2. Department of Clinical Laboratory, Shanghai Children's Hospital, Shanghai Jiao Tong University, Shanghai 200040, China
Objective To detect the effects of microwave on calcium levels in primary hippocampal neurons and primary cardiomyocytes by the real-time microwave exposure combined with laser scanning confocal microscopy.
Methods The primary hippocampal neurons and primary cardiomyocytes were cultured and labeled with probes, including Fluo-4 AM, Mag-Fluo-AM, and Rhod-2, to reflect the levels of whole calcium[Ca2+], endoplasmic reticulum calcium[Ca2+]ER, andmitochondrial calcium[Ca2+]MIT, respectively. Then, the cells were exposed to a pulsed microwave of 2.856 GHz with specific absorption rate (SAR) values of 0, 4, and 40 W/kg for 6 min to observe the changes in calcium levels.
Results The results showed that the 4 and 40 W/kg microwave radiation caused a significant decrease in the levels of[Ca2+], [Ca2+]ER, and[Ca2+]MIT in primary hippocampal neurons. In the primary cardiomyocytes, only the 40 W/kg microwave radiation caused the decrease in the levels of[Ca2+], [Ca2+]ER, and[Ca2+]MIT. Primary hippocampal neurons were more sensitive to microwave exposure than primary cardiomyocytes. The mitochondria were more sensitive to microwave exposure than the endoplasmic reticulum.
Conclusion The calcium efflux was occurred during microwave exposure in primary hippocampal neurons and primary cardiomyocytes. Additionally, neurons and mitochondria were sensitive cells and organelle respectively.
Microwaves, which are widely used in telecommunications, military affairs, industries, and medicine, have attracted great attention for their negative effects on human health. Mechanisms of microwave-induced injures have been studied for many years, but have not yet been fully revealed. The central nervous system (CNS) has been considered one of the most sensitive targets of microwave exposure[1, 2]. Besides, the cardiovascular system is also sensitive to microwave radiation[3]. Research has found that microwaves could induce the impairment of learning and memory[2]. Neuronal changes, such as structural alterations, abnormalities of electrical activities, and disorders of energy metabolism, were major factors for microwave-induced CNS injuries[4-7]. Moreover, microwave radiation could induce myocardial cell apoptosis by interfering with oxidative stress and cardiac energy metabolism[8]. Calcium levels have been found to be closely associated with oxidative stress and apoptosis[9-11].
Calcium is a universal second messenger involved in the regulation of various cellular processes, including electrical signaling, contraction, secretion, memory, gene transcription, and cell death. In neurons, calcium efflux is mediated by the plasma membrane calcium ATPase (PMCA), the sodium-calcium exchanger (NCX), and the sarco-/endoplasmic reticulum calcium ATPase (SERCA)[12]. In the heart, the NCX is involved in one of the main calcium efflux mechanisms in cardiomyocytes by transporting three Na+ ions into the cytoplasm in exchange for one Ca2+, which moves out of the cytoplasm[13, 14]. Besides, the PMCA and SERCA are also important in transporting calcium out of cardiomyocytes. Calcium ions play an important role in the activity-dependent synaptic plasticity in neurons and the contractile activity in cardiovascular system; calcium efflux might decrease the functions of synaptic plasticity and myocardial contraction.
So far, the effects of microwave radiation on the calcium levels have still been controversial. Kumar once reported that chronic exposure to a non-modulated microwave of 2.45 GHz (power density, 0.033 mW/cm2; specific absorption rate, 0.023 W/Kg) for 2 h daily for 1 month could induce the increase of intracellular calcium levels in mice[15]. Additionally, chronic exposure to microwave radiation of 9.9 GHz at a power density of 0.125 mW/cm2 and specific absorption rate of 1.0 W/kg for 2 h/day for 35 days could increase the calcium ion efflux in male Wistar rats[16]. No clear indications of mobile phones radiation (simulated GSM mobile phone signals, 915 MHz and 2 W/kg) were reported to be associated with any changes in calcium levels or calcium signaling in lymphocytes[17]. This difference might be attributed to the different parameters of microwave radiation (frequency, power density, specific absorption rate, and exposure time) and the methods for detecting the calcium ion levels. The aforementioned studies mainly focused on the calcium detection after microwave exposure. The changes of calcium ion levels often occurred in a short time, and therefore, there was a lag in the observation after microwave exposure. In our study, we have designed a real-time pulsed microwave exposure system, which could be combined with laser scanning confocal microscopy (LSCM), for observing the changes of calcium levels in primary hippocampal neurons and primary cardiomyocytes.
