2. Hunan Provincial Institute of Emergency Medicine, Hunan Provincial Key Laboratory of Emergency and Critical Care Metabonomics
Acute kidney injury (AKI) continues to be a global public health concern due to it's high morbidity, increased costs, and high mortality, with about 13.3 million patients and 1.7 million deaths per year[1].So far, other than dialysis, there is no other reliable therapeutic intervention that can improve survival. A number of preparations have been shown for their protective effects, including some clinical drugs, herbs, cytokines, growth factors and et al. However, none of them has been successfully applied in clinic[2].AKI is mainly characterized by rapid loss of renal function due to damage of renal tubular epithelial cells, thus, the regeneration of damaged renal tubular epithelial cells by transplanting of stem cells could be a promising therapeutic strategy. Over the past few years, several studies have shown that the bone marrow-derived mesenchymal stem cells (MSCs) transplantation can attenuate the ischemia kidney injury[3].However, aftter being transplanted into the ischemia kidney, MSCs face a complex environment with numerous toxic factors such as the local hypoxia, the oxidative stress, and the inflammation that can result in low cell survival and thus reduces the therapeutic effects[4].
We hypothesize that factors in the AKI environment dynamically change over time of kidney reperfusion, and there should be an optimal time point for the growth of transplanted MSCs. In this research, the proliferation and differentiation of MSCs were observed by cell culture in vitro with different kidney injury microenvironment conditions, and the optimal time was discussed.
Methods Experimental animalsThe Male specific-pathogen-free(SPF) Sprague-Dawley rats weighting 220~300g were purchased from SJA Laboratory Animal Co., Ltd. (Changsha, China). The rats were housed under specific-pathogen-free conditions of normal room light (12/12h day/night cycle) with free access to food and water and at the room temperature (23±2℃). All procedures performed were reviewed and approved by the Institutional Animal Ethics Committee and conformed to the Guidelines of Laboratory Animal Care and Use Committee of Hunan Normal University (Changsha, China).
Surgical procedure to induce kidney ischemia/reperfusion (I/R) injuryAnimal kidney I/R models were made for AKI[5].Rats were anesthetized with an intraperitoneal injection of 40mg/kg pentobarbital sodium. After medial laparotomy, bilateral kidney arteries and veins were clipped by aneurysm clips for 45 min till the color of kidneys turn to dark. Then the clips were removed, the kidneys were reperfused till the color turn to red. At 0h, 12h, 24h, 48h, 96h following the initiation of reperfusion, bilateral kidneys were excised and used to prepare homogenate.
Preparation of kidney homogenateAfter experimental model were successfully established, kidneys were removed at different reperfusion times. One kidney was used for qRT-PCR preparation, the other one on the opposite side was used for homogenate referring to previous reports[6].Cortices were obtained and cut into pieces on a clean bench after weighing. Homogenization extraction was obtained by using a tissue homogenizer in an ice bath. Mix kidney homogenate with PBS to make 10% kidney homogenate.The homogenate was centrifuged at 12 000r/min for 15 min at 4℃ environment.The supernatants was passed through a 0.22μm filter to remove debris and bacterium, and stored at -70℃.
Isolation and Charaterization of MSCRat bone marrow MSCs were isolated from 7 weeks old Sprague-Dawley rat femurs as previously described[7].Briefly, donor rats were sacrificed and femurs were aseptically dissected out. The edge of each femur was cut, and Dulbecco's Modified Eagel's Medium (DMEM; Gibco) containing 10% FBS plus penicillin/streptomycin was injected into the bone marrow using a 25G syringe. Bone marrow cells were flushed from the shaft of the bone with DMEM and then filtered through a 40μm Cell Strainer to produce the single cell suspension. Mononuclear cells were isolated by ficoll-Paque (sigma) density-gradient centrifugation from filtered bone marrow cells. Cells were then seeded into cell culture flask in DMEM containing 10% FBS and penicillin/streptomycin. 48h post-seeding, nonadherent cells were removed and the medium was replaced with fresh medium every 48h. The MSCs were harvested with 0.25% trypsin and 0.02% EDTA and passaged when MSCs approached 80% confluence. The MSC at passage 4~6 were used for the in vitro experiments. The expression of MSC surface markers was ananlyzed by flow cytometry. Anti-CD-34-APC, anti-CD45-PE, anti-CD29-APC and nti-CD90-PE were purchased from BD. The pluripotency of MSCs for differentiation into osteoblasts was verified using previously reported methods[8].
