学研究所, 南京 210095;
3. 江苏农牧科技职业学院, 泰州 225300
2. Institute of Sheep Industry Science, Nanjing Agricultural University, Nanjing 210095, China;
3. Jiangsu Agri-animal Husbandry Vocational College, Taizhou 225300, China
脂联素(adiponectin,Adp)是由脂肪细胞特异分泌的因子,具有调节机体新陈代谢[1]、炎症[2]和免疫应答[3]等生物学作用。Adp生物学功能的发挥是通过脂联素受体(adiponectin receptor,AdpR)介导完成的。迄今为止,已克隆出3种AdpR:AdpR1、AdpR2和T-钙粘蛋白(T-cadherin)。AdpR1和AdpR2在下丘脑[4]、垂体[5]、睾丸[6-7]、卵巢[8]中均有表达,提示下丘脑-垂体-性腺轴(hypothalamus-pituitary-gonad axis,HPG)可能是Adp发挥作用的重要靶器官。Ocon等[6]研究发现,AdpR1主要存在于公鸡睾丸间质细胞、曲精细管管壁肌样细胞,而AdpR2主要表达于间质细胞、支持细胞、精子细胞、精子中。同时,研究者也发现,AdpR1和AdpR2存在于牛精子中[9]。
睾丸间质细胞中存在AdpR1和AdpR2。提示Adp可能与睾酮(testosterone,T)分泌有关。人注射T后,血浆中Adp水平下降[10]。Tsou等[11]证实随着男性青春期发育和T水平的增加,血浆中的Adp水平下降,说明T水平可能与Adp水平呈负相关,这一结论被Adamczak等[12]研究证实。但是,后来Landry等[13]研究发现,Adp能够促进T的合成。这一结论也得到国内外相关研究证实[14-15]。关于血浆中Adp水平与T含量之间相关性仍然是个有争议的问题。因此,明确Adp水平与机体T含量之间的关系具有重要的意义。
T合成与分泌受HPG的调节。这是由于下丘脑通过分泌促性腺激素释放激素(gonadotropin releasing hormone,GnRH),最终作用于睾丸分泌T,促进睾丸的发育。AdpR1和AdpR2存在于下丘脑中,提示Adp可能通过介导下丘脑功能的发挥对HPG进行调节[16]。同时下丘脑分泌的促性腺激素抑制激素(gonadotropin inhibitory hormone,GnIH)负反馈抑制GnRH的分泌。据此推测,Adp可能与GnRH和GnIH之间存在一定的相关性。
目前关于AdpR1和AdpR2在生殖器官中的表达主要集中在啮齿类和哺乳动物上,虽然已有研究表明,AdpR1和AdpR2在绵羊繁殖器官中表达[17],但二者在绵羊不同发育阶段生殖器官中如何变化,还未见报道。因此,本研究以不同发育阶段的湖羊为研究对象,采用qRT-PCR技术从mRNA水平探究AdpR1、AdpR2及T合成关键基因在不同发育阶段湖羊生殖器官中的表达规律;采用ELISA技术测定不同发育阶段湖羊血清中Adp、T、GnRH和GnIH含量,分析Adp与GnRH、GnIH、T及T合成关键基因的相关性;应用免疫组化技术将AdpR1在湖羊生殖器官中进行定位,为进一步研究AdpR1在性腺发育过程中如何发挥作用提供理论依据。
1 材料与方法 1.1 试验动物及样品采集本试验所需湖羊由江苏省泰州市海伦羊业有限公司提供。选取3月龄((16.07±0.20)kg)、9月龄((40.07±2.75)kg)、24月龄((76.65±4.38)kg)发育正常的健康湖羊各5只,共15只,进行常规饲养管理。屠宰前禁食12 h,给予正常饮水,颈静脉采血,3 000 r·min-1离心15 min,吸取上清,-20 ℃保存;屠宰后,采集下丘脑、垂体、睾丸、附睾头、体、尾组织,迅速置于液氮中,-80 ℃保存;从每只羊的睾丸、附睾头、体、尾的相同部位采集组织样品,约0.5 cm×0.5 cm×0.2 cm,4.0%多聚甲醛固定。
1.2 试验方法 1.2.1 ELISA检测严格按照绵羊脂联素(Adp)、睾酮(T)、促性腺激素释放激素(GnRH)、促性腺激素抑制激素(GnIH)ELISA试剂盒(Kmals,上海)说明书分别测定不同发育阶段湖羊血清中Adp、T、GnRH和GnIH浓度。
