哺乳动物卵母细胞和早期胚胎在体外发育过程中会产生活性氧(reactive oxygen species,ROS),一定浓度的ROS对维持细胞的正常功能至关重要[1]。有研究证明, 将成熟末期的卵母细胞短时间暴露于过氧化氢中会改善胚胎的后续发育能力[2], 但过量的ROS可以导致DNA、蛋白质损伤,脂质过氧化以及线粒体损伤[3-5]。因此,维持ROS动态平衡在胚胎发育中至关重要。胚胎体外生产过程中,常在培养液中添加抗氧化剂,如抗坏血酸、褪黑素、谷胱甘肽等优化培养体系,从而降低胞内ROS,缓解氧化应激对胚胎的伤害,提高胚胎的生产效率和胚胎质量[6]。
谷胱甘肽(glutathione)是由谷氨酸、半胱氨酸和甘氨酸组成的三肽,是体内主要的非酶类抗氧化剂,主要以两种形式存在,即氧化型谷胱甘肽(oxidized glutathione,GSSG)和还原型谷胱甘肽(reduced glutathione,GSH)。GSH占谷胱甘肽总量的99%左右[7],参与细胞的抗氧化应激、DNA和蛋白质合成以及氨基酸的转运[8]。GSH促进雄原核的形成[9]、提高胚胎致密化[10],对早期胚胎发育到囊胚阶段起关键作用。研究表明, 在牛胚胎培养液中添加GSH,可清除胚胎内ROS,提高胚胎的抗氧化能力,从而有效提高囊胚率和囊胚质量[11]。
检测GSH含量的传统方法有分光光度法、电化学方法和荧光分光光度法等,适用于血清、细胞和组织中GSH的测定[12-14]。GSH在水溶液和空气中易氧化,因而传统方法因准确率低和稳定性差而难以广泛应用,且电化学方法中前期电解液及标准液配制复杂[15-16]。对于胚胎中的GSH,最常用的测定方法是荧光染色法,可保证胚胎完整性,避免氧化过程,且简便快速;但该法也存在染色不均、荧光强度衰减等问题[17]。高效液相色谱技术(high performance liquid chromatography,HPLC)也可定量检测胚胎中GSH含量,操作方便,但无法对GSH进行追踪[18]。近年来,稳定同位素标记与液质联用技术的迅速发展,实现了对标靶物质的追踪和定量,且灵敏度高,已被广泛用于医学、食品和动植物检验检疫等领域[19]。用C15和N13标记GSH中的甘氨酸获得稳定同位素标记物GSH-13C215N(GSX),常用于GSH含量的精确测定和代谢研究[20]。
本研究向牛体外受精胚胎的培养液中添加外源GSH,研究其对胚胎发育的影响;采用荧光染色法和液质联用技术(liquid chromatography mass spectrometry,LC/MS)定量分析早期受精卵中GSH的含量,确定LC/MS方法的可靠性;另外,在培养液中添加GSH的同位素标记物GSX,利用LC/MS对受精卵内GSX含量变化进行跟踪,为探索外源GSH到胚胎内的转运机制提供研究方法。
1 材料与方法 1.1 试验材料所用试剂如无特殊说明均购自西格玛奥德里奇(上海)贸易有限公司;公牛精液购自山东奥克斯生物技术有限公司。超声破碎仪Vibra-CellTM购自美国Sonics公司,液相色谱仪LC-20购自日本岛津公司,质谱仪QTRAP®5500 LC/MS购自美国AB SCIEX公司。
1.2 试验方法 1.2.1 牛体外受精胚胎的制备体外受精(in vitro fertilization,IVF)胚胎的生产流程参照文献[11]。用35~38 ℃生理盐水清洗卵巢2~3遍,抽取2~6 mm卵泡中的卵泡液,体式显微镜下挑选带有3层以上卵丘细胞的卵丘-卵母细胞复合体(cumulus-oocyte complexes,COCs),用成熟液(含10% FBS、0.01 IU·mL-1 FSH、1 IU·mL-1 LH、1 μg·mL-1 E2、10 μg·mL-1肝素、40 ng·mL-1 IGF、50 ng·mL-1 EGF的TCM 199培养液)洗涤后,于成熟液中38.5 ℃、100%湿度条件下培养22~24 h。38 ℃水浴解冻冷冻精液,置于含6 mL洗精液(含4 μg·mL-1 BSA、10 mmol·L-1咖啡因的BO液[21])中,1 800 r·min-1离心两次,每次8 min。用洗精液调整精子密度为2×106·mL-1,取50 μL精子悬浮液加入50 μL受精滴(含4 mg·mL-1 BSA的BO液)中。每个受精滴中移入15枚MII期COCs,于38.5℃、100%湿度条件下培养。受精后8 h(hour post-insemination, hpi),脱去卵丘细胞,移入前期培养液(含6 mg·mL-1 BSA、1 mmol·L-1 L-谷氨酰胺、2% EAA、1% NEAA的mCR1培养液)中培养,2 d后移入后期培养液(含10% FBS、6 mg·mL-1 BSA、1 mmol·L-1 L-谷氨酰胺、2% EAA、1% NEAA的mCR1培养液[21])中培养,之后隔天半量换液,培养7~8 d囊胚。从胚胎移入前期培养液开始计算为0 h,分别于48、120和168 h记录卵裂的胚胎数、桑椹胚和囊胚数,计算相应的发育率。其中处理组于前期和后期培养液中添加3 mmol·L-1 GSH或GSX,对照组则不添加GSH或GSX。每组100个胚胎,3个重复。