MSC cultureThe culture medium of MSC was low-glucose DMEM (Gibco) supplemented with 10%FBS (Invitrogen), penicillin (100U/mL, Invitrogen) and streptomycin (100μg/mL, Invitrogen). The MSC were maintained at 37℃ in a humidified 5% CO2 incubator. The medium was replaced with fresh medium every 48h. When cells reached 80% of confluence, The MSCs were harvested with 0.25% trypsin and 0.02% EDTA and passaged. Cells were cryopreserved at early (< P3) passages. Following thawing in a water-bath at 37℃, cells were centrifuged and resuspended in preheated DMEM culture medium. MSCs were expanded and used only before passage P5.
Analysis of MSCs proliferationThe proliferation inspection was performed by CCK8 assay and cell number counting[9].Briefly, the MSCs were inoculated into 96-well plates or 6-well plates. Following cell confluence, the medium was replaced with medium containing 10% kidney homogenate undergoing 0, 12, 24, 48, 72, 96h reperfusion treatment, and medium without containing homogenate was used as controls. After incubation at 37℃ in a humidified 5% CO2 incubator for different time points, the proliferation of MSCs in 96 well plates was detected by CCK8 assay kit according to the manufacturer's protocol (Dojin Laboratorie), MSCs in 6 well plates were digested into single cell suspension and counted using a blood counting chamber.
Analysis of MSCs differentiationTwo methods were used to analyze the differentiation of MSCs.First, changes in cell shape were observed with an inverted biomicroscope (Leica DMI3000B) on the 1st, 7th and 15th day.Secondly, expression of CK-18 was detected to reflect the differentiation of MSCs on the 1st, 7th and 15th day of cell culture.
Quantitative RT-PCR and primersTotal RNA from kidney tissue or MSCs was isolated using the Trizol reagent (Invitrogen, USA). 1μg of RNA was then reversely transcribed into complementary DNA (cDNA) using the PrimeScriptTM RT Reagent Kit (TaKaRa, Japan) according the manufacturer's instructions and previous reports[10].The qRT-PCR was performed to measure the expression of CK18 in MSCs with a Stepone Plus Real-Time PCR system (ABI, USA) by TB Green® Fast qPCR Mix kit (Takara, Japan). GAPDH was used as an endogenous control. The sequence of forward and reverse primers for CK18 and GAPDH were as follow:CK18 Forward primer:5'-AATCAGGGACGCTGAGACCACA-3' and CK18 reverse primer: 5'-GCTCCATCTGTGCCTTGTATCG-3'; GAPDH Forward primer:5'-CATCACTGCCACCCAGAAGACTG-3' and GAPDH reverse primer:5'-ATGCCAGTGAGCTTCCCGTTCAG-3'. Each sample was tested three times, and the CK18 levels were normalized against GAPDH.
Statistical analysisAll experiments were conducted at least for three times. Data were presented as the mean±standard deviation.One-way analysis of variance (ANOVA) was used to compare the results among the groups. LSD used for multiple comparisons if equal variances assumed, and Dunnett's used if it not assumed. A p-value of less than 0.05 was considered significant. All statistical analyses were conducted with IBM SPSS Statistics, version22.0 (SPSS, Chicago, IL).
RESULTS MSCs identification Expression of cell surface markersFlow cytometry was used to characterize the expression of cell surface markers in the MSCs.Results showed that cell surface markers expressed by MSCs were positive for CD29, CD90, which was 99.3% and 96.2% respectively, and negative for CD34, CD45, which was 0.170% and 0.036% respectively (Figure 1).