1.2.2 总RNA提取及反转录按照Trizol Reagent试剂盒(Invitrogen公司,美国)说明书分别提取不同发育阶段(n=5)湖羊下丘脑、垂体、睾丸、附睾头、体、尾总RNA,DEPC水(Invitrogen,美国)溶解,核酸蛋白测定仪ND-2000(NanoDrop Technologies,美国)测定总RNA浓度和纯度,同时采用1.5%凝胶电泳检测RNA质量。调整其浓度为1 μg·μL-1左右,反转录体系为40 μL,按照PrimeScript® RT reagent Kit With gDNA Eraser试剂盒(TaKaRa,大连)说明书进行操作。反转录产物cDNA -20 ℃保存。
1.2.3 引物设计与合成根据NCBI上绵羊(Ovis aries)的相关基因序列,利用Primer 5.0软件设计引物(表 1),由南京擎科生物有限公司合成引物序列。
|
|
表 1 用于qRT-PCR引物序列 Table 1 Primer sequences used for qRT-PCR |
以反转录的cDNA为模板,对AdpR1、AdpR2、LHR、StAR、3β-HSD、GAPDH基因进行qRT-PCR扩增。反应体系为20 μL:SYBR Green Master mix (Roche,德国)10 μL,上下游引物(10 μmol·L-1)各0.6 μL,cDNA 1 μL,ddH2O 7.8 μL。反应条件及步骤严格按照试剂盒说明书进行。
1.2.5 免疫组化采用石蜡对已固定好的组织进行包埋,切片厚度为6 μm,37 ℃烤片24 h,常规脱蜡流程按照SABC试剂盒操作说明进行。其中,Rabbit anti-AdpR1(华安生物,杭州;1:100),4 ℃过夜,PBS每5 min洗3次;Goat anti-Rabbit(博士德,武汉;1:100),37 ℃ 30 min,用PBS每5 min洗3次;SABC(博士德,武汉;1:100),37 ℃ 30 min,PBS每5 min洗3次。同时设置阴性对照,用PBS代替一抗,并用Nikon生物显微镜观察。
1.3 数据分析各目的基因相对表达量采用△△CT法计算,相对表达水平为2-△△CT,各目的基因相对表达量均应经GAPDH校正。其中各目的基因mRNA表达量分别以3月龄表达量均值为对照,其余(9和24月龄)以相对于对照的倍数作图。比较AdpR1、AdpR2 mRNA表达量时,以AdpR1 3月龄表达量为对照。其余月龄相对于其倍数作图。数据采用SPSS 24.0统计软件进行分析,结果表示为“平均值±标准误”。差异显著性采用One-way ANOVA和T-test分析,相关性分析采用Bivariate correlation进行,显著水平为P<0.05。
2 结果 2.1 AdpR1在9月龄湖羊生殖器官中的定位如图 1A所示,AdpR1定位于湖羊睾丸间质细胞、精母细胞、精子细胞和曲精细管管壁肌样细胞。在附睾头、体、尾上皮细胞中均检测到AdpR1阳性信号,即细胞核被DAB染成深色(图 1B~D);阴性对照未检测到任何阳性信号,即细胞核没有被DAB染色,因此细胞核为浅色(图 1E)。
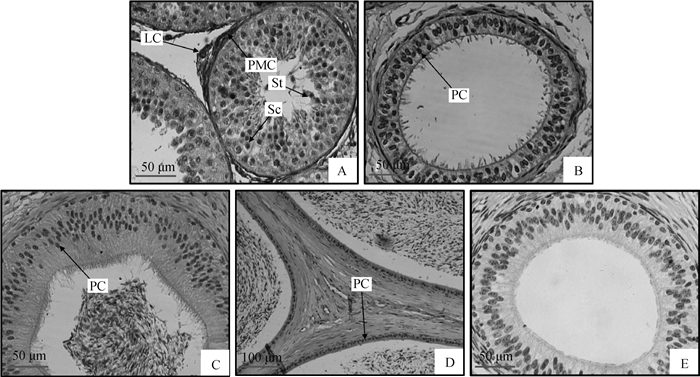
|
A~D.睾丸、附睾头、附睾体、附睾尾;E.阴性对照(A~C和E 400×; D 200×)。PMC.曲精细管管壁肌样细胞;LC.间质细胞;Sc.精母细胞;St.精子细胞;PC.主细胞(上皮细胞) A-D.Testis, caput, corpus and cauda, respectively; E. Negative control (A-C, E 400×; D 200×). PMC. Peritubular myoid cells; LC. Leydig cells; Sc. Spermatocyte; St. Spermatid, PC.Principal cells 图 1 9月龄湖羊生殖器官中AdpR1蛋白表达 Figure 1 Immunohistochemical location of AdpR1 protein in the reproductive tracts of 9 month Hu sheep |
由图 2可知,湖羊血清中Adp和T(结果已另文发表)含量呈先上升后下降趋势,表现为性成熟后(9和24月龄)血清中Adp和T浓度显著高于性成熟前(3月龄)(P<0.05),但9与24月龄间差异不显著(P>0.05);血清GnRH含量同样呈先上升后下降趋势,3和24月龄差异不显著(P>0.05);血清GnIH含量在湖羊9月龄时达到最高,24月龄时最低,其中3与9月龄差异不显著(P>0.05),但显著高于24月龄(P<0.05)。

|
