2. 中国农业科学院哈尔滨兽医研究所禽传染病研究室, 哈尔滨 150001;
3. 马里兰大学帕克分校动物与禽类科学系, 马里兰州 20742, 美国
2. Division of Avian Infectious Diseases, Harbin Veterinary Research Institute of Chinese Academy of Agricultural Sciences, Harbin 150001, China;
3. Department of Animal & Avian Sciences, University of Maryland, College Park, Maryland 20742, United States
马立克病(Marek’s disease, MD)是由马立克病病毒(Marek’s disease virus, MDV)引起的恶性T淋巴细胞增生性肿瘤疾病;它是一种严重危害养鸡业和家禽健康的传染病[1]。MDV属于马立克病毒属,又称为禽疱疹病毒2型。体内感染MDV可以分为四个阶段,分别为病毒溶细胞感染(感染病毒后的3至6 d),潜伏感染(感染病毒后的7至10 d),溶细胞感染第二阶段(感染病毒后的10至14 d)和肿瘤转化(感染病毒后的21 d)[2-3]。越来越多的微小RNA (microRNA, miRNA)在病毒引起的肿瘤性疾病中扮演重要角色,其中包括miRNA-181a[4]、miRNA-26a[5-6]、miRNA-219[7]等。
miRNA是小的单链非编码RNA,长度约22个核苷酸,在调节细胞增殖、分化、凋亡、发育和肿瘤发生等生物过程中扮演重要角色[8]。miRNA通过与靶基因mRNA的3′-非翻译区(3′-untranslated region, 3′-UTR)结合影响转录或者mRNA的翻译,从而在转录后水平上发挥作用[9]。现在,越来越多与MD肿瘤发生密切相关的宿主miRNA和病毒miRNA被挖掘出来。gga-miR-155只在MDV转化的肿瘤细胞系中表达下调,这可以作为MD肿瘤细胞中特异性表达的标志[10]。MDV编码的Mdv1-miR-M4-5p和gga-miR-155具有高度同源性,对MDV的原癌性具有至关重要的作用[11];并且它能通过靶向潜伏转化生长因子β结合蛋白1(Latent TGF-β binding protein 1, LTBP1)激活原癌基因c-Myc,从而抑制TGF-β信号通路[12]。Z.J.Li等[13]发现了79个感染MDV后表达呈显著差异的miRNA,并发现两类可以作为MDV感染和MD肿瘤发生指示标志的miRNA。gga-miR-26a在MDV引发的肿瘤中低表达[14],在禽类转化淋巴细胞系中介导白细胞介素2[5](interleukin-2, IL-2)和NIMA相关激酶6[6](NIMA related kinase 6, NEK6)的表达,并能抑制MD淋巴瘤细胞增殖。gga-miR-181a通过抑制类v-myb成髓细胞瘤病毒原癌基因同源体1(v-myb myeloblastosis viral oncogene homolog-like 1, MYBL1)使MSB1细胞增殖发生抑制[15]。
我们利用Solexa深度测序分析了MD肿瘤组和非感染组的miRNA表达谱[16],发现gga-miR-140-3p在MDV引发的肿瘤组织中表达异常。本研究检测该miRNA对MD肿瘤细胞MSB1增殖、迁移的影响以及对与侵袭密切相关的MMP2、MMP9基因转录水平的影响。
1 材料与方法 1.1 样品收集试验鸡群和马立克病毒毒株(MDV-GA)由中国农业科学院哈尔滨兽医研究所提供。试验选择150只无特定病原体(specific pathogen free, SPF)的白来航鸡,分成2组,攻毒组100只,对照组50只。在1日龄时攻毒组个体腹腔注射MDV-GA(2 000 PFU)国际标准毒株0.2 mL。对照组注射同等剂量的无病毒稀释液。两组鸡分开饲养在不同的负压隔离器中,其他试验条件一致,试验全期56 d。MDV感染后30 d,剩余66只MDV感染鸡和28只未感染对照组鸡。在感染MDV的第30~56天,一共有47只鸡形成肿瘤,另外19只鸡状态良好,未形成肿瘤。在感染后31~55 d,连续观察鸡群状态,对外观表现为严重病症的个体进行屠宰,同时屠宰未感染对照组个体。个体屠宰后收集脾、肝和各组织来源的MD淋巴瘤。所有组织保存在RNA保存液中用于RNA的提取。
1.2 细胞培养和miRNA转染MDV转化淋巴细胞系,MDCC-MSB1,由C. Itakura博士友情馈赠,培养在含有10%胎牛血清(Invitrogen公司)的RPMI-1640培养基中,放置于温度37 ℃,相对湿度95%, 含有5% CO2的培养箱中。FuGENE HD转染试剂(Promega公司)用于miRNA转染,用于转染的miRNA激动剂(agomir)和阴性对照(Negative control, NC)剂量分别为100 nmol·L-1。gga-miR-140-3p agomir和NC购自上海吉玛公司。