The intracellular free calcium ions participate in a large variety of cell functions, including the regulation of CNS functions and control of heart muscle cell contraction[12, 18]. Free calcium ions are critical coordinators of various aspects of body physiology and are closely related with energy metabolism, neurotransmitter release, enzyme activation, intracellular signal transduction, and gene expression[12]. Calcium ions are mainly stored in the endoplasmic reticulum (ER) and mitochondria (MIT). The calcium ions in the endoplasmic reticulum ([Ca2+]ER) play an important role in controlling cell activation, regulating signaling pathways, post-transcriptional modification of protein molecules, and maintaining intracellular environmental calcium balance[19, 20]. The mitochondrial calcium ([Ca2+]MIT) plays an important role in regulating mitochondrial metabolism, maintaining mitochondrial ATP production, and balancing the calcium ion levels in the intracellular environment[21]. Mitochondrial calcium is also closely related with reactive oxygen species (ROS) production, which plays a critical role in mitochondria-mediated cell death[22]. The endoplasmic reticulum and mitochondria cannot be considered as static structures, as they intimately communicate, forming very dynamic platforms, termed mitochondria-associated membranes. In particular, the ER transmits proper Ca2+ signals to the mitochondria, which decode them into specific inputs to regulate essential functions, including metabolism, energy production, and apoptosis[23, 24]. Therefore, in our study, we not only detected the concentration of whole calcium by Fluo-4 AM labeling, but also measured the concentrations of calcium in the ER and MIT to clarify the effects of microwave radiation on the changes in calcium ion levels.
MATERIALS AND METHODS Primary Hippocampal Neuron CulturesPostnatal Wistar rats younger than 12 h old were purchased from the Experimental Animal Center of Beijing Institute of Radiation Medicine (Beijing, China). All protocols were approved by the Institutional Animal Care and Use Committee. Firstly, the hippocampi were microdissected and digested with 0.25% trypsin-ethylene diamine tetraacetic acid (EDTA) (Gibco, USA) at 37 ℃ for 20 min. Then, the cells were suspended in Dulbecco's Modified Eagle Medium (DMEM) containing 10% fetal bovine serum (FBS) (Gibco, USA), 10% horse serum (Gibco, USA), 1% L-glutamine (Invitrogen, USA), and 1% penicillin-streptomycin solution (HyClone, USA), and plated at a density of 5 × 105 cells/mL on 0.1 mg/mL poly-D-lysine (Sigma, USA)-coated 15-mm confocal dishes. After one day in vitro (DIV), the plating medium was replaced by Dulbecco's modified eagle media (DMEM) containing 10% horse serum, 1% L-glutamine, 1% penicillin-streptomycin solution, 2% B27, and 1% N2. To inhibit the growth of glial cells, 3 μg/mL cytarabine (Sigma, USA) was added at three DIV after plating and half of the medium was changed after 24 h. Afterwards, half of the medium was changed every four days.
Primary Cardiomyocyte CulturesPostnatal Wistar rats younger than 12 h were chosen and disinfected using 75% ethanol. The hearts were separated and soaked in 1% PBS to remove the blood. Then, the ventricles were selected and cut into 1-mm3 blocks. The tissue blocks were digested with 0.25% trypsin-EDTA (Gibco, USA) at 37 ℃ for 8 min and the upper layer suspension was discarded. Then, the remaining tissues were continued to be digested with 0.25% trypsin-EDTA at 37 ℃ for 8 min and the upper layer suspension was collected, and the action of trypsin was terminated by the addition of the same amount of culture medium (10% fetal bovine serum, 1% L-glutamine, 1% penicillin-streptomycin solution, and 88% DMEM). The remaining tissues were continued to be digested 3-5 times using the aforementioned steps until all the tissues were digested. The collected suspension was centrifuged at 1, 000 rpm for 10 min. The cells were plated at a cell density of 5 × 105 cells/mL on the 0.1 mg/mL poly-D-lysine-coated 15-mm confocal dishes. Afterwards, the medium was changed every two days.
GroupsThe two types of cells were divided randomly into three groups, the 0 W/kg exposure group, the 4 W/kg microwave exposure group, and the 40 W/kg microwave exposure group. To eliminate the self-differences, every group must be observed using at least three different fields of vision from three different dishes.
Ethical AuthorizationThe experiments were approved by an Ethical Committee of the Academy of Military Medical Science, and the authorization number was IACUC-AMMS-2010-007.
Calcium Labeling and Confocal ObservationSpecific fluorescent probes, Fluo-4 AM (Thermo Fisher Scientific, USA), Mag-Fluo-AM, and Rhod-2 (Genmed Scientifics Inc., USA) were used to label the whole calcium [Ca2+], endoplasmic reticulum calcium [Ca2+]ER, and mitochondrial calcium [Ca2+]MIT, respectively. All labeling protocols were in strict accordance with the experimental instructions.
Specifically, for [Ca2+] labeling, 45.6 μL of dimethyl sulfoxide (DMSO) was used to dissolve 50 μg of Fluo 4-AM, and the 1-mmol/L stock solutions were prepared. Then, the stock solution was diluted to 1:200 using Hanks' balanced salt solution (HBSS), and the working solutions were prepared. Two-hundred μL of the working solution was added to each confocal dish; the dishes were then maintained at 37 ℃ for 30 min. Then, the working solution was removed and the dishes were washed with HBSS three times. Under the confocal microscope, the labeled calcium appears green when the excitation wavelength was 488 nm.