在胚胎内ROS、GSH和GSX含量分析中,分别收集受精后8、18、24、32 h受精卵用于检测。
1.2.2 牛囊胚细胞染色培养7 d的囊胚用0.1% BSA-PBS洗3遍后,2%多聚甲醛固定1 h,洗3遍后,置于20 μmol·L-1 Hoechst 33342中室温孵育10 min,用0.1% BSA-PBS清洗后压片,荧光显微镜下拍照, 并统计囊胚总细胞数。为防止荧光淬灭,该过程需在避光条件下进行。每组30个胚胎,共3个重复。
1.2.3 牛胚胎内ROS含量的检测采用2, 7-dichlorofluoresccein diacetate(DCFH-DA)荧光染色剂测定胚胎内ROS含量[22]。将8、18、24、32 hpi的受精卵(包括对照组和处理组)在0.1% BSA-PBS中洗3遍,移入50 μmol·L-1的染色液中,避光,38.5 ℃孵育30 min,清洗3遍后,在荧光显微镜(Nikon TE-2000)下观察并拍照,激发光波长为450~490 nm。使用Image J软件(1.47,National Institutes of Health)计算荧光密度。每组10个受精卵,3个重复。
1.2.4 采用荧光染色法检测牛胚胎中GSH含量采用4-chloromethyl-6, 8-difluoro-7-hydroxycoumarin(Cell Tracker Blue CMF2HC,Life Technology)对8、18、24、32 hpi的受精卵(包括对照组和处理组)染色,测定其GSH含量。受精卵用0.1% BSA-PBS清洗3遍后,置于10 μmol·L-1染色液中,38.5 ℃避光孵育15 min后取出,0.1% BSA-PBS清洗3遍后在荧光显微镜观察并拍照,激发光的波长为330~380 nm。使用Image J软件计算荧光密度。
1.2.5 液质联用仪(LC/MS)检测牛受精卵中GSH和GSX的含量检测所用色谱条件:色谱柱HSS T3 (1.7 μm×100 mm× 2.1 mm),流动相A(水,0.1%甲酸)和B(乙腈),流速0.3 mL·min-1,进样量5.0 μL,柱温40 ℃。质谱条件:采用正离子模式,离子源的喷雾电压(ion spray voltage,IS)5 000 V,雾化温度(temperature,TEM)400 ℃,气帘气压力(curtain gas,CUR)30 kPa,碰撞气(collision gas,CAD)Medium,雾化气(ion source gas1,GS1)55 kPa,辅助气(ion source gas2,GS2)55 kPa。
标准曲线绘制:精确称取GSH或GSX适量,用甲醇分别配制浓度为1 mg·mL-1的储备液;甲醇逐级稀释,得到0.125、0.250、0.500、1.250、2.500、5.000 μmol·L-1的系列工作液,上样量为5 μL,得出与标准液浓度相对应的峰面积值,绘制标准曲线。以待测物GSH或GSX浓度为横坐标,以峰面积为纵坐标,用加权最小二乘法进行线性回归。
样品前处理:分别收集8、18、24、32 hpi的受精卵(对照组和处理组),用500 μL冷甲醇破碎提取1 h,加200 μL水混匀后于4 ℃,13 200 r·min-1离心10 min,取上清检测。每组30个受精卵,共3个重复。分别收集处理组受精卵(18、24、32 hpi)的培养液50 μL,加入冷甲醇50 μL,再加50 μL水混匀,4 ℃、13 200 r·min-1离心10 min,取上清检测。每组重复3次。
1.2.6 数据分析囊胚细胞计数使用Photoshop CS5(Adobe,美国)进行,使用SPSS 17.0软件进行单因素方差分析,结果使用“平均值±标准误”表示,P < 0.05表示差异显著。
2 结果 2.1 外源GSH对牛体外受精胚胎发育的影响本研究共使用588枚牛卵母细胞,体外受精后的胚胎分别培养于含GSH(处理组)和不含GSH(对照组)的培养液中,发育至囊胚(表 1)。结果显示,GSH处理组和对照组间胚胎的卵裂率无显著差异(P>0.05),但处理组胚胎的桑椹胚率(58.67% vs. 48.46%)、囊胚率(50.83% vs. 34.88%)以及囊胚总细胞数(102.26 vs. 76.27)均显著高于对照组(P < 0.05),说明体外受精胚胎培养液中添加GSH可提高牛体外受精胚胎的囊胚率和囊胚质量。
|
|
表 1 GSH对牛IVF胚胎体外发育的影响 Table 1 Effects of GSH on the development of bovine IVF embryos |
采用DCFH-DA染色法检测210枚受精卵中ROS的含量(图 1)。处理组18、24和32 hpi受精卵中ROS含量均极显著或显著低于对照组(P < 0.01,P < 0.05),随着培养时间的延长,对照组中受精卵的ROS含量呈先上升后下降,在18 hpi时达最高;处理组受精卵的ROS含量则逐渐降低。可见在培养液中添加GSH可以清除受精卵中ROS。