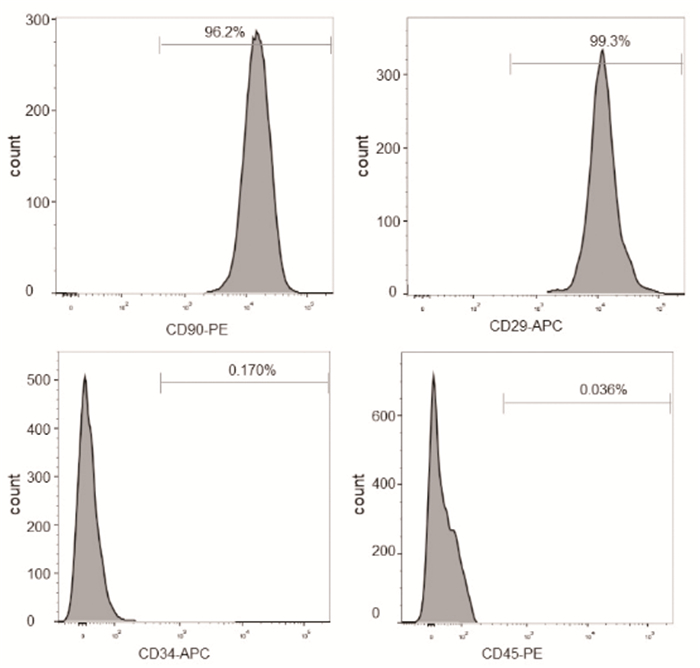
|
Figure 1 Cell surface markers expressed by isolated MSCs Osteogenic differentiation |
After 2 weeks of osteogenic orientation culture of the isolated MSCs, results showed that the mineralization of calcium was good, and the ALP staining was dark purple (Figure 2).
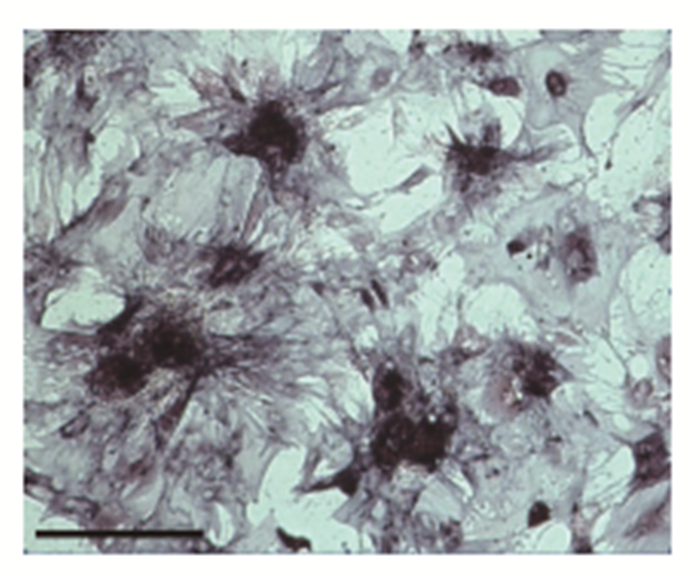
|
Figure 2 Osteogenic differentiation of MSCs(bar=100um) |
Number of MSCs of each group had been increasing from 1st day to 7th day of culture. Comparing within groups, there was no significantly difference on 1st day, (F=0.65, P=0.69), But significant differences were shown from 3rd to 7th day (F=10.27, P=0.00 at 3rd day, F=20.39, P=0.00 at 5th day, F=26.37, P=0.00 at 7th day, respectively). Cell numbers of reperfusion groups were lower, comparing with controls, especially in 24h reperfusion group which showed the lowest number. Comparatively, the cell number of 72h reperfusion group was much closer to controls(Figure 3, Table 1).
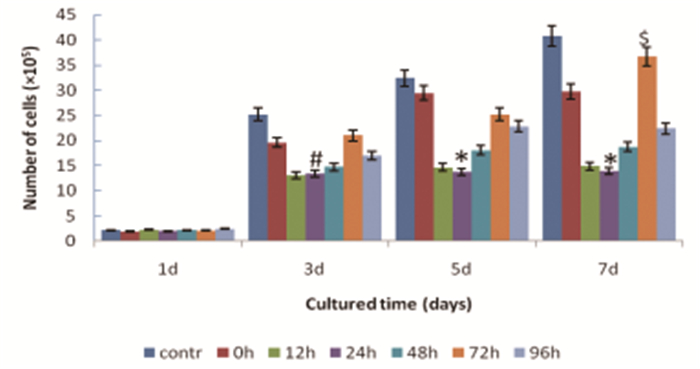
|
Figure 3 Proliferation of MSCs from 1st to 7th cultured day in different groups.*Denotes statistically significant difference comparing with all other groups, P < 0.01.#Significant difference comparing with control group, P < 0.05.$No significant difference comparing with control group, P>0.05.contr:control group, 0h: reperfusion at 0h group, 12h: reperfusion at 12h group, 24h: reperfusion at 24h group, 48h: reperfusion at 48h group, 72h:reperfusion at 72h group, 96h: reperfusion at 96h group. |
| Table 1 Cell count of MSCs(×105) from 1st to 7th cultured day in different groups |
CCK-8 detection was performed to appraise the effect of kidney homogenate of I/R injury on stem cell proliferation. From 1st day to 3rd day of cell culture, CCK-8 level increased in all groups, and no significant difference was found among the groups, all P>0.05. From 5th d to 7th d, CCK-8 level decreased in all groups except the control group. There was significant difference among groups, F=3.893, P=0.000 on 5th d and F=10.458, P=0.000 on 7th d. Results comparing between groups demonstrated that CCK-8 OD value of 24h reperfusion group was lower than that of other groups (Figure 4, Table 2).