For [Ca2+]ER labeling, 500 μL of cleaning fluid was added to each dish to wash the cell debris. Then, 200 μL of the diluted dyeing solution (1:10) was added to each dish, and the dishes were incubated at 37 ℃ for 60 min. The dyeing solution was then removed and 200 μL of cleaning fluid and 2 μL of permeated fluid were added, followed by incubation at 37 ℃ for 4 min. Then, the dishes were washed with the cleaning fluid and maintained in HBSS for confocal observation. For [Ca2+]MIT labeling, 500 μL of cleaning fluid was added to wash the debris. Then, 200 μL of the diluted dyeing solution (1:10) was added, followed by incubation at 37 ℃ for 60 min. Then, each dish was washed with the cleaning fluid and HBSS was added for observation. Due to the differences of excitation wavelengths for the [Ca2+]ER and [Ca2+]MIT (490 nm and 550 nm, respectively), we used the co-dyeing method to observe the calcium in the ER and MIT simultaneously.
Real-time Microwave Exposure System and DosimetryThe real-time pulsed microwave exposure system was reported in our published manuscript[25]. The system comprised a signal generator, pulse amplifier, circulator, directional coupler, waveguide cell, power meter, and the laser scanning confocal microscope. The Agilent E4417A EPM-P series power meter (Agilent Technologies, Santa Clara, CA) and the signal generator were connected, allowing for a continuous monitoring of the power level, which was adjusted according to the required specific absorption rate (SAR) (0, 4, and 40 W/kg) (Figure 1). The pulse width, peak power, and frequency for the two exposure groups (4 and 40 W/kg) were the same (2 μs, 60 W, and 2.856 GHz respectively). The pulse interval times of the 4 and 40 W/kg were 360 μs and 36 μs, respectively.
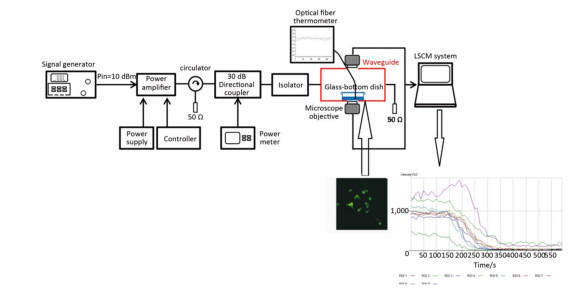
|
Download:
|
| Figure 1 The real-time microwave exposure system. The signal generator, amplifier, circulator, bidirectional coupler, and customized waveguide are the main parts. The fluorescence measurement system was mainly composed of the customized waveguide and the laser scanning confocal microscope. The glass-bottom dish containing cells is placed above the opening window on the bottom side of the waveguide. Cells were labeled with the probes, and the fluorescent signals were recorded and analyzed. | |
In our experiment, confocal observation was started 2 min before the microwave exposure. The confocal observation was uninterrupted during six minutes of microwave exposure until two minutes after microwave radiation.
Statistical AnalysisAll data were expressed as the mean ± SEM. A repeated measure of two-way ANOVA was used. Post-hoc test was performed using S-N-K method. The software used was SPSS 19.0. Differences were considered to be significant if P < 0.05.
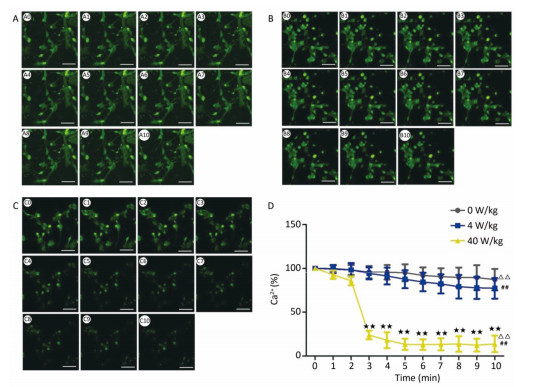
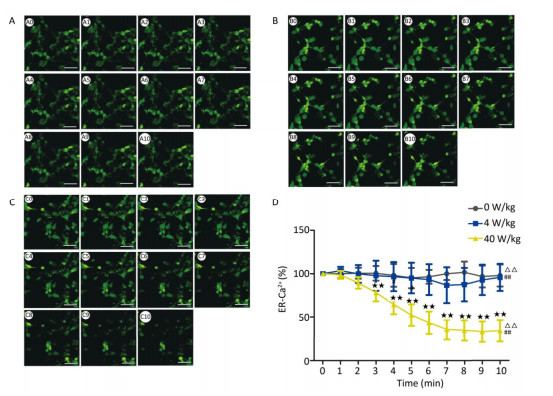
RESULTS Decrease of [Ca2+], [Ca2+]ER, and [Ca2+]MIT in Primary Hippocampal NeuronsCompared to the 0 W/kg group, the [Ca2+], [Ca2+]ER, and [Ca2+]MIT concentrations in primary hippocampal neurons decreased significantly in the 4 and 40 W/kg groups (P < 0.01) (Figures 1-3). When the microwave exposure source started to emit the pulsed microwave, the fluorescence density became weak. Compared to the 4 W/kg group, the 0 and 40 W/kg groups showed significant changes (P < 0.01) (Figures 2-4), indicating that there was a significant dose-effect relationship with the increase of exposure dose.
