2. National Institute for Communicable Disease Control and Prevention, State Key Laboratory for Infectious Disease Prevention and Control, Chinese Center for Disease Control and Prevention, Beijing 102206, China;
3. Center for Disease Control and Prevention of Xinjiang Uygur Autonomous Region, Urumqi 830011, Xinjiang, China;
4. Medical Laboratory Center, the First Affiliated Hospital of Xinjiang Medical University, Urumqi 830054, Xinjiang, China;
5. Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, Hangzhou 310058, Zhejiang, China
Streptococcus Pneumoniae (S. pneumoniae) infections can result in serious invasive pneumococcal disease (IPD)[1]. IPD usually results in a high case fatality rate of 5%-50% worldwide. In China, about 30, 000 children die of pneumococcal disease each year. Patients with pneumococcal disease and healthy carriers are all sources of infection.
Studying carriage and serotype distribution of S. pneumoniae will improve our understanding of the epidemiology of S. pneumoniae as well as support the introduction and measurement of the impact of widespread pneumococcal vaccination. Cross-sectional surveys are usually performed to investigate pneumococcal carriage by culturing and agglutination reactions. Real-time PCR methodology could be an optimal choice, and the pneumococcal lytA gene was considered a good target gene for S. pneumoniae species testing by real-time PCR.
The use of pneumococcal vaccines (PVC) has resulted in profound declines in IPD, pneumonia, and otitis media[2, 3]. In China, PCVs have not been adopted into the national immunization schedules; therefore, data on pneumococcal carriage are limited. Population-based studies are warranted. Understanding of pre-vaccine carriage rates of S. pneumoniae will provide a baseline estimate, which permits evaluation of the effect of upcoming PCV mass immunization and facilitates monitoring of serotype replacement in Xinjiang, China.
Ethics Statement Exemption The study consent and questionnaire forms were all submitted and approved by the Xinjiang Autonomous Region center for disease control and prevention ethical review committee. Each participant was informed in writing before they were sampled and provided a signed consent form. For children, the consent form was signed by their guardian.
Study Sites and Population Aksu City and Yining City were chosen as the sentinel sites for this study. Aksu City is located in the south of Xinjiang and adjoins with the desert of Taklamakan. Yining City is located in the northwest of Xinjiang and on the northern side of the Ili River in the Dzungarian Basin. The ethnic composition, quality of healthcare, and living conditions are different in the two cities. Healthy individuals and those without acute respiratory infections before the enrollment date were included in the study. The study population were recruited from kindergartens, vaccination clinics, and schools. Based on a 5% maximum permissible error, we estimated a 50% carriage rate[4] at a 5% significance level.
Laboratory Testing Each participant was swabbed at the oropharynx using fiber swabs (Classiq Swabs, COPAN Italy S.p.A.). Initially, real-time PCR targeting lytA gene was performed to identify the S. pneumoniae species. Twelve serotypes (4, 5, 6A/B, 7A/F, 9V/A, 10A/B, 14, 15, 18B/C, 19A, 19B/F, and 23F) were detected in the lytA-positive samples (CT value was < 38). Primers and probes for real-time PCR are listed in Supplementary Table S1[5] (available in www.besjournal.com). A negative control (ultrapure water) and a positive control (DNA extracted from cultures positive for S. pneumoniae serotype) were included in every run. PCR reaction conditions were as follows: 1 cycle of 95 ℃ for 30 s, 50 cycles of 95 ℃ for 5 s, and 60 ℃ for 30 s. The result was considered positive when the CT value was < 38.
|
|
Supplementary Table S1 Primers and Probes for S. pneumoniae Serotypes by Real-time PCR |
Statistical Analysis Data were analyzed using SPSS for Windows, version 17.0 (SPSS Inc., Chicago, IL, USA). The values (%) for categorical variables such as region, age, ethnicity, gender, and other pneumococcal carriage rates, and constituent ratios were assessed using chi-square test to test for statistical significance of compared variables. P-values < 0.05 were considered statistically significant. The significance level was adjusted for multiple testing of age groups using the Bonferroni correction; therefore, the P-value for statistical significance was P = 0.003 (0.05/15).
Carriage Rate of S. pneumonia A total of 513 healthy individuals (female, 294, 57.3%; male, 219, 42.7%) were enrolled in this study, including 250 from Aksu City and 263 from Yining City. Participants' age ranged from 2 months to 53 years. All participants were not vaccinated with pneumococcal vaccine.