不同小写字母表示不同月龄之间差异显著(P<0.05) Values with different small letter superscripts mean significant difference among different ages (P < 0.05) 图 2 不同月龄湖羊血清中Adp、GnRH和GnIH浓度的变化 Figure 2 The concentrations of Adp, GnRH and GnIH in serum at different developmental ages of Hu sheep |
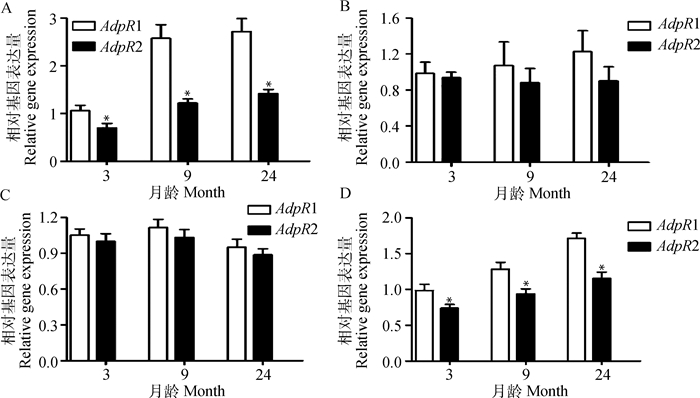
采用qRT-PCR技术对不同发育阶段湖羊性腺轴中AdpR1和AdpR2 mRNA表达规律的检测结果如图 3所示。AdpR1和AdpR2 mRNA在9月龄湖羊性腺轴组织中呈现不同的变化趋势,二者在睾丸中的表达水平显著高于其他组织(P<0.05,图 3A和3B);AdpR1在睾丸和附睾尾中mRNA表达量随月龄的增大而升高,均表现为性成熟后(9和24月龄)显著高于性成熟前(3月龄)(P<0.05,图 3C和3I),其中睾丸中AdpR1 mRNA表达量在9与24月龄间差异不显著(P>0.05,图 3C);AdpR1在不同月龄湖羊附睾头和附睾体中mRNA表达量差异不显著(P>0.05,图 3E和3G);AdpR2在不同月龄湖羊睾丸、附睾头和附睾体中mRNA表达量差异不显著(P>0.05,图 3D、3F和3H);AdpR2 mRNA在24月龄附睾尾中的表达量显著高于3和9月龄(P<0.05),但3与9月龄差异不显著(P>0.05,图 3J)。

|
不同小写字母表示不同组织或不同月龄之间差异显著(P<0.05)。A、B.AdpR1和AdpR2在9月龄湖羊性腺轴中mRNA表达;C、E、G、I.AdpR1在不同月龄湖羊睾丸、附睾头、体、尾中mRNA表达;D、F、H、J.AdpR2在不同月龄湖羊睾丸、附睾头、体、尾中mRNA表达 Values with different small letter superscripts mean significant different among different tissues or different ages (P < 0.05)A, B.The mRNA expression of AdpR1 and AdpR2 in the gonadal-axis of 9 month Hu sheep, respectively. C, E, G, I.The mRNA expression of AdpR1 in the testis, epididymis caput, corpus and cauda at different developmental stages of Hu sheep, respectively; D, F, H, J.The mRNA expression of AdpR2 in the testis, epididymis caput, corpus and cauda at different developmental stages of Hu sheep, respectively 图 3 AdpR1和AdpR2在不同月龄湖羊性腺轴中mRNA表达 Figure 3 The mRNA expression of AdpR1 and AdpR2 in the gonadal-axis at different developmental stages of Hu sheep |
从图 4可知,各个月龄睾丸和附睾尾中AdpR1 mRNA表达量均显著高于AdpR2 (P<0.05)(图 4A, D);虽然各个月龄附睾头和附睾体中AdpR1 mRNA表达量均高于AdpR2,但差异不显著(P>0.05)(图 4B,C)。
|
A~D.睾丸、附睾头、体、尾。*.同一月龄AdpR1与AdpR2 mRNA间差异达显著(P<0.05) A-D.Indicate testis, epididymis caput, corpus and cauda, respectively.*. Significant difference (P < 0.05) between AdpR1 and AdpR2 mRNA abundance at the same ages 图 4 AdpR1和AdpR2在不同月龄湖羊生殖器官中mRNA表达水平的比较 Figure 4 Comparison of the mRNA expression levels between AdpR1 and AdpR2 in reproductive organs of Hu sheep at different developmental stages |