|
Download:
|
| Figure 2 Changes of [Ca2+] concentration in primary hippocampal neurons. A, B, and C represent the 0, 4, and 40 W/kg microwave exposure groups, respectively. A0-A10, B0-B10, and C0-C10 show the time changes from 0 min to 10 min for the three groups, respectively. D indicates the statistical results. Statistical significances (repeated measures ANOVA): compared with the 0 W/kg group, #P < 0.05, ##P < 0.01; compared to the 4 W/kg group, △P < 0.05, △△P < 0.01. Statistical significances (Post-hoc analysis): compared with the 0 W/kg group, ★P < 0.05, ★★P < 0.01 at a corresponding time point after microwave exposure. Scale bars = 50 μm. | |
|
Download:
|
| Figure 3 Changes of [Ca2+]ER concentration in primary hippocampal neurons. A, B, and C represent the 0, 4, and 40 W/kg microwave exposure groups, respectively. A0-A10, B0-B10, and C0-C10 show the time changes from 0 min to 10 min for the three groups, respectively. D indicates the statistical results. Statistical significances (repeated measures ANOVA): compared with the 0 W/kg group, #P < 0.05, ##P < 0.01; compared to the 4 W/kg group, △P < 0.05, △△P < 0.01. Statistical significances (Post-hoc analysis): compared with the 0 W/kg group, ★P < 0.05, ★★P < 0.01 at a corresponding time point after microwave exposure. Scale bars = 50 μm. | |

|
Download:
|
| Figure 4 Changes of [Ca2+]MIT concentration in primary hippocampal neurons. A, B, and C represented the 0, 4, and 40 W/kg microwave exposure groups, respectively. A0-A10, B0-B10, and C0-C10 show the time changes from 0 min to 10 min for the three groups, respectively. D indicates the statistical results. Statistical significances (repeated measures ANOVA): compared with the 0 W/kg group, #P < 0.05, ##P < 0.01; compared to the 4 W/kg group, △P < 0.05, △△P < 0.01. Statistical significances (Post-hoc analysis): compared with the 0 W/kg group, ★P < 0.05, ★★P < 0.01 at a corresponding time point after microwave exposure. Scale bars = 50 μm. | |
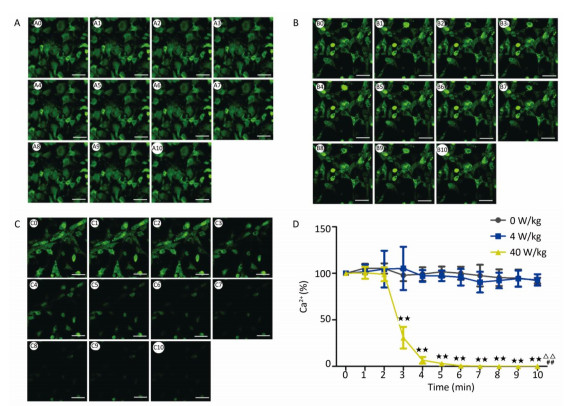
The changes of the [Ca2+], [Ca2+]ER, and [Ca2+]MIT concentrations for the primary cardiomyocytes were different from those for the primary hippocampal neurons. Compared to the 0 W/kg group, significant decreases were found only in the 40 W/kg group (P < 0.01) (Figures 5-7). When compared to the 4 W/kg group, the [Ca2+], [Ca2+]ER, and [Ca2+]MIT concentrations were found to be significantly decreased in the 40 W/kg group (P < 0.01) (Figures 4-6).