1.3 RNA提取和实时荧光定量PCR(qRT-PCR)利用Trizol试剂(Invitrogen公司)按照说明书要求提取冰冻组织或细胞总RNA。利用cDNA synthesis kit (miRACLE公司)反转录miRNA; qPCR miRNA kit(miRACLE公司)用于miRNA定量检测, 每组8个样本。用于检测内对照5S的上游引物序列为5′-ACCGGGTGCTGTAGGCTTAA-3′; 检测gga-miR-140-3p的上游引物序列为5′-AGGGTAGAACCACGGACAAAA-3′。反应条件为95 ℃预变性10 min,95 ℃变性10 s,57 ℃退火20 s,72 ℃延伸1 min,qRT-PCR反应在Invitrogen公司的ABI 7500仪器上进行40个循环。
利用EasyScript First-Strand cDNA Synthesis SuperMix (TransGen公司)将总RNA反转为cDNA用于基因表达水平的检测。使用Power SYBR Green PCR Master Mix (Invitrogen公司)在ABI 7500仪器上进行qRT-PCR,每组三个生物学重复。引物信息如下:β-actin上游引物为5′-GAGAAATTGTGCGTGACATCA-3′,下游引物为5′-CCTGAACCTCTCATTGCCA-3′;MMP2上游引物为5′-TGAAACAGGAGATTTGGAT-3′,下游引物为5′-CATTTTGGCTTTCTTGGA-3′, MMP9上游引物为5′-ACCTGGACCGTGCCGTGAT-3′, 下游引物为5′-TGCCTCGCCGCTGTAAAT-3′。基因的相对表达量以5S和β-actin为参考基因通过2-ΔΔCt方法表达为倍数变化。
1.4 细胞增殖检测在96孔板中接种95 μL细胞悬液(3×104·孔-1),进行转染。在转染后的24、36、48、60和72 h利用CCK-8检测细胞增殖。检测时每孔加入10 μL CCK-8溶液,在细胞培养箱内继续孵育2 h,用分光光度计在450 nm测定吸光度。每组设置5个重复。
1.5 细胞迁移检测所有细胞培养试剂和Transwell chamber (8 mm孔径; BD Bioscience公司)放在37 ℃温育。在24孔板中接种500 μL细胞悬液(1.5×105·孔-1),进行转染。转染48 h后,收集细胞,用PBS和无血清培养基先后洗涤一次,用无血清培养基悬浮细胞,计数。在Transwell下室加入600 μL含20%血清的培养基,上室加入100 μL细胞悬液,每孔5×104个细胞,继续在培养箱培养16 h,收集下室的细胞进行计数。每组设置2个重复。
1.6 统计分析所有数据的表达形式为“x±sx”。用SAS软件按照Student’s t检验进行统计分析。当P < 0.05时表示数据之间差异显著;当P < 0.01时表示数据之间差异极显著。
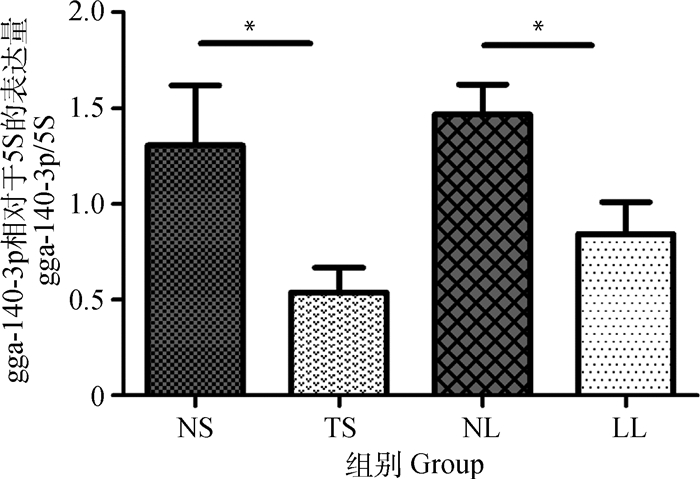
2 结果 2.1 gga-miR-140-3p在不同组织中的差异表达gga-miR-140-3p在不同组织中的表达量见图 1。与非感染组脾相比,gga-miR-140-3p在肿瘤化脾中显著下调(P < 0.05);与非感染组肝相比,gga-miR-140-3p在肝淋巴瘤中也呈现显著低表达(P < 0.05)(图 1)。结果表明该miRNA在MD肿瘤化组织中表达下调。
|
NS.非感染脾;TS.肿瘤化脾;NL.非感染肝;LL.肝淋巴瘤。n=8, *.P < 0.05 NS. Non-infected spleen; TS. Tumorous spleen; NL. Non-infected liver; LL. MD lymphoma from liver. n=8. *.P < 0.05 图 1 不同组织中gga-miR-140-3p表达水平 Figure 1 Differential expression of gga-miR-140-3p in different tissues |
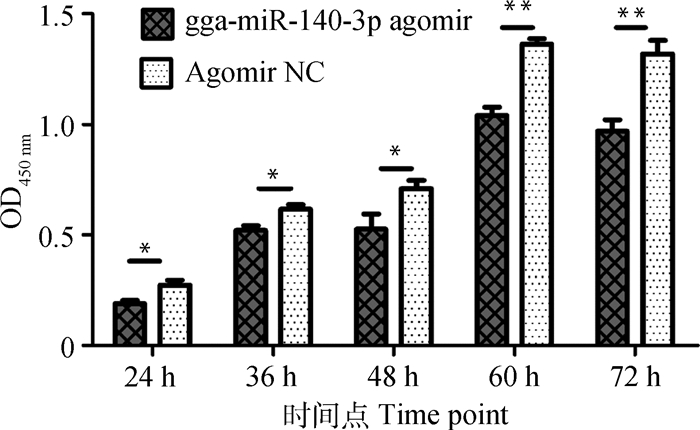
过表达gga-miR-140-3p对MSB1细胞增殖影响的检测结果见图 2。与对照组相比,转染gga-miR-140-3p agomir后的24、36、48 h细胞吸光值显著(P < 0.05)降低,60和72 h吸光值极显著(P < 0.01)降低(图 2)。这表明该miRNA能显著抑制MD肿瘤淋巴细胞增殖。