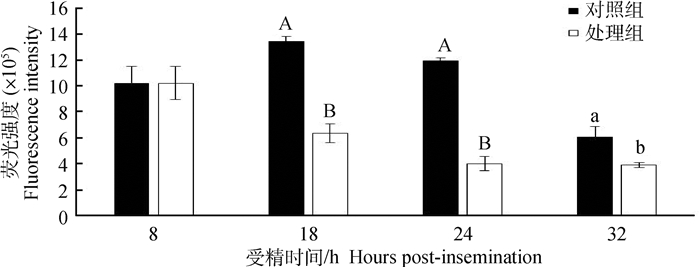
|
处理组和对照组之间所标大写字母相异表示差异极显著(P < 0.01),所标小写字母相异表示差异显著(P < 0.05),所标字母相同表示差异不显著(P>0.05)。下同 Different capital letters mean extremely significant difference (P < 0.01), different small letters mean significant difference (P < 0.05), same letter means no significant difference between the treatment group and the control group (P>0.05).The same as below 图 1 GSH处理对不同发育阶段的牛受精卵中ROS含量的影响 Figure 1 Effects of GSH on the intracellular ROS contents in bovine zygotes at different stages |
通过LC/MS法分别检测梯度稀释的GSH或GSX标准液,获得GSH的线性回归方程y=6.67e+5x(e表示10的科学计数、x表示GSH浓度、y表示峰面积),相关系数r=0.999 7,并绘制标准曲线(图 2A);GSX的线性回归方程为y=1.14e+6x(e表示10的科学计数、x表示GSX浓度、y表示峰面积),相关系数r=0.999 5,其标准曲线见图 2B。GSH在0.125~5.000 μmol·L-1线性关系良好,保留时间为2.04 min;GSX在0.125~5.000 μmol·L-1线性关系良好,保留时间为2.05 min(图 2C)。
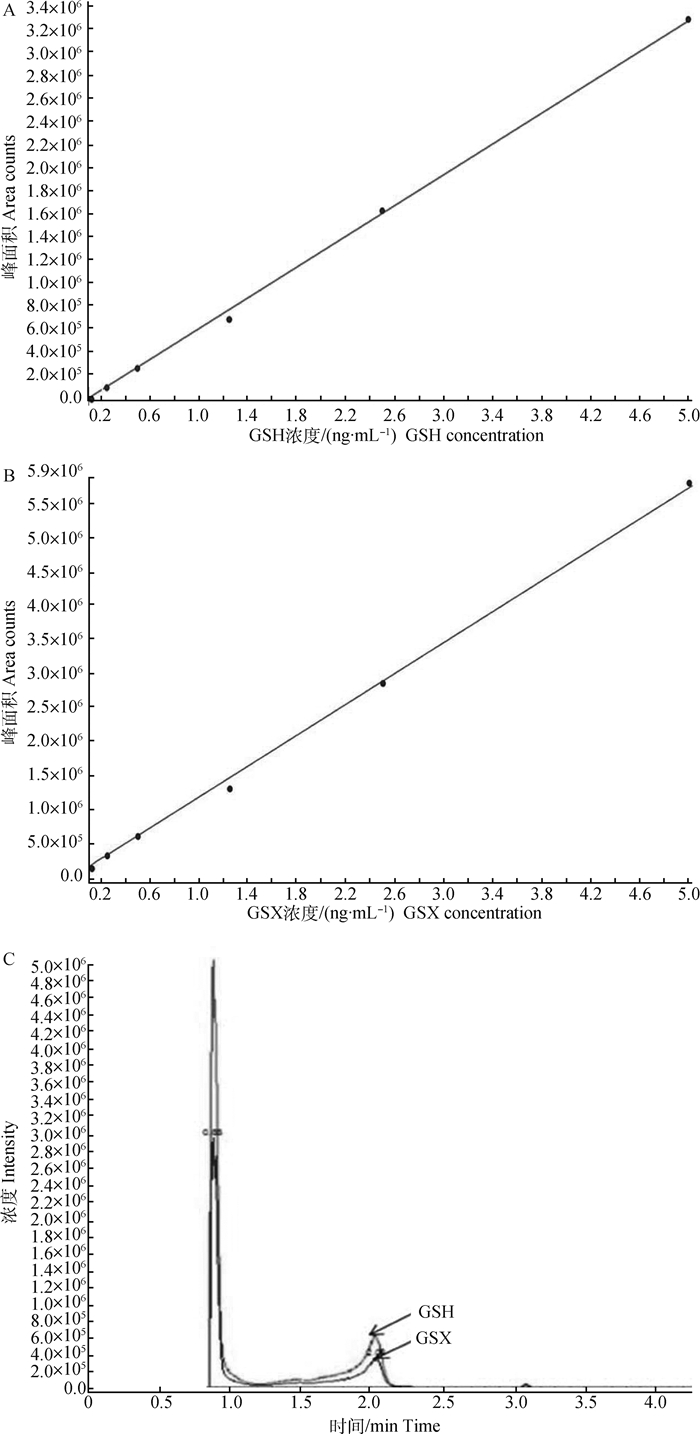
|
图 2 LC/MS法测定GSH(A)和GSX(B)的标准曲线及图谱(C) Figure 2 Standard curves of GSH(A)and GSX(B) by LC/MS and their chromatograms (C) |
210枚受精卵培养于含外源GSH的培养液中,采用荧光染色法对受精卵中GSH的含量进行检测(图 3A)。在18、24和32 hpi 3个发育阶段,处理组受精卵的GSH含量显著高于对照组(P < 0.05)。结果表明,培养液中添加GSH可以提高受精卵内GSH的含量。
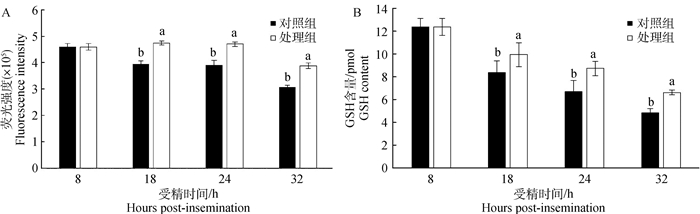
|
图 3 荧光染色法和液质联用法检测不同发育阶段牛受精卵的GSH含量 Figure 3 Intracellular GSH levels in bovine zygotes at different stages by fluorescence staining and LC/MS |
通过LC/MS法检测相同处理的630枚受精卵的GSH含量,结果表明,3个发育阶段的处理组受精卵的GSH含量均显著高于对照组(图 3B)(P < 0.05)。另外,各组中受精卵的GSH含量随体外培养时间的延长均呈现下降趋势,对照组中18 hpi受精卵中GSH含量从8.37 pmol降到32 hpi的4.84 pmol,而处理组受精卵中GSH含量则从9.93 pmol降低到6.63 pmol。比较GSH含量的两种测定方法,结果一致。
2.5 外源GSX对牛受精卵内GSH、GSX及培养液中GSX含量的影响经过外源GSX处理的受精卵,采用LC/MS方法对其GSX和GSH含量进行检测(图 4),处理组受精卵中GSH含量随着发育时间的延长呈下降趋势;但均显著高于各阶段(18、24、32 hpi)的对照组受精卵(P < 0.05,图 4A)。处理组的受精卵中均检测到GSX的存在,受精卵GSX含量维持在0.9~1.3 pmol(图 4B)。随培养时间的延长,培养液中GSX的浓度呈显著下降趋势(P < 0.05,图 5)。可见培养液添加GSX后,受精卵内GSX含量较低,但内源GSH的含量显著升高;同时培养液中GSX浓度随培养时间延长大幅下降。
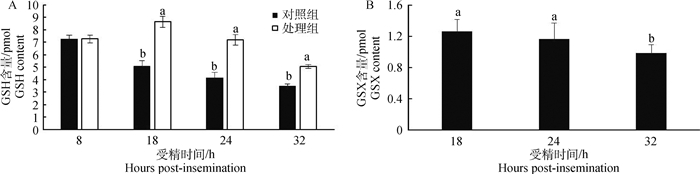
|
A.GSH; B.GSX 图 4 外源GSX对不同发育阶段牛受精卵内GSH和GSX含量的影响 Figure 4 Effect of exogenous GSX on intracellular GSH and GSX levels in bovine zygotes at different stages |
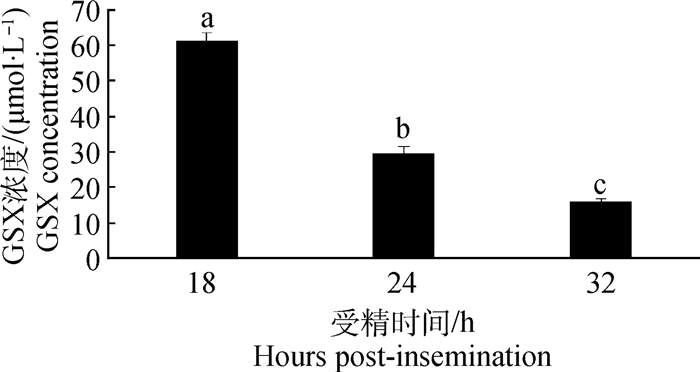
|
图 5 牛受精卵培养液中外源GSX的浓度变化 Figure 5 Concentration of exogenous GSX in culture media of bovine zygotes at different stages |