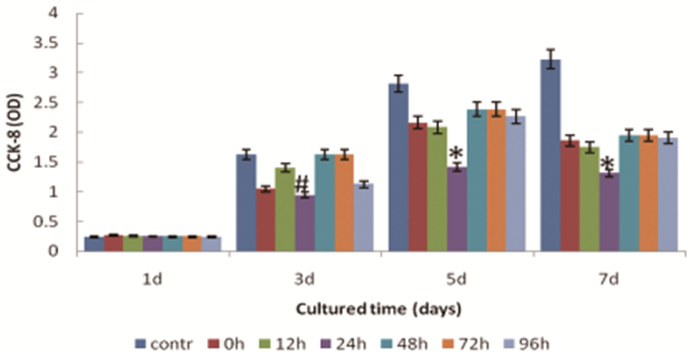
|
Figure 4 CCK-8 of MSCs cultured with I/R injury kidney homogenate at different reperfusion times.*Denotes statistically significant difference comparing with all other groups, P < 0.01.#Significant difference comparing with control group, P < 0.05.contr:control group, 0h:reperfusion at 0h group, 12h:reperfusion at 12h group, 24h:reperfusion at 24h group, 48h:reperfusion at 48h group, 72h:reperfusion at 72h group, 96h:reperfusion at 96h group. |
| Table 2 CCK-8 of MSCs cultured with I/R injury kidney homogenate at different reperfusion time point |
At the start of cell culture, the MSCs were observed under an inverted microscope growing in long spindles. On 7th day of culture, some MSCs in reperfusion group presented ellipsoidal and characterized by having short spindles comparing with control groups. On 15th day of culture, more MSCs were round and ellipsoidal characterized by having short fat spindles. Moreover, the shape of many MSCs of reperfusion at 72h group was round and ellipsoidal, and the cells had a cobble like appearance (Figure 5).
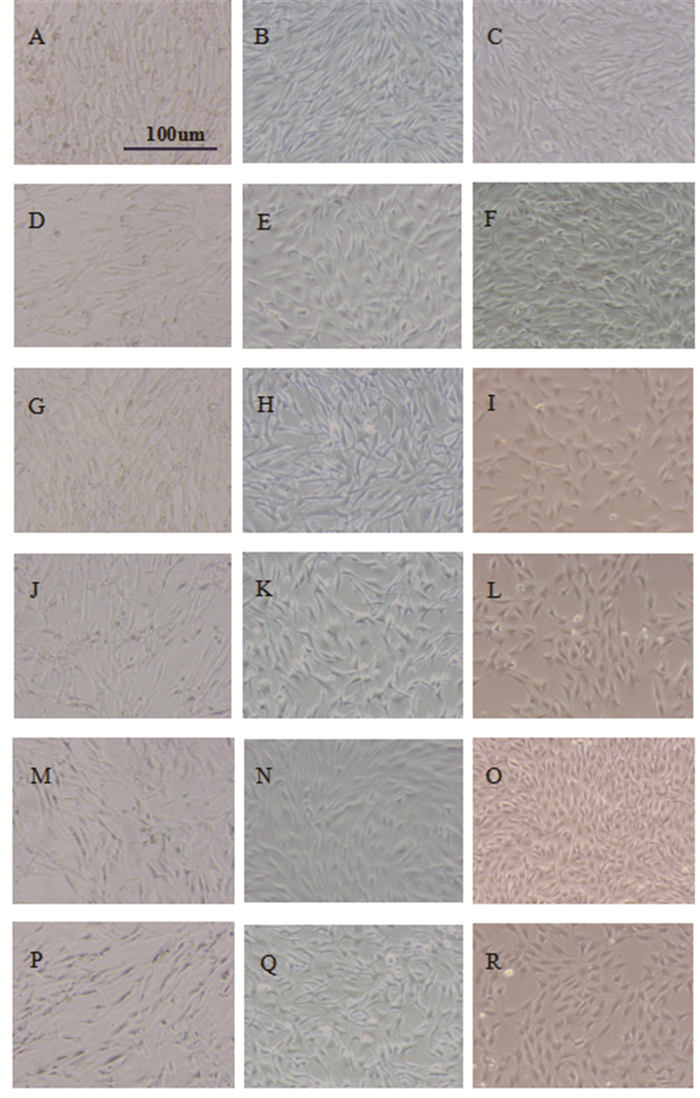
|
Figure 5 Morphological changes of the MSCs cultured with I/R kidney homogenate at different reperfusion time points(×100).A.control group, day 1;B.control group, day 7;C.control group, day 15.D.reperfusion at 0h group, day 1;E.reperfusion at 0h group, day 7;F.reperfusion at 0h group, day 15.G.Reperfusion at 24h group, day 1;H.reperfusion at 24h group, day 7;I.reperfusion at 24h group, day 15.J.reperfusion at 48h group, day 1;K.reperfusion at 48h group, day 7;L.reperfusiona at 48h group, day 15.M.reperfusion at 72h group, day 1;N.reperfusion at 72h group, day 7;O.reperfusion at 72h group, day 15.P.reperfusion at 96h group, day 1;Q.reperfusion at 96 h group, day 7;R.reperfusion at 96h group, day 15. |
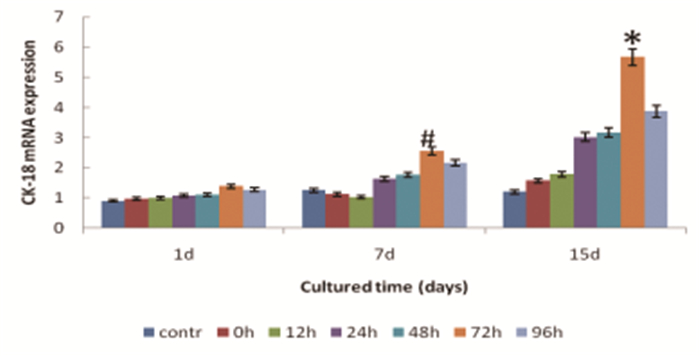
CK-18 expression was shown to be time-dependent. From 1st day to 15th day, the positive expression rate of CK-18 of BMSC increased. Moreover, the increasing extent of CK-18 in reperfusion at 72h group shown to be the highest both on the 7th and 15th ay(F=10.408 on 7th day and F=23.048 on 15th day respectively, all P < 0.01) (Figure 6, Table 3).
|