|
n=5,*.P < 0.05,* *.P < 0.01 图 2 gga-miR-140-3p对MSB1细胞增殖的影响 Figure 2 Effect of gga-miR-140-3p on MSB1 cell proliferation |
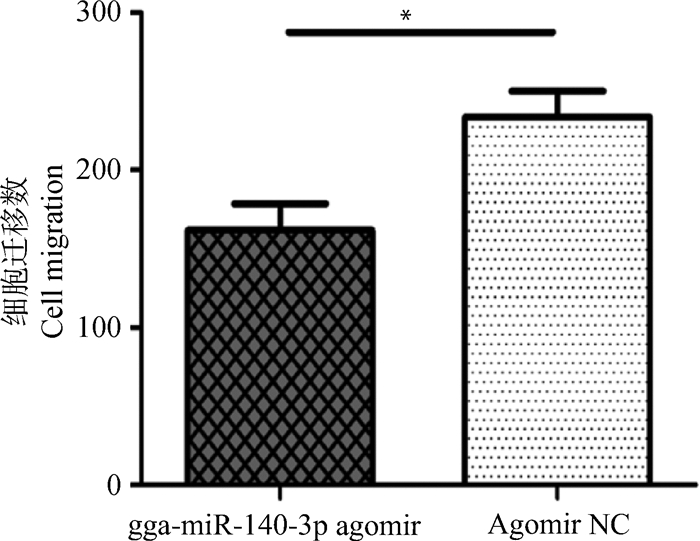
过表达gga-miR-140-3p对MSB1细胞迁移影响的检测结果见图 3。与对照组相比,转染gga-miR-140-3p agomir后,Transwell下室中的细胞数目显著(P < 0.05)较低(图 3)。这表明该miRNA能显著抑制MSB1细胞迁移。
|
n=2,*.P < 0.05 图 3 gga-miR-140-3p对MSB1细胞迁移的影响 Figure 3 Effect of gga-miR-140-3p on MSB1 cell migration |
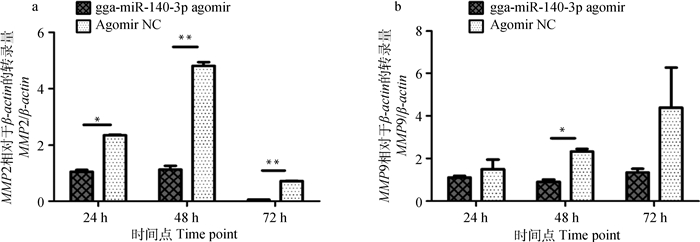
过表达gga-miR-140-3p对MMP2、MMP9转录量影响的检测结果见图 4。转染gga-miR-140-3p agomir后,MMP2转录量在24 h显著(P < 0.05)降低,48和72 h极显著(P < 0.01)降低(图 4a);MMP9转录量在48 h显著(P < 0.05)降低(图 4b)。这表明该miRNA能够影响与侵袭相关基因的转录,从而可能影响MD肿瘤细胞侵袭。
|
n=3,*.P < 0.05,* *.P < 0.01 图 4 gga-miR-140-3p对MMP2和MMP9转录的影响 Figure 4 Effect of gga-miR-140-3p on MMP2 and MMP9 mRNA expression level |
作为一种短的非编码RNA,miRNA参与肿瘤发生的报道日趋增多[17-21]。在前期试验中,通过Solexa高通量测序发现gga-miR-140-3p在MDV引发的肿瘤化脾及肝淋巴瘤中表达异常[16],提示该miRNA或许能够作为MD肿瘤发生的生物标记。MDCC-MSB1细胞是一种由感染MDV BC-1毒株引发的脾淋巴瘤制备的细胞系,主要由CD4+ T细胞组成,可以作为研究MD肿瘤转化的细胞模型[22-23]。为揭示gga-miR-140-3p在MD肿瘤转化过程中的作用,我们研究了它对肿瘤细胞MSB1功能的影响,结果表明该miRNA在一定程度上能够影响MD肿瘤细胞增殖和迁移,同时能影响与侵袭密切相关基因的转录水平。
miR-140在多种癌症中表达紊乱,通过靶向不同靶基因发挥各种各样的作用。miR-140-3p在肺鳞状细胞癌[24]、卵巢癌[25]、鳞状上皮细胞癌[26]和基质细胞瘤[27]中表达下调,扮演肿瘤抑制因子的作用;然而在脊髓脊索瘤[28]中表达上调,扮演原癌miRNA的角色。F. Tian等[29]报道,与非感染组相比,感染MDV后gga-miR-140在MD易感系72脾中表达下调。L. Lian等[30]报道在感染MDV 14 d后,gga-miR-140-3p在感染脾中的表达量显著降低,表明该miRNA表达异常在肿瘤转化前期就已发生,肿瘤细胞在早期已经开始渗透入脾。我们的研究结果也显示gga-miR-140-3p在MDV引发的肿瘤化脾中表达下调。在感染MDV的鸡肌肉成纤维细胞(chicken embryo fibroblast, CEF)中,gga-miR-140-3p呈现高表达趋势[31];然而,我们发现gga-miR-140-3p在感染MDV的肿瘤化脾和肝淋巴瘤中表达比非感染组低。造成该miRNA表达差异的原因可能是因为CEF和脾、肝的细胞组成成分不一致,CEF主要是肌肉成纤维细胞,而脾和肝中含有大量的淋巴细胞。在骨肉瘤细胞U-2 OS和结肠癌细胞HCT116中,过表达miR-140能够诱导p53和p21表达,同时伴随细胞周期G1和G2期阻滞;进一步研究发现,该miRNA是通过靶向组蛋白去乙酰酶4(histone deacetylase 4, HDAC4)介导细胞周期进程[32]。在骨肉瘤细胞MG63中,过表达miR-140使细胞周期阻滞在G0/G1期,同时细胞迁移发生抑制[33]。过表达gga-miR-140-3p显著抑制MSB1细胞增殖,提示该miRNA可能参与MD肿瘤细胞增殖。不过增殖发生抑制的原因是由细胞周期阻滞还是凋亡抑制引发还需要进一步研究。过表达miR-140-3p抑制肺癌细胞A549和H1299的增殖、迁移和侵袭[34]。在非小细胞肺癌裸鼠中,miR-140-3p靶向ATP酶磷脂运输8A1(ATPase phospholipid transporting 8A1, ATP8A1)诱导凋亡从而抑制肿瘤细胞增殖,并且该miRNA抑制细胞迁移和侵袭[35]。