GSH是哺乳动物卵母细胞和胚胎中主要的非酶类抗活性氧防御系统之一。众多的抗氧化物质如维生素C、褪黑素、半胱胺、巯基乙醇和N-乙酰-L-半胱氨酸中,关于GSH的抗氧化作用研究较多[23],且褪黑素、维生素C和小分子硫醇类抗氧化剂均能诱导卵母细胞中GSH的合成,提高胚胎内GSH含量,从而发挥其抗氧化的功能[24-26]。研究证明,猪和水牛卵母细胞的成熟液中添加GSH,可提高体外成熟率,减少胚胎发育中的个体差异[27-28]。此外,内源GSH可减轻过氧化氢对小鼠2-细胞胚胎发育的阻滞作用,提高小鼠孤雌胚胎的囊胚率和孵化率,显著降低线粒体空泡及细胞凋亡现象[29]。卵母细胞中高浓度的GSH对合子中雄原核的形成和植入前胚胎的发育也具有重要作用[30]。在卵母细胞成熟过程中,包裹其外周的卵丘细胞在GSH合成中发挥关键作用;而在体外受精胚胎的培养过程中,脱去卵丘的受精卵自身无法合成GSH,因而植入前早期胚胎中的GSH含量显著降低[31]。本研究在牛体外受精胚胎的培养液中添加3 mmol·L-1 GSH或GSX(同位素标记的GSH)以补偿胚胎中GSH的消耗,结果胚胎的囊胚率和囊胚质量显著提高,与本课题组前期的研究结果一致[11]。
早期胚胎在发育过程中会经历特定的ROS释放过程,并维持动态平衡[32]。在卵母细胞受精和胚胎第一次卵裂时ROS的含量增加,耗氧量呈先上升后下降趋势;在7~11 hpi ROS到达峰值,22~25 hpi出现较小幅度的波动[33]。在本研究中,18 hpi时雌雄原核形成产生大量ROS,受精卵处于第一个细胞周期的S期,胚胎细胞进行DNA的合成,ROS水平显著升高,与小鼠3T3细胞的观察结果一致[34]。随后ROS含量呈下降趋势;32 hpi时胚胎处于2-细胞期,ROS含量降低。随着受精的完成,胚胎内发生一系列的细胞分裂过程,GSH含量也随着分裂进程而逐渐降低,到囊胚阶段GSH含量约为卵母细胞中的1/10[35]。斑马鱼胚胎在30 hpi内,GSH含量呈显著下降趋势[22]。牛受精卵中GSH含量低于8-~16-细胞胚胎,此时更易受到氧化应激的影响[36]。本研究利用荧光染色法和LC/MS法检测了受精后至第1次分裂期的胚胎内GSH含量的变化,该过程中GSH含量也呈现下降趋势。
利用HPLC技术对斑马鱼胚胎发育过程中GSH含量的动态变化进行分析,从而确定了其氧化还原状态的变化趋势[18];但该方法灵敏度低,只能到纳克级的水平[37]。随着质谱技术的发展,液质联用逐渐成为物质定量分析技术的主流[38-40]。液质联用将色谱对复杂样品的高分离能力,与质谱的高选择性、高灵敏度及能够提供相对分子质量与结构信息的优点相结合,在某些药物、食品分析和代谢物质分析等许多领域得到了广泛的应用,实现了对牛胚胎内、外GSH含量的精确测定[41]。GSX是用稳定同位素13C和15N标记甘氨酸残基的GSH(GSH-13C215N),常在液质联用基础上用于GSH的示踪和定量分析研究,例如抗利什曼虫药(antileishmanial)2-取代喹啉在人和大鼠肝细胞中的代谢分析[42];职业性诱喘物二苯基甲烷二异氰酸酯(methylene diphenyl diisocyanate)的生物转化研究[43];另外,还被用于捕捉活性代谢物[44]。本研究使用相同浓度的GSX代替GSH处理牛胚胎,根据LC/MS结果,GSH和GSX在测定范围内线性关系较好,色谱图峰型良好,保留时间分别在2.04和2.05 min,说明该法专属性强,可定量分析胚胎内GSH的变化,也可用于外源GSH促进胚胎发育的机理研究。在0.88 min出现干扰峰,可能是因为受精卵中存在与GSH和GSX结构相似的物质。LC/MS还用于检测血浆中儿茶酚胺、羟基红花黄色素以及单胺类神经递质等,灵敏准确、特异性强[45-47]。
完整的GSH并不能被动物细胞直接吸收,而外源GSH在淋巴细胞的跨膜转运的过程中,GSH会被细胞膜上的GGT分解成γ-半胱氨酸甘氨酸和γ-谷氨酰胺,通过γ-谷氨酰循环进行跨膜转运到细胞内,然后重新合成GSH并发挥作用[48]。也有研究发现在小肠刷状缘膜囊泡中,完整的GSH可以通过双侧膜和基底膜进入肠细胞,不需要GGT参与[49]。然而,外源GSH进入胚胎的途径仍不清楚,因此,笔者利用GSH同位素标记物GSX处理胚胎,通过LC/MS技术跟踪胚胎内、外GSX的变化,发现添加GSX的处理组胚胎中GSH含量大幅增长,而GSX含量却在0.9~1.3 pmol,相当于GSH含量的1/5,可见完整GSH直接进入胚胎内的可能性较小,可能是通过γ-谷氨酰循环途径进入胚胎,这仍需要进一步的研究证实。
4 结论本研究通过在牛体外受精胚胎培养液中添加GSH,降低了胚胎内ROS含量,提高了胚胎的体外发育率和胚胎质量;采用荧光染色法和LC/MS方法检测受精卵中GSH含量,证明了LC/MS检测GSH方法的可行性、特异性和准确性;另外,用LC/MS方法追踪了GSH的同位素标记物GSX在不同发育阶段受精卵中的变化,为后期探索外源GSH到胚胎内的转运途径提供研究思路。