由图 5可知,湖羊睾丸中LHR和StAR mRNA的表现为先上升后下降趋势,且各组之间存在显著差异(P<0.05),二者都是以9月龄表达量最高,3月龄表达量最低;3β-HSD mRNA在不同发育阶段湖羊睾丸中呈现出先上升后下降趋势,且性成熟后(9和24月龄)显著高于性成熟前(3月龄)(P<0.05),但9与24月龄之间差异不显著(P>0.05)。
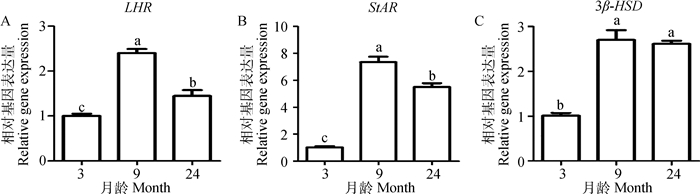
|
不同小写字母表示不同月龄之间差异显著(P<0.05) Values with different small letter superscripts mean significant difference among different ages (P < 0.05) 图 5 LHR(A)、StAR(B)和3β-HSD(C)在不同月龄湖羊睾丸中mRNA表达水平 Figure 5 The mRNA expression of LHR(A), StAR (B)and 3β-HSD (C) in the testis of Hu sheep at different ages |
由表 2可知,湖羊血清中T分别与Adp和GnRH水平显著正相关(P<0.05);AdpR1 mRNA表达水平与StAR、3β-HSD mRNA表达水平显著正相关(P<0.05)。虽然Adp含量与GnRH正相关,与GnIH负相关,但均差异不显著(P>0.05)。
|
|
表 2 不同月龄湖羊血清激素与相关基因的相关性分析 Table 2 Correlation of serum hormones and related genes at different developmental ages of Hu sheep |
Adp作为一种具有多种生理功能的脂肪细胞因子,通过内分泌方式循环到血液中,继而发挥各种功能。检测血清Adp水平临床上对胰岛素抵抗[18]、卵巢综合征[19]等疾病的发生进行预测。而且越来越多研究表明,脂联素对动物的生殖功能具有重要影响[20-21]。本研究首先测定了不同发育阶段湖羊血清Adp含量,结果发现,性成熟(9和24月龄)湖羊血清中Adp水平显著高于性成熟前(3月龄)。李云等[22]研究发现,性成熟皖南花猪血清中Adp含量显著高于性成熟前。这一现象与前人在公鸡[6]上的报道一致。这说明Adp在动物机体发育过程中扮演着重要角色。
Adp功能的发挥需要通过其受体(AdpR1/2)介导来完成。前期研究表明,AdpR1和AdpR2在猪[14]、鸡[6-7]、大鼠[23]睾丸中表达。本试验首先利用免疫组化技术对AdpR1在睾丸和附睾中进行了定位,研究发现,AdpR1主要存在于睾丸间质细胞、精母细胞、精子细胞、曲精细管管壁肌样细胞以及附睾头、体、尾中的上皮细胞中。而Ocón-Grove等[6]研究发现,AdpR1主要表达于公鸡睾丸间质细胞和曲精细管管壁肌样细胞。这种差异可能与物种差异有关。附睾是精子成熟的场所,精子在附睾中的成熟不仅受到精子自身因素的作用,还受到附睾微环境的影响[24]。附睾上皮细胞能够分泌种类不同的蛋白质及免疫抑制分子,以形成不断变化的附睾微环境,精子在附睾内转运过程中,附睾液与精子相互作用是精子逐步获得成熟时所具备的功能[25]。已有研究发现,AdpR1和AdpR2在牛[9]和绵羊[26]精子中有表达。提示Adp可能对精子成熟有一定的影响,但具体机制还需要进一步研究。
AdpR1和AdpR2 mRNA在绵羊睾丸和附睾有表达[17],但是二者在发育过程中是如何变化的,目前还未见报道。因此,本试验进一步采用qRT-PCR技术探究了AdpR1和AdpR2 mRNA在不同发育阶段湖羊睾丸和附睾中的表达模式。研究发现,AdpR1 mRNA在睾丸和附睾尾中表达量呈上升趋势,且具有显著的变化。这与前人发现的AdpR1 mRNA在不同发育阶段鸡[6]和皖南花猪[14]睾丸中变化趋势一致。而AdpR2 mRNA在附睾尾中呈上升趋势,而在其他生殖器官中无显著变化。同时,笔者发现AdpR1 mRNA表达量在睾丸和附睾中表达量均高于AdpR2,其中AdpR1 mRNA在睾丸和附睾尾中具有显著变化。提示Adp在睾丸和附睾中的作用主要通过AdpR1介导。