迁移作为肿瘤转化的重要特征之一,我们利用Transwell进行迁移试验检测过表达gga-miR-140-3p对MSB1迁移能力的影响。考虑到MSB1属于悬浮细胞,细胞无法黏附在Transwell上室的膜上,而是会移动到下室的培养基中,所以我们采用细胞计数的方法来反映细胞迁移情况,过表达gga-miR-140-3p显著抑制MSB1细胞迁移。金属基质蛋白酶(Matrix metalloproteinases, MMPs)能够降解基底膜的主要组成成分——Ⅳ型胶原蛋白,参与肿瘤细胞侵袭和转移,其中MMP2和MMP9是MMP家族的重要成员[36-39]。定量检测MMP2和MMP9基因表达可以间接反映肿瘤细胞的侵袭能力。过表达gga-miR-140-3p抑制MMP2和MMP9的表达,提示该miRNA可能通过抑制细胞侵袭影响MD肿瘤转化过程。
4 结论gga-miR-140-3p作为在MD肿瘤中异常表达的miRNA,能够影响MD肿瘤细胞的增殖、迁移和侵袭,提示该miRNA可能通过影响肿瘤细胞功能参与肿瘤转化过程,该miRNA可以作为MD肿瘤发生的指示标志。
| [1] | BIGGS P M. The Leeuwenhoek Lecture, 1997. Marek's disease herpesvirus:oncogenesis and prevention[J]. Philos Trans R Soc Lond B Biol Sci, 1997, 352(1364): 1951–1962. DOI: 10.1098/rstb.1997.0181 |
| [2] | CALNEK B W. Marek's disease-a model for herpesvirus oncology[J]. Crit Rev Microbiol, 1986, 12(4): 293–320. |
| [3] | CALNEK B W. Pathogenesis of Marek's disease virus infection[J]. Curr Top Microbiol Immunol, 2001, 255: 25–55. |
| [4] | BHATTACHARYA S D, GARRISON J, GUO H T, et al. Micro-RNA-181a regulates osteopontin-dependent metastatic function in hepatocellular cancer cell lines[J]. Surgery, 2010, 148(2): 291–297. DOI: 10.1016/j.surg.2010.05.007 |
| [5] | XU H T, YAO Y X, SMITH L P, et al. MicroRNA-26a-mediated regulation of interleukin-2 expression in transformed avian lymphocyte lines[J]. Cancer Cell Int, 2010, 10: 15. DOI: 10.1186/1475-2867-10-15 |
| [6] | LI X, LIAN L, ZHANG D X, et al. gga-miR-26a targets NEK6 and suppresses Marek's disease lymphoma cell proliferation[J]. Poult Sci, 2014, 93(5): 1097–1105. DOI: 10.3382/ps.2013-03656 |
| [7] | FAVREAU A J, SATHYANARAYANA P. miR-590-5p, miR-219-5p, miR-15b and miR-628-5p are commonly regulated by IL-3, GM-CSF and G-CSF in acute myeloid leukemia[J]. Leuk Res, 2012, 36(3): 334–341. DOI: 10.1016/j.leukres.2011.09.027 |
| [8] | BARTEL D P. MicroRNAs:genomics, biogenesis, mechanism, and function[J]. Cell, 2004, 116(2): 281–297. DOI: 10.1016/S0092-8674(04)00045-5 |
| [9] | BRENNECKE J, STARK A, RUSSELL R B, et al. Principles of microRNA-target recognition[J]. PLoS Biol, 2005, 3(3): e85. DOI: 10.1371/journal.pbio.0030085 |