Figure 6 The RNA expression of CK-18 of MSCs cultured with I/R injury kidney homogenate.*Denotes statistically significant difference comparing with other groups, P < 0.01.#Significant difference comparing with control group, P < 0.05.contr: control group, 0h: reperfusion at 0h group, 12h:reperfusion at 12h group, 24h: reperfusion at 24h group, 48h: reperfusion at 48h group, 72h: reperfusion at 72h group, 96h: reperfusion at 96h group. |
| Table 3 The mRNA expression of CK-18 of MSCs cultured with I/R injury kidney homogenate at different reperfusion time point |
MSCs transplantation for AKI is a promising technology in cell biological engineering, in which MSCs are transplanted into the damaged kidney and consequently improve kidney function[11]. However, studies showed that the number of MSCs that omed and implanted in the kidney injury site for repair is very small.This may be due to the harsh microenvironment of the damage, which is not conducive to the replication and multiplication of MSCs[12].
However, injury microenvironment is not static in the time course of injury, it is presumed that there might exist an optimal time point for transplatation which may avoid the harsh period. Xiaoyan Liu et al believed that MSC should be administered immediately after 1h post I/R [13], because there are fewer damage factors produced at this time.Whereas Chen Yihuan et al supposed that 2~4 weeks after injury was the best time for MSCs transplantation[14], which was possibly attributed to the time point chosen after the fading of deleterious factors. Wang LQ believed that stem cell transplantation at 24h would have beneficial effects, because at that time a large number of benign factors in the damage environment increased, thus balanced the toxic factors in the host environment[15]. In fact, once the I/R occurs, a burst of toxic cytokines are generated, which do not support the growth of MSCs[16].Whereas, in chronic phase of injury, MSCs transplantation may contend with the disadvantage of scar tissue formation, which may adversely affect implanted cells[15]. So, the optimal time window of injury micro-environment for survival of transplanted MSCs should be chosen after fading of deleterious milieu and before scar formation.