AdpR1在睾丸间质细胞中表达,推测Adp可能对T的生成有一定的影响。T的合成受HPG调节。而下丘脑是HPG的主要控制中心,通过分泌GnRH和GnIH对动物机体生殖功能进行调节。笔者进一步检测了不同发育阶段血清中T[27]、GnRH和GnIH含量发现,血清中T含量表现为性成熟后(9月龄)显著高于性成熟前(3月龄),而GnRH和GnIH均表现为9月龄最高。这可能是由于湖羊在性成熟过程中,随着血清中GnRH含量的增加,经过一系列内分泌调节,导致T含量升高,进而促进睾丸发育,当T含量积累到一定程度,就会反馈抑制GnRH的释放,进而导致GnIH升高。而当湖羊达到24月龄后,睾丸已经发育完全,对T的依赖性有所减弱,进而导致GnRH和GnIH降低。前期研究表明,随着男性青春期的发育和T水平的增加,血浆Adp水平下降[11]。这说明Adp与T含量之间有一定的相关性。但也有研究却得出相反结论,Adp对T的生成有促进作用[13, 15]。本研究发现,在特定的发育阶段,血清中Adp和T含量显著正相关,说明Adp能够促进T生成,这一发现与邵康等[14]在皖南花猪上研究一致。前期研究已证实,Adp能够抑制下丘脑GT1-7神经细胞GnRH的分泌[28]。李云等[22]研究表明,Adp与GnRH显著负相关。但是,本研究发现,Adp与GnRH呈正相关。这可能与本研究选取的湖羊发育阶段跨度太大有关。因此,二者相互作用关系还需要进一步研究证实。
T合成途径中也受多种类固醇合成酶的调控。StAR和3β-HSD是睾丸间质细胞中类固醇激素合成途径的2个最重要的限速酶[29]。StAR和3β-HSD mRNA表达量的高低直接影响T的生成[30]。本研究发现,StAR和3β-HSD mRNA在9月龄表达量最高,表明此阶段是T快速合成时期。StAR mRNA显著变化与邵康等[14]发现在皖南花猪性成熟前后表达趋势一致。如前所述,AdpR1 mRNA在睾丸中有显著的变化,表现为性成熟后(9和24月龄)显著高于性成熟前(3月龄)。且AdpR1分别与StAR和3β-HSD mRNA表达量显著正相关。在特定的发育阶段,AdpR1与T合成限速酶有类似的表达量上升模式,提示它们有协同升高T的相互作用。
4 结论AdpR1 mRNA表达量在不同发育阶段湖羊生殖器官中具有显著性变化,且在各个阶段睾丸和附睾尾中均显著高于AdpR2,提示Adp在湖羊生殖器官中的作用主要由AdpR1介导。同时,AdpR1与StAR和3β-HSD mRNA表达量显著正相关,提示AdpR1介导Adp在湖羊睾丸发育过程中促进T生成。
| [1] | KYRIAKAKIS E, CHARMPILAS N, TAVERNARAKIS N. Differential adiponectin signalling couples ER stress with lipid metabolism to modulate ageing in C. elegans[J]. Sci Rep, 2017, 7(1): 5115. DOI: 10.1038/s41598-017-05276-2 |
| [2] | HANSEN C S, VISTISEN D, JØRGENSEN M E, et al. Adiponectin, biomarkers of inflammation and changes in cardiac autonomic function:whitehall Ⅱ study[J]. Cardiovasc Diabetol, 2017, 16: 153. DOI: 10.1186/s12933-017-0634-3 |
| [3] | OBEID S, WANKELL M, CHARREZ B, et al. Adiponectin confers protection from acute colitis and restricts a B cell immune response[J]. J Biol Chem, 2017, 292(16): 6569–6582. DOI: 10.1074/jbc.M115.712646 |
| [4] | KAMINSKI T, SMOLINSKA N, MALESZKA A, et al. Expression of adiponectin and its receptors in the porcine hypothalamus during the oestrous cycle[J]. Reprod Domest Anim, 2014, 49(3): 378–386. DOI: 10.1111/rda.2014.49.issue-3 |
| [5] | SMOLINSKA N, DOBRZYN K, MALESZKA A, et al. Expression of adiponectin and adiponectin receptors 1(AdipoR1) and 2(AdipoR2) in the porcine uterus during the oestrous cycle[J]. Anim Reprod Sci, 2014, 146(1-2): 42–54. DOI: 10.1016/j.anireprosci.2014.02.001 |