| [1] | ANATHY V, ROBERSON E, CUNNIFF B, et al. Oxidative processing of latent Fas in the endoplasmic reticulum controls the strength of apoptosis[J]. Mol Cell Biol, 2012, 32(17): 3464–3478. DOI: 10.1128/MCB.00125-12 |
| [2] | VANDAELE L, THYS M, BIJTTEBIER J, et al. Short-term exposure to hydrogen peroxide during oocyte maturation improves bovine embryo development[J]. Reproduction, 2010, 139(3): 505–511. DOI: 10.1530/REP-09-0430 |
| [3] | BAIN N T, MADAN P, BETTS D H. The early embryo response to intracellular reactive oxygen species is developmentally regulated[J]. Reprod Fert Dev, 2011, 23(4): 561–575. DOI: 10.1071/RD10148 |
| [4] | YANG H W, HWANG K J, KWON H C, et al. Detection of reactive oxygen species (ROS) and apoptosis in human fragmented embryos[J]. Hum Reprod, 1998, 13(4): 998–1002. DOI: 10.1093/humrep/13.4.998 |
| [5] | FAVETTA L A, JOHN E J S, KING W A, et al. High levels of p66shc and intracellular ROS in permanently arrested early embryos[J]. Free Rad Biol Med, 2007, 42(8): 1201–1210. DOI: 10.1016/j.freeradbiomed.2007.01.018 |
| [6] | SOVERNIGO T C, ADONA P R, MONZANI P S, et al. Effects of supplementation of medium with different antioxidants during in vitro maturation of bovine oocytes on subsequent embryo production[J]. Reprod Domest Anim, 2017, 52(4): 561–569. DOI: 10.1111/rda.2017.52.issue-4 |
| [7] | AKERBOOM T P M, BILZER M, SIES H. The relationship of biliary glutathione disulfide efflux and intracellular glutathione disulfide content in perfused rat liver[J]. J Biol Chem, 1982, 257(8): 4248–4252. |
| [8] | MEISTER A, TATE S S. Glutathione and related γ-glutamyl compounds:Biosynthesis and utilization[J]. Annu Rev Biochem, 1976, 45: 559–604. DOI: 10.1146/annurev.bi.45.070176.003015 |
| [9] | ZUELKE K A, JEFFAY S C, ZUCKER R M, et al. Glutathione (GSH) concentrations vary with the cell cycle in maturing hamster oocytes, zygotes, and pre-implantation stage embryos[J]. Mol Reprod Dev, 2003, 64(1): 106–112. DOI: 10.1002/(ISSN)1098-2795 |
| [10] | PERREAULT S D, BARBEE R R, SLOTT V L. Importance of glutathione in the acquisition and maintenance of sperm nuclear decondensing activity in maturing hamster oocytes[J]. Dev Biol, 1988, 125(1): 181–186. DOI: 10.1016/0012-1606(88)90070-X |