The total carriage rate of S. pneumoniae was 70.4% (361/513). Results of the chi-square test showed that there were statistically significant differences between region, ethnicity, and occupation (P < 0.001). The carriage rate of S. pneumoniae in Aksu City (81.2%, 203/250) was higher than that in Yining (60.1%, 158/263) (P < 0.001). The carriage rate of S. pneumoniae was highest among kindergarten children (80.4%) and lowest among medical workers (33.3%). Significant differences were observed among scattered children (66.3%), kindergarten children (80.4%), and students (75.8%) when compared with medical workers (33.3%) (P < 0.001, Table 1).
|
|
Table 1 The Carriage Rate of S. pneumoniae in Yining and Aksu City |
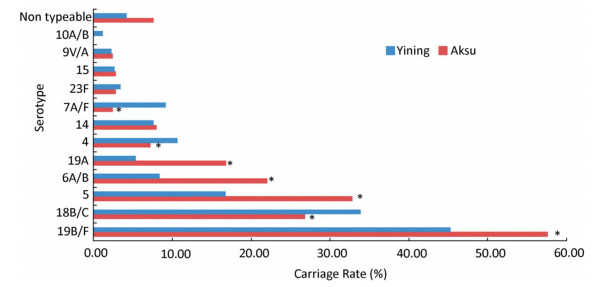
Serotype Distributions of S. pneumoniae In 361 lytA-positive individuals, 331 specimens were serotyped using real-time PCR, which targeted 12 serotype-specific primers and probes. The other 30 specimens were all negative for the 12 serotypes. Serotypes 19B/F, 18B/C, 5, and 6A/B were the major serotypes of S. pneumoniae, accounting for 51.3%, 30.4%, 24.6%, and 15.0%, respectively (Figure 1).
|
Download:
|
| Figure 1 The carriage rate of pneumococcal serotypes. Red bar represents the carriage rate in Aksu City, and the blue bar represents the carriage rate in Yining City. Statistically, the carriage rate for each serotype was compared between the two cities (*P < 0.05). | |
The carriage rates of serotypes 19B/F, 5, 6A/B, 19A, 18B/C, 4, and 7A/F were significantly different between the two cities. The carriage rates of 19B/F, 5, 6A/B, and 19A were higher in Aksu than in Yining; carriage rates of serotypes 18B/C, 4, and 7A/F were higher in Yining than in Aksu. However, there was no statistically significant difference between serotypes 14, 23F, 15, 9V/A, and 10A/B.
The carriage rate for each age group was higher in Aksu City than that observed in Yining City. In addition, it was significantly different between age groups (P < 0.001), increasing from 67.1% (57/85) in a group of individuals aged < 3 years, peaking at 88.6% (70/79) in those aged 6-10 years, and progressively decreasing to 51.1% (48/94) in those aged ≥ 21 years. In 12 serotypes, most of the high carriage rates were evident among individuals aged 3-5 years, 6-10 years, and 11-15 years.
Co-colonization with Multiple Serotypes A total of 67 (20.24%) samples had only one serotype in the 331 samples that could be serotyped. Co-colonization with two to six serotypes was identified in 264 specimens (79.8%). The proportion of samples with two to six serotypes decreased successively, where 20.5% (n = 105) had two co-colonizations, 18.3% (n = 94) had three co-colonizations, 9.4% (n = 48) had four co-colonizations, 2.9% (n = 15) had five co-colonizations, and 0.4% had six co-colonizations (n = 2).
A total of 107 multiple serotype combinations were identified. Two and three multiple serotypes were common (n = 199). Major serotypes in co-carried specimens were 19B/F, 18B/C, 5, and 6A/B, accounting for 71.0%, 46.7%, 40.2%, and 28.0%, respectively. The most common combinations for two serotypes were 19B/F + 18B/C (9.7%, n = 32), 19B/F + 5 (3.9%, n = 13), and 19B/F + 6A/B (3.6%, n = 12). The major combinations for three serotypes were 19B/F + 5 + 18B/C (5.4%, n = 18), 19B/F + 18B/C + 6A/B (2.7%, n = 9), 19A + 19B/F + 18B/C (2.4%, n = 8), and 19B/F + 5 + 6A/B (2.4%, n = 8).
Discussion Colonization with S. pneumoniae is a prerequisite to invasive infections. In this study, the carriage rate of the pneumococcal lytA gene was 70.4%. The prevalence rate of S. pneumoniae carriage in healthy populations has been shown to vary from 27% in developed countries to 85% in other developing countries[6]. The difference in serotype carriage is related to genetic variables, socioeconomic conditions (housing and healthcare provision), family size, overcrowded living conditions, and number of siblings, which all affect transmission[7, 8]. One reason for the high carriage in this population is due to the lack of pneumococcal vaccination. Secondly, oropharyngeal sampling was more effective than nasopharyngeal sampling in evaluating pneumococcal carriage.