| [6] | OCÍN-GROVE O, KRZYSIK-WALKER W S, MADDINENI S R, et al. Adiponectin and its receptors are expressed in the chicken testis:influence of sexual maturation on testicular ADIPOR1 and ADIPOR2 mRNA abundance[J]. Reproduction, 2008, 136(5): 627–638. DOI: 10.1530/REP-07-0446 |
| [7] | OCON-GROVE O, KRZYSIK-WALKER S, MADDINENI S R, et al. Expression of adiponectin and its receptors, Adipor1 and Adipor2, in the chicken testis[J]. Biol Reprod, 2007, 77(Suppl_1): 171. |
| [8] | OLIVEIR B S P, COSTA J A S, GOMES E T, et al. Expression of adiponectin and its receptors (AdipoR1 and AdipoR2) in goat ovary and its effect on oocyte nuclear maturation in vitro[J]. Theriogenology, 2017, 104: 127–133. DOI: 10.1016/j.theriogenology.2017.08.013 |
| [9] | KASIMANICKAM V R, KASIMANICKAM R K, KASTELIC J P, et al. Associations of adiponectin and fertility estimates in Holstein bulls[J]. Theriogenology, 2013, 79(5): 766–777. DOI: 10.1016/j.theriogenology.2012.12.001 |
| [10] | PAGE S T, HERBST K L, AMORY J K, et al. Testosterone administration suppresses adiponectin levels in men[J]. J Androl, 2005, 26(1): 85–92. |
| [11] | TSOU P L, JIANG Y D, CHANG C C, et al. Sex-related differences between adiponectin and insulin resistance in schoolchildren[J]. Diabetes Care, 2004, 27(2): 308–313. DOI: 10.2337/diacare.27.2.308 |
| [12] | ADAMCZAK M, RZEPKA E, CHUDEK J, et al. Ageing and plasma adiponectin concentration in apparently healthy males and females[J]. Clin Endocrinol, 2005, 62(1): 114–118. DOI: 10.1111/cen.2005.62.issue-1 |
| [13] | LANDRY D, PARÉ A, JEAN S, et al. Adiponectin influences progesterone production from MA-10 Leydig cells in a dose-dependent manner[J]. Endocrine, 2015, 48(3): 957–967. DOI: 10.1007/s12020-014-0456-y |
| [14] |
邵康, 周杰, 吴小雪, 等. 猪睾丸中脂联素受体与LHR、CYP11A1、StAR基因表达的发育变化及其相关性研究[J]. 畜牧兽医学报, 2011, 42(12): 1680–1685.