In our study, MSCs were cultured with the kidney homogenate at different time points after I/R to imitate the dynamic microenvironment of AKI. The results showed that injury kidney homogenate have a time dependant manner effect on MSCs culture, and reperfusion at 72h time point exhibited optimal proliferation and differentiation effect comparing with other time points, which proved that 72h after I/R injury would be the optimal time point for the survival of transplanted MSCs.
A large number of studies have confirmed that the AKI microenvironment has many factors that affect the proliferation and differentiation of transplanted MSCs[17].However, the potential mechanism under it remains unclear.
Oxidative stress is a well-known risk factor in AKI microenvironment[18].Low basal levels of active oxygen radicals may be beneficial for maintaining stem cell pluripotency. Whereas high levels of it may inhibit both proliferation and differentiation of stem cells[19].Studies already proved that MSCs usually undergo apoptotic cell death when exposed to high level of oxidative stress, or they may lose their ability to divide[20].
Moreover, acute phase of I/R injury could stimulate the expression of inflammatory factors which aggravates renal injuries that are not beneficial for stem cells proliferation[21].Meanwhile, by up-regulation of growth factors, inflammatory factors enhance differentiation of stem cells as well.[22].
Although AKI microenvironment is adverse to the proliferation of MSCs with oxidative stress and inflammatory state, it retains the capability to promote differentiation of MSCs[23].Benign cytokines as HGF, VEGF, BM7 would be the potential factors produced in the injury microenvironment, which could promote the differentiation of MSCs[24, 25]. Liu NM studied the effect of kidney homogenate supernatant on the differentiation of MSCs. Results demonstrated that positive expression rate of CK18 of MSCs increased significantly when co-cultured with I/R kidney homogenate[26].Our experiments also proved the promotional effect of AKI microenvironment on stem cells. Results showed that MSCs cultured in I/R group at all time points performed better differentiation comparing with control groups.
In addition, the injury microenvironment is not always constant, including toxic cytokines and benign cytokines, which are both dynamically changing.
MDA is a well-known peroxidation marker of polyunsaturated fatty acids in oxidative stress and is capable of inducing apoptosis of cells[27].Studies reached a consensus that MDA increased immediately after I/R injury and presented a time dependant manner[28]. Xue Y et al studied the time course of MDA level in kidney after I/R injury in rats.It demonstrated that MDA began to increase at the start of reperfusion, and peaked at 24 hours, decrease to baseline at almost 1 week after reperfusion[29].
IL-6, one of the important inflammatory factors, which expressed in a time dependant manner following I/R injury. Timothy M.Williams found that there were kinetic changes of IL-6 in I/R kidney injury and proved that IL-6 level was significantly higher following injury at all time-points from 6th to 7th day post reperfusion, and the growth was the most marked from first to 3rd day[30].
VEGF is released during I/R injury, which helps to attenuat inflammation and oxidative stress, and ultimately improves renal insufficiency. It is one of the protective cytokines of MSCs[31].Compared with MDA and IL-6, VEGF arise relatively slow. In Hao P's experiments, the kinetic profile of VEGF in I/R kidney of rabbits was detected. By the time of reperfusion at 12h, 24h and 48h, it increased gradually and peaked at 48h[32].Considering the results of our cell culture, We presumed that 72h post I/R would should be the best time point for proliferation and differentiation of MSCs.Our study was limited to investigate the optimal time point for survival of MSCs in the acute injury microenvironment with cell culture in vitro. However, the therapeutic windows for MSC transplantation vary widely according to different animal models, the severity of tissues injury, the paracrine action of MSCs itself and so on. So, the optimal time for MSCs transplantation and the underlying mechanism remains to be investigated in vivo.
Disclosure statementThe authors declare there is no conflict of interest.