|
Download:
|
| Figure 5 Changes of [Ca2+] concentration in cardiomyocytes. A, B, and C represent the 0, 4, and 40 W/kg microwave exposure groups, respectively. A0-A10, B0-B10, and C0-C10 show the time changes from 0 min to 10 min for the three groups, respectively. D indicates the statistical results. Statistical significances (repeated measures ANOVA): compared with the 0 W/kg group, #P < 0.05, ##P < 0.01; compared to the 4 W/kg group, △P < 0.05, △△P < 0.01. Statistical significances (Post-hoc analysis): compared with the 0 W/kg group, ★P < 0.05, ★★P < 0.01 at a corresponding time point after microwave exposure. Scale bars = 50 μm. | |

|
Download:
|
| Figure 6 Changes of [Ca2+]ER concentration in cardiomyocytes. A, B, and C represent the 0, 4, and 40 W/kg microwave exposure groups, respectively. A0-A10, B0-B10, and C0-C10 show the time changes from 0 min to 10 min for the three groups, respectively. D indicates the statistical results. Statistical significances (repeated measures ANOVA): compared with the 0 W/kg group, #P < 0.05, ##P < 0.01; compared to the 4 W/kg group, △P < 0.05, △△P < 0.01. Statistical significances (Post-hoc analysis): compared with the 0 W/kg group, ★P < 0.05, ★★P < 0.01 at a corresponding time point after microwave exposure. Scale bars = 50 μm. | |

|
Download:
|
| Figure 7 Changes of [Ca2+]MIT concentration in cardiomyocytes. A, B, and C represent the 0, 4, and 40 W/kg microwave exposure groups, respectively. A0-A10, B0-B10, and C0-C10 show the time changes from 0 min to 10 min for the three groups, respectively. D indicates the statistical results. Statistical significances (repeated measures ANOVA): compared with the 0 W/kg group, #P < 0.05, ##P < 0.01; compared to the 4 W/kg group, △P < 0.05, △△P < 0.01. Statistical significances (Post-hoc analysis): compared with the 0 W/kg group, ★P < 0.05, ★★P < 0.01 at a corresponding time point after microwave exposure. Scale bars = 50 μm. | |
From the above results, we found that the concentrations of [Ca2+], [Ca2+]ER, and [Ca2+]MIT decrease after the low-dose microwave exposure of 4 W/kg in the primary hippocampal neurons. However, the concentrations of [Ca2+], [Ca2+]ER, and [Ca2+]MIT in the primary cardiomyocytes did not change after the 4 W/kg microwave exposure. These results indicate that the primary hippocampal neurons were more sensitive to microwave exposure than the primary cardiomyocytes.
The Mitochondria Were More Sensitive to Microwave Exposure than Endoplasmic ReticulumTo clarify the sensitivity of different cell regions to microwave radiation, the whole calcium concentrations, and the concentrations of calcium in the endoplasmic reticulum and mitochondria were compared under the same exposure dose and in the same cell types. After the 40 W/kg microwave exposure, compared to the whole calcium concentrations, the calcium ion concentrations in the endoplasmic reticulum and mitochondria showed significant differences (P < 0.01) (Figure 8). Compared to the calcium ions in the endoplasmic reticulum, the whole calcium and mitochondria calcium concentrations showed significant differences (P < 0.01) (Figure 7). Moreover, among the [Ca2+], [Ca2+]ER, and [Ca2+]MIT, the most pronounced decrease of calcium ions was found to be in the mitochondria. This indicates that the mitochondria were more sensitive to microwave radiation than the endoplasmic reticulum.

|
Download:
|
| Figure 8 The relative fluorescence intensity of whole calcium concentration, and endoplasmic reticulum and mitochondria calcium ion concentrations after 40 W/kg microwave exposure in the primary hippocampal neurons. Statistical significances (repeated measures ANOVA): compared with the whole calcium concentration, #P < 0.05, ##P < 0.01; compared to the endoplasmic reticulum calcium concentration, △P < 0.05, △△P < 0.01. Statistical significances (Post-hoc analysis): compared with the whole calcium, ★P < 0.05, ★★P < 0.01 at a corresponding time point after microwave exposure. | |
In the central nervous system, it was reported that the intracellular calcium concentration plays important roles in the triggering of neurotransmitter release and the regulation of short-term plasticity[26]. Besides, calcium ions play crucial signaling roles in many forms of activity-dependent synaptic plasticity in postsynaptic neurons[27]. Research has found that in the cardiovascular system, calcium ions regulate mitochondrial adenosine triphosphate (ATP) production and contractile activity, and thus, play a pivotal role in matching the energy supply and demand in cardiac muscles[28]. Moreover, the imbalance of calcium handling might promote heart failure and arrhythmias[29].
Ca2+ is a double-edged sword for both life and death. The calcium efflux or influx plays different physiological roles in cells. In neurons, sources of calcium influx are calcium-permeable a-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and N-methyl-D-aspartate acid receptor (NMDAR), voltage-gated calcium channels (VGCC), nicotinic acetylcholine receptors (nAChR), and transient receptor potential type C (TRPC) channels. Calcium release from internal stores is mediated by inositol trisphosphate receptors (IP3R) and ryanodine receptors (RyR). Calcium efflux is mediated by the plasma membrane calcium ATPase (PMCA), the sodium-calcium exchanger (NCX), and the sarco-/endoplasmic reticulum calcium ATPase (SERCA). Additionally, the mitochondria are important for neuronal calcium homeostasis[12]. In cardiomyocytes, the VGCC channels are the main sources of calcium influx. The calcium efflux is mainly mediated by the NCX and PMCA.