| [10] | YAO Y X, ZHAO Y G, SMITH L P, et al. Differential expression of microRNAs in Marek's disease virus-transformed T-lymphoma cell lines[J]. J Gen Virol, 2009, 90(Pt 7): 1551–1559. |
| [11] | ZHAO Y G, YAO Y X, XU H T, et al. A functional MicroRNA-155 ortholog encoded by the oncogenic Marek's disease virus[J]. J Virol, 2009, 83(1): 489–492. DOI: 10.1128/JVI.01166-08 |
| [12] | CHI J Q, TENG M, YU Z H, et al. Marek's disease virus-encoded analog of microRNA-155 activates the oncogene c-Myc by targeting LTBP1 and suppressing the TGF-β signaling pathway[J]. Virology, 2015, 476: 72–84. DOI: 10.1016/j.virol.2014.11.027 |
| [13] | LI Z J, ZHANG Y P, LI Y, et al. Distinct expression pattern of miRNAs in Marek's disease virus infected-chicken splenic tumors and non-tumorous spleen tissues[J]. Res Vet Sci, 2014, 97(1): 156–161. DOI: 10.1016/j.rvsc.2014.04.003 |
| [14] | BURNSIDE J, OUYANG M, ANDERSON A, et al. Deep sequencing of chicken microRNAs[J]. BMC Genomics, 2008, 9: 185. DOI: 10.1186/1471-2164-9-185 |
| [15] | LIAN L, LI X, ZHAO C F, et al. Chicken gga-miR-181a targets MYBL1 and shows an inhibitory effect on proliferation of Marek's disease virus-transformed lymphoid cell line[J]. Poult Sci, 2015, 94(11): 2616–2621. DOI: 10.3382/ps/pev289 |
| [16] | LIAN L, QU L J, CHEN Y M, et al. A systematic analysis of miRNA transcriptome in Marek's disease virus-induced lymphoma reveals novel and differentially expressed miRNAs[J]. PLoS One, 2012, 7(11): e51003. DOI: 10.1371/journal.pone.0051003 |
| [17] | LI X, LIAN L, ZHANG D X, et al. gga-miR-26a targets NEK6 and suppresses Marek's disease lymphoma cell proliferation[J]. Poult Sci, 2014, 93(5): 1097–1105. DOI: 10.3382/ps.2013-03656 |
| [18] | LIAN L, LI X, ZHAO C F, et al. Chicken gga-miR-181a targets MYBL1 and shows an inhibitory effect on proliferation of Marek's disease virus-transformed lymphoid cell line[J]. Poult Sci, 2015, 94(11): 2616–2621. DOI: 10.3382/ps/pev289 |