| [11] | SUN W J, PANG Y W, LIU Y, et al. Exogenous glutathione supplementation in culture medium improves the bovine embryo development after in vitro fertilization[J]. Theriogenology, 2015, 84(5): 716–723. DOI: 10.1016/j.theriogenology.2015.05.001 |
| [12] | MOKRASCH L C, TESCHKE E J. Glutathione content of cultured cells and rodent brain regions:A specific fluorometric assay[J]. Anal Biochem, 1984, 140(2): 506–509. DOI: 10.1016/0003-2697(84)90201-X |
| [13] |
曹新志, 魏明. 应用OPA柱前衍生法测定西红柿中的谷胱甘肽[J]. 食品科学, 1998, 19(4): 52–55.
CAO X Z, WEI M. Determination of glutathione in tomatoes by OPA pre-column derivation[J]. Food Science, 1998, 19(4): 52–55. (in Chinese) |
| [14] |
黄彦生, 智艳芳, 孔沈燕, 等. 用荧光分光光度法测量冠心病患者血浆谷胱甘肽[J]. 光谱学与光谱分析, 2006, 26(5): 936–940.
HUANG Y S, ZHI Y F, KONG S Y, et al. Plasma glutathione of patients with coronary heart disease measured by fluorospectrophotometer[J]. Spectroscopy and Spectral Analysis, 2006, 26(5): 936–940. DOI: 10.3321/j.issn:1000-0593.2006.05.038 (in Chinese) |
| [15] |
廖飞, 杨晓, 康格非, 等. 紫外吸收碘量法测定微量维生素C和还原型谷胱甘肽[J]. 重庆医科大学学报, 2003, 28(3): 372–373.
LIAO F, YANG X, KANG G F, et al. Trace vitamin C and glutathione was determined by iodometry with UV absorbance[J]. Journal of Chongqing Medical University, 2003, 28(3): 372–373. DOI: 10.3969/j.issn.0253-3626.2003.03.038 (in Chinese) |
| [16] |
张静, 吉宏武, 周静, 等. 荧光分光光度法测定海洋生物中的谷胱甘肽[J]. 广东海洋大学学报, 2005, 25(4): 32–34.
ZHANG J, JI H W, ZHOU J, et al. Determining glutathione in several marine organisms by fluorescence spectrophotometetry[J]. Journal of Zhanjiang Ocean University, 2005, 25(4): 32–34. DOI: 10.3969/j.issn.1673-9159.2005.04.008 (in Chinese) |
| [17] |
范崇东, 王淼, 卫功元, 等. 谷胱甘肽测定方法研究进展[J]. 生物技术, 2004, 14(1): 68–70.
FAN C D, WANG M, WEI G Y, et al. Research progress on the determination of glutathione[J]. Biotechnology, 2004, 14(1): 68–70. (in Chinese) |
| [18] | TIMME-LARAGY A R, GOLDSTONE J V, IMHOFF B R, et al. Glutathione redox dynamics and expression of glutathione-related genes in the developing embryo[J]. Free Rad Biol Med, 2013, 65: 89–101. DOI: 10.1016/j.freeradbiomed.2013.06.011 |
| [19] |
朱亚玲.枸杞中谷胱甘肽的含量测定研究[D].延吉: 延边大学, 2010.