However, so far, the effects of microwave radiation on calcium in brain cells are still controversial. Most studies have suggested that microwave radiation could lead to calcium efflux. As early as 1979, there was a study about the effects of electric fields on the brain chemistry of cats that were awake, which found that between 6 and 20 Hz, there was a highly significant increase in the calcium efflux[30]. Since then, studies about the effects of microwave radiation on calcium ions continue to be performed. Shelton reported that at low pulse repetition frequencies and low power densities, microwaves did not alter the 45Ca2+ efflux in rat neural tissues[31]. In a study of microwave exposure on human neuroblastoma cells, a significant increase in the efflux of calcium ions (45Ca2+) as compared to unexposed control cultures occurred at two SAR values: 0.05 and 1 W/Kg[32]. Chronic exposure of rats to microwave radiation (9.9 GHz, 0.125 mW/cm2, 1.0 W/kg, for 2 h/day for 35 days) could significantly increase the calcium ion efflux[16]. However, the opposite observation was also reported. Kesari exposed Wistar rats to microwaves at a frequency of 2.45 GHz (0.21 mW/cm2, 0.14 W/kg) for 2 h a day for 45 d. A significant increase in calcium ion concentration was observed in the whole brain of the exposed group of animals, compared to sham exposed group[33]. There are only a few studies about the effects of microwave exposure on calcium levels in cardiomyocytes. Only in 1991, it was reported that a microwave field with a surface density power stream of about 4 mW/cm2 increased the rate of Ca2+ uptake by the sarcoplasmic reticulum in the heart. In summary, these inconsistent results might be associated with the variation in doses and microwave exposure times and the observed cell or tissue types. Moreover, the observations were all recorded after microwave exposure. The simultaneous detection of calcium ions during microwave exposure was only reported in one study. Cranfield delivered GSM mobile phone signals (915 MHz, 2 W/kg) via a coaxial applicator to a perfused chamber where cells were adhered to a thin glass coverslip; imaging was performed using LSCM. Both continuous wave (CW) and pulsed wave (PW) RF (on both phytohemagglutinin-activated and nonactivated cells) were studied. There is no clear indication that radiofrequency emissions from mobile phones are associated with any changes in calcium levels or calcium signaling in lymphocytes[17]. The negative results might be due to the low SAR of microwave exposure.
In our previous studies, we have successfully established the real-time microwave radiation system connected with a laser scanning confocal microscope and found that 4 W/kg microwave radiation could not change the calcium ion concentration in PC12 cells[25]. The dose of microwave exposure and the cell type might be main reasons for the negative results. Therefore, in our study, the real-time microwave exposure system was used to observe the dynamic changes of calcium ions in other two different cell types under three different exposure doses. We found that the concentrations of [Ca2+], [Ca2+]ER, and [Ca2+]MIT significantly decreased in the primary hippocampal neurons after the exposure to 4 and 40 W/kg microwave radiation, which indicated that a significant calcium efflux occurred after microwave radiation. Meanwhile, compared to the 4 W/kg groups, there were significant differences in [Ca2+], [Ca2+]ER, and [Ca2+]MIT concentrations in the primary hippocampal neurons and the primary cardiomyocytes in the 40 W/kg exposure groups, which indicated that a clear dose-effect relationship existed. In the primary cardiomyocytes, the 4 W/kg microwave radiation did not induce the changes in calcium ion concentrations, indicating that the primary hippocampal neurons might be more sensitive to microwave radiation than the primary cardiomyocytes. Moreover, we also found that the [Ca2+]MIT concentration changed more significantly than the [Ca2+]ER concentration, which indicated that the mitochondria were more sensitive to microwave radiation than the endoplasmic reticulum.
In the CNS, microwave radiation could impair the synaptic plasticity and hippocampal structure and function[34, 35]. Moreover, microwave radiation can cause electrophysiological, histological, and ultrastructural changes in the heart[36]. However, the underlying mechanism for this has not yet been clearly elucidated; especially, the roles of calcium ions in microwave-induced injuries are still unclear. Our study found that a significant calcium efflux occurred once the microwave source was turned on. During the exposure time, the efflux continued to occur. Two minutes after microwave exposure, the fluorescence intensities showed recovery trends, but did not recover to the normal values. Calcium ions play important roles in maintaining cell functions; the efflux of numerous calcium ions might induce the decrease of calcium concentrations and impair the normal physiological functions. With regards to the important roles of calcium transients in cardiomyocytes, we also detected the effects of pulsed microwave on intracellular calcium transients in cardiomyocytes, and observed a calcium efflux after the 40 W/kg microwave exposure. These results were consistent with those from a study about the effects of electromagnetic fields on cardiomyocytes isolated from neonatal Sprague-Dawley rats. Exposure to rectangular-wave pulsed extremely low-frequency electromagnetic fields (ELF-EMF) at four different frequencies (15 Hz, 50 Hz, 75 Hz, and 100 Hz) and at a flux density of 2 mT, was found to enhance the activities of NCX and SERCA2a and decrease the amplitude of calcium transients and calcium level in the sarcoplasmic reticulum[37]. These results indicate that the activities of NCX and SERCA should be studied in the future to explain the calcium efflux process.
The pulsed microwave exposure system combined with the laser scanning confocal microscope provided a new approach for dynamically monitoring the changes in ion concentrations or other fluorescently labeled molecules. The new system could avoid missing the real-time changes during microwave exposure, which could be widely used in the field of electromagnetic biology.