| [19] | HAN B, LIAN L, LI X, et al. Chicken gga-miR-130a targets HOXA3 and MDFIC and inhibits Marek's disease lymphoma cell proliferation and migration[J]. Mol Biol Rep, 2016, 43(7): 667–676. DOI: 10.1007/s11033-016-4002-2 |
| [20] | ZHAO C F, LI X, HAN B, et al. Gga-miR-219b targeting BCL11B suppresses proliferation, migration and invasion of Marek's disease tumor cell MSB1[J]. Sci Rep, 2017, 7: 4247. DOI: 10.1038/s41598-017-04434-w |
| [21] | TIAN F, LUO J, ZHANG H M, et al. MiRNA expression signatures induced by Marek's disease virus infection in chickens[J]. Genomics, 2012, 99(3): 152–159. DOI: 10.1016/j.ygeno.2011.11.004 |
| [22] | AKIYAMA Y, KATO S. Two cell lines from lymphomas of Marek's disease[J]. Biken J, 1974, 17(3): 105–116. |
| [23] | NAZERIAN K. An updated list of avian cell lines and transplantable tumours[J]. Avian Pathol, 1987, 16(3): 527–544. DOI: 10.1080/03079458708436402 |
| [24] | TAN X G, QIN W Y, ZHANG L, et al. A 5-microRNA signature for lung squamous cell carcinoma diagnosis and hsa-miR-31 for prognosis[J]. Clin Cancer Res, 2011, 17(21): 6802–6811. DOI: 10.1158/1078-0432.CCR-11-0419 |
| [25] | MILES G D, SEILER M, RODRIGUEZ L, et al. Identifying microRNA/mRNA dysregulations in ovarian cancer[J]. BMC Res Notes, 2012, 5: 164. DOI: 10.1186/1756-0500-5-164 |
| [26] | SAND M, SKRYGAN M, GEORGAS D, et al. Microarray analysis of microRNA expression in cutaneous squamous cell carcinoma[J]. J Dermatol Sci, 2012, 68(3): 119–126. DOI: 10.1016/j.jdermsci.2012.09.004 |
| [27] | SAND M, SKRYGAN M, SAND D, et al. Expression of microRNAs in basal cell carcinoma[J]. Br J Dermatol, 2012, 167(4): 847–855. DOI: 10.1111/bjd.2012.167.issue-4 |
| [28] | ZOU M X, HUANG W, WANG X B, et al. Identification of miR-140-3p as a marker associated with poor prognosis in spinal chordoma[J]. Int J Clin Exp Pathol, 2014, 7(8): 4877–4885. |
| [29] | TIAN F, LUO J, ZHANG H M, et al. MiRNA expression signatures induced by Marek's disease virus infection in chickens[J]. Genomics, 2012, 99(3): 152–159. DOI: 10.1016/j.ygeno.2011.11.004 |