ZHU Y L.Determination of glutathione in Lycium barbarum L[D].Yanji: Yanbian University, 2010.(in Chinese) http://cdmd.cnki.com.cn/Article/CDMD-10184-2010132481.htm |
| [20] |
钟华, 张慧, 许海平. 谷胱甘肽的测定方法进展[J]. 氨基酸和生物资源, 2014, 36(1): 23–26.
ZHONG H, ZHANG H, XU H P. Progress on determination of glutathione[J]. Amino Acids & Biotic Resources, 2014, 36(1): 23–26. (in Chinese) |
| [21] |
郭芹芹, 李凤, 孙尉峻, 等. 牛体外受精胚胎抗氧化相关的长链非编码RNA表达谱[J]. 畜牧兽医学报, 2017, 48(7): 1229–1240.
GUO Q Q, LI F, SUN W J, et al. Long non-coding RNA profiling in bovine embryos treated with glutathione during in vitro culture[J]. Acta Veterinaria et Zootechnica Sinica, 2017, 48(7): 1229–1240. (in Chinese) |
| [22] | OZAWA M, HIRABAYASHI M, KANAI Y. Developmental competence and oxidative state of mouse zygotes heat-stressed maternally or in vitro[J]. Reproduction, 2002, 124(5): 683–689. DOI: 10.1530/rep.0.1240683 |
| [23] | FORMAN H J. Glutathione-from antioxidant to post-translational modifier[J]. Arch Biochem Biophys, 2016, 595: 64–67. DOI: 10.1016/j.abb.2015.11.019 |
| [24] | HIDAKA T, FUKUMOTO Y, YAMAMOTO S, et al. Variations in bovine embryo production between individual donors for OPU-IVF are closely related to glutathione concentrations in oocytes during in vitro maturation[J]. Theriogenology, 2018, 113: 176–182. DOI: 10.1016/j.theriogenology.2018.03.002 |
| [25] | YANG M H, TAO J L, CHAI M L, et al. Melatonin improves the quality of inferior bovine oocytes and promoted their subsequent IVF embryo development:Mechanisms and results[J]. Molecules, 2017, 22(12): 2059. DOI: 10.3390/molecules22122059 |
| [26] | XIANG Q Q, XU B F, DING Y L, et al. Oxidative stress response induced by Butachlor in Zebrafish Embryo/Larvae:The protective effect of vitamin C[J]. Bull Environ Contam Toxicol, 2018, 100(2): 208–215. DOI: 10.1007/s00128-017-2245-9 |
| [27] | JEON Y, YOON J D, CAI L, et al. Supplementation of zinc on oocyte in vitro maturation improves preimplatation embryonic development in pigs[J]. Theriogenology, 2014, 82(6): 866–874. DOI: 10.1016/j.theriogenology.2014.06.021 |
| [28] |
韩岩, 尹多, 张涛, 等. 谷胱甘肽对卵母细胞成熟及胚胎发育影响的研究进展[J]. 江苏农业科学, 2010(3): 269–270.
HAN Y, YIN D, ZHANG T, et al. Research progress on effects of glutathione on maturation and embryo development of oocyte[J]. Jiangsu Agricultural Sciences, 2010(3): 269–270. DOI: 10.3969/j.issn.1002-1302.2010.03.108 (in Chinese) |
| [29] |
余朝阳.抗氧化剂对小鼠孤雌胚胎体外发育的影响[D].唐山: 华北理工大学, 2017.
YU Z Y.Effects of antioxidants on the mouse parthenogenetic embryo development in vitro[D].Tangshan: North China University of Science and Technology, 2017. http://cdmd.cnki.com.cn/Article/CDMD-10081-1017741568.htm |
| [30] | DE MATOS D G, FURNUS C C. The importance of having high glutathione (GSH) level after bovine in vitro maturation on embryo development:Effect of β-mercaptoethanol, cysteine and cystine[J]. Theriogenology, 2000, 53(3): 761–771. DOI: 10.1016/S0093-691X(99)00278-2 |
| [31] | GARDINER C S, REED D J. Glutathione redox cycle-driven recovery of reduced glutathione after oxidation by tertiary-butyl hydroperoxide in preimplantation mouse embryos[J]. Arch Biochem Biophys, 1995, 321(1): 6–12. |
| [32] | SIRISTATIDIS C, VOGIATZI P, VAROUNIS C, et al. The effect of reactive oxygen species on embryo quality in IVF[J]. In vivo, 2016, 30(2): 149–153. |
| [33] | LOPES A S, LANE M, THOMPSON J G. Oxygen consumption and ROS production are increased at the time of fertilization and cell cleavage in bovine zygotes[J]. Hum Reprod, 2010, 25(11): 2762–2773. DOI: 10.1093/humrep/deq221 |
| [34] | TAKAHASHI Y, OGRA Y, SUZUKI K T. Synchronized generation of reactive oxygen species with the cell cycle[J]. Life Sci, 2004, 75(3): 301–311. DOI: 10.1016/j.lfs.2003.12.014 |
| [35] | GARDINER C S, REED D J. Synthesis of glutathione in the preimplantation mouse embryo[J]. Arch Biochem Biophys, 1995, 318(1): 30–36. |
| [36] | MORALES H, TILQUIN P, REES J F, et al. Pyruvate prevents peroxide-induced injury of in vitro preimplantation bovine embryos[J]. Mol Reprod Dev, 1999, 52(2): 149–157. DOI: 10.1002/(ISSN)1098-2795 |
| [37] |
樊跃平, 于健春, 余跃, 等. 谷胱甘肽的生理意义及其各种测定方法比较、评价[J]. 中国临床营养杂志, 2003, 11(2): 136–139.