AcknowledgementsThis work was supported by Innovation Platform and Talent Plan of Hunan Provincial Science and Technology Department(NO.2015JC3121).
| [1] |
Mehta RL, Cerda J, Burdmann EA, et al. International Society of Nephrology's 0 by25 initiative for acute kidney injury (zero preventable deaths by 2025):a human rights case for nephrologyp[J]. Lancet, 2015, 385: 1-28. |
| [2] |
Yuan Yang, Meifang Song, Yu Liu, et al. Renoprotective approaches and strategies in acute kidney injury[J]. Pharmacology & Therapeutics, 2016, 163: 58-73. |
| [3] |
Kidder D. Mesenchymal stem cells attenuate ischemic acute kidney injury by inducing regulatory T cells through splenocyte interactions[J]. Kidney Int, 2014, 85(4): 981-982. |
| [4] |
Park MH, Baek B, Jin HK, et al. Novel peptides derived from neuropeptide Y prevent chemotherapy-induced bone marrow damage by regulating hematopoietic stem cell microenvironment[J]. Animal Cells Syst (Seoul), 2018, 22(5): 281-288. |
| [5] |
Fleig SV, Humphreys BD. Rationale of mesenchymal stem cell therapy in kidney injury[J]. Nephron Clin Pract, 2014, 127(1-4): 75-80. |
| [6] |
Si XY, Li JJ, Yao T, et al. Transforming growth factor-β1 in the microenvironment of ischemia r eperfusion-injured kidney enhances the chemotaxis of mesenchymal stem cells to stromal cell-derived factor-1 through upregulation of surface chemokine (C-X-C motif) receptor 4[J]. Mol Med Rep, 2014, 9(5): 1794-1798. |
| [7] |
Selek O, Buluç L, Muezzinoĝlu B, et al. Mesenchymal stem cell application improves tendon healing via anti-apoptotic effect (Animal study)[J]. Acta Orthop Traumatol Turc, 2014, 48(2): 187-195. |
| [8] |
Shao D, Lu M, Xu D, et al. Carbon dots for tracking and promoting the osteogenic differentiation of mesenchymal stem cells[J]. Biomater Sci, 2017, 5(9): 1820-1827. |
| [9] |
Ding X, Zhu M, Xu B, et al. Integrated trilayered silk fibroin scaffold for osteochondral differentiation of adipose-derived stem cells[J]. ACS Appl Mater Interfaces, 2014, 6(19): 16696-16705. |
| [10] |
Fujimura T, Fujimoto T, Itaya-Hironaka A, et al. Significance of Interleukin-6/STAT Pathway for the Gene Expression of REG Iα, a New Autoantigen in Sjögren's Syndrome Patients, in Salivary Duct Epithelial Cells[J]. Clin Rev Allergy Immunol, 2017, 52(3): 351-363. |
| [11] |
Ashwani KG, Sachin HJ, Naresh KT, er al. Fetal kidney stem cells ameliorate cisplatin induced acute renal failure and promote renal angiogenesis[J]. World J Stem Cells, 2015, 7(4): 776-788. |
| [12] |
Saidi RF, Moghadasali R, Shekarchian S. Utilization of mesenchymal stem cells in kidney transplantation:from bench to bedside[J]. Iran J Kidney Dis, 2019, 13(4): 213-224. |
| [13] |
Xiaoyan Liu, Jieru Cai, Xiaoyan Jiao, et al. Therapeutic potential of mesenchymal stem cells in acute kidney injury is affected by administration timing[J]. Acta Biochim Biophys Sin, 2017, 49(4): 338-348. |
| [14] |
Chen Y, Teng X, Chen W, et al. Timing of transplantation of autologous bone marrow derived mesenchymal stem cells for treating myocardial infarction[J]. Sci China Life Sci, 2014, 57(2): 195-200. |
| [15] |
Wang LQ, Lin ZZ, Zhang HX, et al. Timing and dose regimens of marrow mesenchymal stem cell transplantation affect the outcomes and neuroinflammatory response after ischemic stroke[J]. CNS Neurosci Ther, 2014, 20(4): 317-326. |
| [16] |
Ghoreyshi M, Mahmoudabady M, Bafadam S, et al. The protective effects of pharmacologic postconditioning of hydroalcoholic extract of nigella sativa on functional activities and oxidative stress injury during ischemia-reperfusion in isolated rat heart[J]. Cardiovasc Toxicol, 2020, 20(2): 130-138. |