SHAO K, ZHOU J, WU X X, et al. The developmental patterns and correlation of adiponectin receptors, LHR, CYP11A1 and StAR mRNA expression in testis of Wannan Hua pigs[J]. Acta Veterinaria et Zootechnica Sinica, 2011, 42(12): 1680–1685. (in Chinese) |
| [15] | LANFRANCO F, ZITZMANN M, SIMONI M, et al. Serum adiponectin levels in hypogonadal males:influence of testosterone replacement therapy[J]. Clin Endocrinol, 2004, 60(4): 500–507. DOI: 10.1111/cen.2004.60.issue-4 |
| [16] | MICHALAKIS K G, SEGARS J H. The role of adiponectin in reproduction:from polycystic ovary syndrome to assisted reproduction[J]. Fertil Steril, 2010, 94(6): 1949–1957. DOI: 10.1016/j.fertnstert.2010.05.010 |
| [17] | RAHMANIFAR F, TABANDEH M R. Adiponectin and its receptors gene expression in the reproductive tract of ram[J]. Small Ruminant Res, 2012, 105(1-3): 263–267. DOI: 10.1016/j.smallrumres.2011.11.019 |
| [18] | KOU H J, ZHENG Q, DENG J, et al. Relationship between Adiponectin, Insulin resistance and atherosclerosis in non-diabetic hypertensive patients and healthy adults[J]. J Am Coll Cardiol, 2017, 70(16): C142. |
| [19] | MAZLOOMI S, ALIZADEH N, AMINZARE M, et al. Serum zinc and adiponectin levels in patients with polycystic ovary syndrome, adjusted for anthropometric, biochemical, dietary intake, and physical activity measures[J]. Biol Trace Elem Res, 2018, 181(2): 388. DOI: 10.1007/s12011-017-0951-0 |
| [20] | KAWWASS J F, SUMMER R, KALLEN C B. Direct effects of leptin and adiponectin on peripheral reproductive tissues:a critical review[J]. Mol Hum Reprod, 2015, 21(8): 617–632. DOI: 10.1093/molehr/gav025 |
| [21] | DOBRZYN K, SMOLINSKA N, KIEZUN M, et al. The effect of estrone and estradiol on the expression of the adiponectin system in the porcine uterus during early pregnancy[J]. Theriogenology, 2017, 88: 183–196. DOI: 10.1016/j.theriogenology.2016.09.023 |
| [22] |
李云, 张凌齐, 周杰, 等. 脂联素及其受体与GnRH和GnIH在皖南花猪下丘脑中的发育性表达及相关性研究[J]. 西北农林科技大学学报:自然科学版, 2013, 41(12): 7–13.