FAN Y P, YU J C, YU Y, et al. Physiological and clinical significance of glutathione and evaluation of various measurements of glutathione[J]. Chinese Journal of Clinical Nutrition, 2003, 11(2): 136–139. DOI: 10.3760/cma.j.issn.1674-635X.2003.02.017 (in Chinese) |
| [38] |
黎奔, 麦葭沂, 钟玲, 等. LC-MS/MS法测定大鼠血浆中的野黄芩苷含量[J]. 现代生物医学进展, 2014, 14(28): 5414–5418, 5450.
LI B, MAI J Y, ZHONG L, et al. Determination of scutellarin in rat plasma by LC-MS/MS[J]. Progress in Modern Biomedicine, 2014, 14(28): 5414–5418, 5450. (in Chinese) |
| [39] |
李武超, 刘敏臣, 马小琼, 等. LC-MS/MS法测定去甲斑蝥素在大鼠体内的组织分布[J]. 北京中医药大学学报, 2014, 37(4): 263–268.
LI W C, LIU M C, MA X Q, et al. Distribution of norcantharidin determined by LC-MS/MS in rat tissues[J]. Journal of Beijing University of Traditional Chinese Medicine, 2014, 37(4): 263–268. DOI: 10.3969/j.issn.1006-2157.2014.04.012 (in Chinese) |
| [40] |
张帆.应用LC-MS/MS分析抗生素在斑马鱼体内的吸收研究[D].北京: 北京协和医学院, 2014.
ZHANG F.Antibiotic absorption in zebrafish using liquid chromatography-tandam mass spectrometry[D].Beijing: Peking Union Medical College, 2014.(in Chinese) http://cdmd.cnki.com.cn/article/cdmd-10023-1015515222.htm |
| [41] | LI A P, HO M C D, AMARAL K, et al. A novel in vitro experimental system for the evaluation of drug metabolism:Cofactor-supplemented permeabilized cryopreserved human hepatocytes (MetMaxTM Cryopreserved Human Hepatocytes)[J]. Drug Metab Dispos, 2018. DOI: 10.1124/dmd.117.079657 |
| [42] | DESRIVOT J, EDLUND P O, SVENSSON R, et al. Metabolism of 2-substituted quinolines with antileishmanial activity studied in vitro with liver microsomes, hepatocytes and recombinantly expressed enzymes analyzed by LC/MS[J]. Toxicology, 2007, 235(1-2): 27–38. DOI: 10.1016/j.tox.2007.03.003 |
| [43] | WISNEWSKI A V, LIU J. Immunochemical detection of the occupational allergen, methylene diphenyl diisocyanate (MDI), in situ[J]. J Immunol Methods, 2016, 429: 60–65. DOI: 10.1016/j.jim.2015.12.008 |
| [44] | HUANG K, HUANG L Y, VAN BREEMEN R B. Detection of reactive metabolites using isotope-labeled glutathione trapping and simultaneous neutral loss and precursor ion scanning with ultra-high-pressure liquid chromatography triple quadruple mass spectrometry[J]. Anal Chem, 2015, 87(7): 3646–3654. DOI: 10.1021/ac504737x |
| [45] | BERGMANN M L, SADJADI S, SCHMEDES A. Analysis of catecholamines in urine by unique LC/MS suitable ion-pairing chromatography[J]. J Chromatogr B, 2017, 1057: 118–123. DOI: 10.1016/j.jchromb.2017.04.011 |
| [46] |
李长印, 储继红, 张军, 等. LC-MS/MS法测定人血浆中羟基红花黄色素A的浓度[J]. 中国药理学通报, 2014, 30(10): 1402–1407.
LI C Y, CHU J H, ZHANG J, et al. Determination of hydroxysafflor yellow A in human plasma by LC-MS/MS analysis[J]. Chinese Pharmacological Bulletin, 2014, 30(10): 1402–1407. DOI: 10.3969/j.issn.1001-1978.2014.10.016 (in Chinese) |
| [47] | GRECO S, DANYSZ W, ZIVKOVIC A, et al. Microdialysate analysis of monoamine neurotransmitters-a versatile and sensitive LC-MS/MS method[J]. Anal Chim Acta, 2013, 771: 65–72. DOI: 10.1016/j.aca.2013.02.004 |
| [48] | JENSEN G L, MEISTER A. Radioprotection of human lymphoid cells by exogenously supplied glutathione is mediated by gamma-glutamyl transpeptidase[J]. Proc Natl Acad Sci U S A, 1983, 80(15): 4714–4717. DOI: 10.1073/pnas.80.15.4714 |
| [49] | LASH L H, JONES D P. Renal glutathione transport:characteristics of the sodium-dependent system in the basal-lateral membrane[J]. J Biol Chem, 1984, 259(23): 14508–14514. |