| [17] |
Liu N, Wang H, Han G, et al. Enhanced proliferation and differentiation of HO-1 gene-modified bone marrow-derived mesenchymal stem cells in the acute injured kidney[J]. Int J Mol Med, 2018, 42(2): 946-956. |
| [18] |
Ham O, Lee SY, Lee CY, et al. let-7b suppresses apoptosis and autophagy of human mesenchymal stem cells transplanted into ischemia/reperfusion injured heart 7by targeting caspase-3[J]. Stem Cell Res Ther, 2015, 6: 147. |
| [19] |
Chen X, Shen WB, Yang P, et al. High Glucose Inhibits Neural Stem Cell Differentiation Through Oxidative Stress and Endoplasmic Reticulum Stress[J]. Stem Cells Dev, 2018, 27(11): 745-755. |
| [20] |
Liu Y, Yang H, Wen Y, et al. Nrf2 inhibits periodontal ligament stem cell apoptosis under excessive oxidative stress[J]. Int J Mol Sci, 2017, 18(5): 1076. |
| [21] |
Liu Z, Yang Q, Wei Q, et al. The protective effect of miR-377 inhibitor against renal ischemia-reperfusion injury through inhibition of inflammation and oxidative stress via a VEGF-dependent mechanism in mice[J]. Mol Immunol, 2019, 106: 153-158. |
| [22] |
Seo E, Kang H, Lim OK, et al. Supplementation with IL-6 and Muscle Cell Culture Conditioned Media Enhances Myogenic Differentiation of Adipose Tissue-Derived Stem Cells through STAT3 Activation[J]. Int J Mol Sci, 2018, 19(6): 1557. |
| [23] |
Patrick Page, Joshua DJ, Alaina Bandstra, et al. Effect of serum and oxygen concentration on gene expression and secretion of paracrine factors by mesenchymal stem cells[J]. Int J Cell Biol, 2014, 2014: 601063. |
| [24] |
Nanmei Liu, Huiling Wang, Guofeng Han, et al. Alleviation of apoptosis of bone marrow-derived mesenchymalstem cells in the acute injured kidney by heme oxygenase-1gene modification[J]. Int J Biochem Cell Biol, 2015, 69: 85-94. |
| [25] |
Ming Hu, Guixian Guo, Qiang Huang. The harsh microenvironment in infracted heart accelerates transplanted bone marrow mesenchymal stem cells injury:the role of injured cardiomyocytes-derived exosomes[J]. Cell Death and Disease, 2018, 9: 357. |
| [26] |
Liu NM, Tian J, Wang WW, Han GF, et al. Effect of erythropoietin on mesenchymal stem cell differentiation and secretion in vitro in an acute kidney injury microenvironment[J]. Genet Mol Res, 2013, 12(4): 6477-6487. |
| [27] |
Yu Liu, Luosheng Tang, Baihua Chen. Effects of antioxidant gene therapy on retinal neurons and oxidative stress in a model of retinal ischemia/reperfusion[J]. Free Radical Biology & Medicine, 2012, 52: 909-915. |
| [28] |
Fonseca I, Reguengo H, Almeida M, et al. Oxidative stress in kidney transplantation:malondialdehyde is an early predictive marker of graft dysfunction[J]. Transplantation, 2014, 97(10): 1058-1065. |
| [29] |
Xue Y, Xu Z, Chen H, et al. Low-energy shock wave preconditioning reduces renal ischemic reperfusion injury caused by renal artery occlusion[J]. Acta Cir Bras, 2017, 32(7): 550-558. |
| [30] |
Williams TM, Wise AF, Layton DS, et al. Phenotype and influx kinetics of leukocytes and inflammatory cytokine production in kidney ischemia/reperfusion injury[J]. Nephrology (Carlton), 2018, 23(1): 75-85. |
| [31] |
Liu Z, Yang Q, Wei Q, et al. The protective effect of miR-377 inhibitor against renal ischemia-reperfusion injury through inhibition of inflammation and oxidative stress via a VEGF-dependent mechanism in mice[J]. Mol Immunol, 2019, 106: 153-158. |
| [32] |
Hao P. Monitoring of renal ischemia reperfusion injury in rabbits by ultrasonic contrast and its relationship with expression of VEGF in renal tissue[J]. Asian Pac J Trop Med, 2016, 9(2): 188-192. |
 2020, Vol. 4
2020, Vol. 4