In summary, our study group found that a significant calcium efflux occurred during the process of microwave exposure. Besides, the neurons might be more sensitive to microwave radiation than the cardiomyocytes. The mitochondria were found to be more sensitive to microwave radiation than the endoplasmic reticulum.
ACKNOWLEDGMENTSThe authors would like to offer special thanks to the editor and referees for their comments and suggestions, which greatly improved the substance and presentation of the paper.
| 1. |
Altunkaynak BZ, Altun G, Yahyazadeh A, et al. Different methods for evaluating the effects of microwave radiation exposure on the nervous system. J Chem Neuroanat, 2016; 75, 62-9. doi:10.1016/j.jchemneu.2015.11.004 |
| 2. |
Shahin S, Banerjee S, Singh SP, et al. 2.45 GHz Microwave Radiation Impairs Learning and Spatial Memory via Oxidative/Nitrosative Stress Induced p53-Dependent/Independent Hippocampal Apoptosis:Molecular Basis and Underlying Mechanism. Toxicol Sci, 2015; 148, 380-99. doi:10.1093/toxsci/kfv205 |
| 3. |
Kantz J, Muller J, Hadeler KP, et al. Insensitivity of cardiovascular function to low power cm-/mm-microwaves. Int J Environ Health Res, 2005; 15, 207-15. doi:10.1080/09603120500105695 |
| 4. |
Zhao L, Peng RY, Wang SM, et al. Relationship between Cognition Function and Hippocampus Structure after Long-term Microwave Exposure. Biomed Environ Sci, 2012; 25, 182-8. |
| 5. |
Suhhova A, Bachmann M, Karai D, et al. Effect of microwave radiation on human EEG at two different levels of exposure. Bioelectromagnetics, 2013; 34, 264-74. doi:10.1002/bem.v34.4 |
| 6. |
Vorobyov V, Janac B, Pesic V, et al. Repeated exposure to low-level extremely low frequency-modulated microwaves affects cortex-hypothalamus interplay in freely moving rats:EEG study. Int J Radiat Biol, 2010; 86, 376-83. doi:10.3109/09553000903567938 |
| 7. |
Zhao L, Yang YF, Gao YB, et al. Upregulation of HIF-1alpha via activation of ERK and PI3K pathway mediated protective response to microwave-induced mitochondrial injury in neuron-like cells. Mol Neurobiol, 2014; 50, 1024-34. doi:10.1007/s12035-014-8667-z |
| 8. |
Zhu W, Cui Y, Feng X, et al. The apoptotic effect and the plausible mechanism of microwave radiation on rat myocardial cells. Can J Physiol Pharmacol, 2016; 94, 849-57. doi:10.1139/cjpp-2015-0537 |
| 9. |
Moine L, De Barboza GD, Perez A, et al. Glutamine protects intestinal calcium absorption against oxidative stress and apoptosis. Comp Biochem Physiol A Mol Integr Physiol, 2017; 212, 64. doi:10.1016/j.cbpa.2017.07.006 |
| 10. |
Palee S, Chattipakorn SC, Chattipakorn N. Liraglutide preserves intracellular calcium handling in isolated murine myocytes exposed to oxidative stress. Physiol Res, 2017; 24, 889-95. |
| 11. |
Choi S, Quan X, Bang S, et al. Mitochondrial calcium uniporter in Drosophila transfers calcium between the endoplasmic reticulum and mitochondria in oxidative stress-induced cell death. J Biol Chem, 2017; 1, 14473-85. |
| 12. |
Grienberger C, Konnerth A. Imaging calcium in neurons. Neuron, 2012; 73, 862-85. doi:10.1016/j.neuron.2012.02.011 |
| 13. |
Shattock MJ, Ottolia M, Bers DM, et al. Na+/Ca2+ exchange and Na+/K+-ATPase in the heart. J Physiol, 2015; 593, 1361-82. doi:10.1113/jphysiol.2014.282319 |
| 14. |
Nita LI, Hershfinkel M, Sekler I. Life after the birth of the mitochondrial Na+/Ca2+ exchanger, NCLX. Sci China Life Sci, 2015; 58, 59-65. doi:10.1007/s11427-014-4789-9 |
| 15. |
Kumar M, Singh SP, Chaturvedi CM. Chronic Nonmodulated Microwave Radiations in Mice Produce Anxiety-like and Depression-like Behaviours and Calcium-and NO-related Biochemical Changes in the Brain. Exp Neurobiol, 2016; 25, 318-27. doi:10.5607/en.2016.25.6.318 |
| 16. |
Paulraj R, Behari J. Biochemical changes in rat brain exposed to low intensity 9.9 GHz microwave radiation. Cell Biochem Biophys, 2012; 63, 97-102. doi:10.1007/s12013-012-9344-3 |
| 17. |
Cranfield CG, Wood AW, Anderson V, et al. Effects of mobile phone type signals on calcium levels within human leukaemic T-cells (Jurkat cells). Int J Radiat Biol, 2001; 77, 1207-17. doi:10.1080/09553000110083960 |