Gillis et al. suggested that simultaneous detection of two genetic targets (lytA and cpsA) is preferred[9]. However, in this study, only 30 of the 361 lytA-positive specimens were identified as non-typeable organisms. In effect, at least 331 positive specimens were serotyped, and this was a conservative estimation. In addition, 5.85% (30/513) of the lytA gene-positive oropharyngeal swabs were negative for all 12 serotypes tested, implying that other serotypes were undetected, or were lytA gene-positive non-typeable organisms.
When assessing the distribution of the organism by occupation, kindergarten children have the highest carriage rate for S. pneumoniae, which is similar to other studies. By contrast, medical workers have the lowest carriage rate, which is a surprising finding. In our study, the medical workers were not clinicians but were all healthcare workers who worked in vaccination clinics. Most of the people they come into contact with are healthy children or adults who have been vaccinated, and not sick patients. In addition, as medical workers, they have a strong perception of healthcare and are likely to be vigilant when it comes to preventing disease. These are the main reasons why the medical workers have the lowest carriage rate. Alternatively, this may also be a result of information bias.
Carriage rates gradually increase by age, peaking at the age of 6-10 years (especially in Aksu City, where the carriage rate is 97.8%). This observation is different from the previous studies performed in some industrialized countries, where the highest carriage rate was noted in children < 5 years. More attention should be focused on children aged 6-10 years.
In this study, we only detected 12 serotypes as not all serotypes are detectable with adapted real-time PCR. Multiple serotypes were most commonly detected, where the proportion of two and three serotypes carried simultaneously in all of specimens was 38.8%. In our study, the carriage of multiple serotypes was the most common phenomenon, with 79.8% of all oropharyngeal swabs having multiple serotypes. Serotypes 19B/F, 18B/C, 5, and 6A/B were the most frequently identified multiple serotypes. Comparatively, the carriage rate of 23F was low, compared with serotypes 18B/C and 5. This result is different from those of the studies conducted in other countries and other provinces of China. In previous studies, serotypes 6A, 6B, 19A, 19F, and 23F were the most frequently isolated serotypes. Our study confirmed that the distribution of S. pneumoniae serotypes varies significantly by both geography and age group. The development of future pneumococcal vaccines should consider specific serotype attributes by target country.
The current data provide a good scientific baseline for introduction of a national pneumococcal vaccine program. Hence, a follow-up study with additional serotypes should be conducted in the future.
Acknowledgements We would like to thank the staff at Center for Disease Control and Prevention of Aksu Prefecture, Xinjiang, China, and the Center for Disease Control and Prevention of Yining City, Xinjiang, China, for their assistance in this study.
Declaration of Interest The authors declare no conflicts of interest.
| 1. | Devine VT, Cleary DW, Jefferies JM, et al. The rise and fall of pneumococcal serotypes carried in the pcv era. Vaccine, 2017, 35: 1293–8. doi:10.1016/j.vaccine.2017.01.035 |
| 2. | Rose M, Zielen S. Impact of infant immunization programs with pneumococcal conjugate vaccine in europe. Expert Rev Vaccines, 2009, 8: 1351–64. doi:10.1586/erv.09.78 |
| 3. | Pilishvili T, Lexau C, Farley MM, et al. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis, 2010, 201: 32–41. doi:10.1086/648599 |
| 4. | Adegbola RA, Deantonio R, Hill PC, et al. Carriage of streptococcus pneumoniae and other respiratory bacterial pathogens in low and lower-middle income countries:A systematic review and meta-analysis. Plos One, 2014, 9: e103293. doi:10.1371/journal.pone.0103293 |
| 5. | Azzari C, Moriondo M, Indolfi G, et al. Realtime pcr is more sensitive than multiplex pcr for diagnosis and serotyping in children with culture negative pneumococcal invasive disease. Plos One, 2010, 5: e9282. doi:10.1371/journal.pone.0009282 |
| 6. | English AI. Pneumococcal vaccines who position paper——2012. Wkly Epidemiol Rec, 2012, 87: 129–44. |
| 7. | Johargy AK, Momenah AM, Ashgar SS, et al. Potential risk of streptococcus pneumoniae in nasopharyngeal carriage during umrah and hajj seasons in makkah, saudi arabia. Journal of Microbial & Biochemical Technology, 2017, 8: 14–20. |
| 8. | Bogaert D, De GR, Hermans PW. Streptococcus pneumoniae colonisation:The key to pneumococcal disease. Lancet Infect Dis, 2004, 4: 144–54. doi:10.1016/S1473-3099(04)00938-7 |
| 9. | Gillis HD, Als L, ElSherif M, et al. Assessing the diagnostic accuracy of pcr-based detection of streptococcus pneumoniae from nasopharyngeal swabs collected for viral studies in canadian adults hospitalised with community-acquired pneumonia:A serious outcomes surveillance (sos) network of the Canadian Immunization Research (CIRN) study. BMJ, 2017, 7: e015008. |
 2018, Vol. 31
2018, Vol. 31