| [30] | LIAN L, ZHANG D X, WANG Q, et al. The inhibitory effects of gga-miR-199-3p, gga-miR-140-3p, and gga-miR-221-5p in Marek's disease tumorigenesis[J]. Poult Sci, 2015, 94(9): 2131–2135. DOI: 10.3382/ps/pev175 |
| [31] | BURNSIDE J, OUYANG M, ANDERSON A, et al. Deep sequencing of chicken microRNAs[J]. BMC Genomics, 2008, 9: 185. DOI: 10.1186/1471-2164-9-185 |
| [32] | SONG B, WANG Y, XI Y, et al. Mechanism of chemoresistance mediated by miR-140 in human osteosarcoma and colon cancer cells[J]. Oncogene, 2009, 28(46): 4065–4074. DOI: 10.1038/onc.2009.274 |
| [33] | GU R, SUN Y F, WU M F, et al. Biological roles of microRNA-140 in tumor growth, migration, and metastasis of osteosarcoma in vivo and in vitro[J]. Tumour Biol, 2016, 37(1): 353–360. DOI: 10.1007/s13277-015-3801-8 |
| [34] | KONG X M, ZHANG G H, HUO Y K, et al. MicroRNA-140-3p inhibits proliferation, migration and invasion of lung cancer cells by targeting ATP6AP2[J]. Int J Clin Exp Pathol, 2015, 8(10): 12845–12852. |
| [35] | DONG W, YAO C P, TENG X P, et al. MiR-140-3p suppressed cell growth and invasion by downregulating the expression of ATP8A1 in non-small cell lung cancer[J]. Tumour Biol, 2016, 37(3): 2973–2985. DOI: 10.1007/s13277-015-3452-9 |
| [36] | STETLER-STEVENSON W G, AZNAVOORIAN S, LIOTTA L A. Tumor cell interactions with the extracellular matrix during invasion and metastasis[J]. Annu Rev Cell Biol, 1993, 9(1): 541–573. DOI: 10.1146/annurev.cb.09.110193.002545 |
| [37] | SUN J, HEMLER M E. Regulation of MMP-1 and MMP-2 production through CD147/extracellular matrix metalloproteinase inducer interactions[J]. Cancer Res, 2001, 61(5): 2276–2281. |
| [38] | LUO Y, LIANG F B, ZHANG Z Y. PRL1 promotes cell migration and invasion by increasing MMP2 and MMP9 expression through Src and ERK1/2 pathways[J]. Biochemistry, 2009, 48(8): 1838–1846. DOI: 10.1021/bi8020789 |
| [39] | TABOURET E, BOUDOURESQUE F, FARINA P, et al. MMP2 and MMP9 as candidate biomarkers to monitor bevacizumab therapy in high-grade glioma[J]. Neuro Oncol, 2015, 17(8): 1174–1176. DOI: 10.1093/neuonc/nov094 |