| 18. |
Dulhunty AF. Excitation-contraction coupling from the 1950s into the new millennium. Clin Exp Pharmacol Physiol, 2006; 33, 763-72. doi:10.1111/cep.2006.33.issue-9 |
| 19. |
Maher P, Van Leyen K, Dey PN, et al. The role of Ca2+ in cell death caused by oxidative glutamate toxicity and ferroptosis. Cell Calcium, 2017; 70, 47-55. |
| 20. |
Di Giuro CML, Shrestha N, Malli R, et al. Na+/Ca2+ exchangers and Orai channels jointly refill endoplasmic reticulum (ER) Ca2+ via ER nanojunctions in vascular endothelial cells. Pflugers Arch, 2017; 469, 1287-99. doi:10.1007/s00424-017-1989-8 |
| 21. |
Mckenzie M, Lim SC, Duchen MR. Simultaneous Measurement of Mitochondrial Calcium and Mitochondrial Membrane Potential in Live Cells by Fluorescent Microscopy. J Vis Exp, 2017, 119. |
| 22. |
Jang S, Javadov S. Association between ROS production, swelling and the respirasome integrity in cardiac mitochondria. Arch Biochem Biophys, 2017; 630, 1-8. doi:10.1016/j.abb.2017.07.009 |
| 23. |
Szymanski J, Janikiewicz J, Michalska B, et al. Interaction of Mitochondria with the Endoplasmic Reticulum and Plasma Membrane in Calcium Homeostasis, Lipid Trafficking and Mitochondrial Structure. Int J Mol Sci, 2017; 18, 1-24. |
| 24. |
Marchi S, Patergnani S, Missiroli S, et al. Mitochondrial and endoplasmic reticulum calcium homeostasis and cell death. Cell Calcium, 2017; 69, 62-72. |
| 25. |
Hu SH, Wang H, Lu L, et al. Real-time Assessment of Cytosolic, Mitochondrial, and Nuclear Calcium Levels Change in Rat Pheochromocytoma Cells during Pulsed Microwave Exposure Using a Genetically Encoded Calcium Indicator. Biomed Environ Sci, 2017; 30, 927-31. |
| 26. |
Neher E, Sakaba T. Multiple roles of calcium ions in the regulation of neurotransmitter release. Neuron, 2008; 59, 861-72. doi:10.1016/j.neuron.2008.08.019 |
| 27. |
Zucker RS. Calcium-and activity-dependent synaptic plasticity. Curr Opin Neurobiol, 1999; 9, 305-13. doi:10.1016/S0959-4388(99)80045-2 |
| 28. |
Wust RC, Helmes M, Martin JL, et al. Rapid frequency-dependent changes in free mitochondrial calcium concentration in rat cardiac myocytes. J Physiol, 2017; 595, 2001-19. doi:10.1113/JP273589 |
| 29. |
Cabassi A, Miragoli M. Altered Mitochondrial Metabolism and Mechanosensation in the Failing Heart:Focus on Intracellular Calcium Signaling. Int J Mol Sci, 2017; 18, 1-14. |
| 30. |
Adey WR. Neurophysiologic effects of radiofrequency and microwave radiation. Bull N Y Acad Med, 1979; 55, 1079-93. |
| 31. |
Shelton WW Jr, Merritt JH. In vitro study of microwave effects on calcium efflux in rat brain tissue. Bioelectromagnetics, 1981; 2, 161-7. |
| 32. |
Dutta SK, Subramoniam A, Ghosh B, et al. Microwave radiation-induced calcium ion efflux from human neuroblastoma cells in culture. Bioelectromagnetics, 1984; 5, 71-8. doi:10.1002/(ISSN)1521-186X |
| 33. |
Kesari KK, Kumar S, Behari J. Pathophysiology of microwave radiation:effect on rat brain. Appl Biochem Biotechnol, 2012; 166, 379-88. doi:10.1007/s12010-011-9433-6 |
| 34. |
Wang LF, Wei L, Qiao SM, et al. Microwave-Induced Structural and Functional Injury of Hippocampal and PC12 Cells Is Accompanied by Abnormal Changes in the NMDAR-PSD95-CaMKⅡ Pathway. Pathobiology, 2015; 82, 181-94. doi:10.1159/000398803 |
| 35. |
Xiong L, Sun CF, Zhang J, et al. Microwave exposure impairs synaptic plasticity in the rat hippocampus and PC12 cells through over-activation of the NMDA receptor signaling pathway. Biomed Environ Sci, 2015; 28, 13-24. |
| 36. |
Zhang X, Gao Y, Dong J, et al. The compound Chinese medicine "Kang Fu Ling" protects against high power microwave-induced myocardial injury. PLoS One, 2014; 9, e101532. doi:10.1371/journal.pone.0101532 |
| 37. |
Wei J, Sun J, Xu H, et al. Effects of extremely low frequency electromagnetic fields on intracellular calcium transients in cardiomyocytes. Electromagn Biol Med, 2015; 34, 77-84. doi:10.3109/15368378.2014.881744 |
 2018, Vol. 31
2018, Vol. 31