LI Y, ZHANG L Q, ZHOU J, et al. Developmental expression and correlation analysis of adiponectin, adiponectin receptors, GnRH and GnIH in hypothalamus of Wannan Hua pigs[J]. Journal of Northwest A&F University:Natural Science Edition, 2013, 41(12): 7–13. (in Chinese) |
| [23] | GUO Z, YAN X, WANG L, et al. Effect of telmisartan or insulin on the expression of adiponectin and its receptors in the testis of streptozotocin-induced diabetic rats[J]. Horm Metab Res, 2016, 48(6): 404–412. DOI: 10.1055/s-00000025 |
| [24] | SULLIVAN R, MIEUSSET R. The human epididymis:its function in sperm maturation[J]. Hum Reprod Update, 2016, 22(5): 574–587. DOI: 10.1093/humupd/dmw015 |
| [25] | BROWNE J A, YANG R, LEIR S H, et al. Expression profiles of human epididymis epithelial cells reveal the functional diversity of caput, corpus and cauda regions[J]. Mol Hum Reprod, 2016, 22(2): 69–82. DOI: 10.1093/molehr/gav066 |
| [26] | KADIVAR A, KHOEI H H, HASSANPOUR H, et al. Correlation of Adiponectin mRNA abundance and its receptors with quantitative parameters of sperm motility in rams[J]. Int J Fertil Steril, 2016, 10(1): 127–135. |
| [27] | YANG H, WANG F, LI F, et al. Comprehensive analysis of long non-coding RNA and mRNA expression patterns in sheep testicular maturation[J]. Biol Reprod, 2018. DOI: 10.1093/biolre/ioy088 |
| [28] | WEN J P, LV W S, YANG J, et al. Globular adiponectin inhibits GnRH secretion from GT1-7 hypothalamic GnRH neurons by induction of hyperpolarization of membrane potential[J]. Biochem Biophys Res Commun, 2008, 371(4): 756–761. DOI: 10.1016/j.bbrc.2008.04.146 |
| [29] | ZHANG G L, DAI D Z, ZHANG C, et al. Apocynin and raisanberine alleviate intermittent hypoxia induced abnormal StAR and 3β-HSD and low testosterone by suppressing endoplasmic reticulum stress and activated p66Shc in rat testes[J]. Reprod Toxicol, 2013, 36: 60–70. DOI: 10.1016/j.reprotox.2012.12.002 |
| [30] | RAUCCI F D, D'ANIELLO A, DI FIORE M M. Stimulation of androgen production byD-aspartate through the enhancement of StAR, P450scc and 3β-HSD mRNA levels in vivo rat testis and in culture of immature rat Leydig cells[J]. Steroids, 2014, 84: 103–110. DOI: 10.1016/j.steroids.2014.03.016 |



