2. Department of Rehabilitation, Hangzhou Sanatorium of PLA, Hangzhou 310007, Zhejiang, China;
3. Zhejiang Forestry Academy, Hangzhou 310023, Zhejiang, China;
4. Hangzhou Forestry Academy, Hangzhou 310022, Zhejiang, China
Chronic heart failure (CHF), which is caused due to the consequences of various cardiovascular diseases (CVDs), is defined as a persistent condition of heart failure and has become an increasing global cause of morbidity and mortality since the recent several decades[1]. Emerging evidence has revealed an association between environmental pollution, especially air pollution, and CHF in urban communities based on a series of observational and epidemiologic studies[2]. Currently available pharmacological therapies provide limited impact on the long-term outlook for patients with CHF. Therefore, it is necessary to develop novel interventional strategies for patients with CHF and related CVDs.
Forest bathing, originally described as Shinrin-yoku in Japan[3], is now attracting increasing attentions due to its health-promotion effects in humans. Our team had initiated the studies on analyzing the effects of forest bathing on human health since 2010 and demonstrated its beneficial effects in normal young subjects[4] and elderly patients with essential hypertension[5], chronic obstructive pulmonary disease[6], and CHF[7], which are primarily in agreement with other reports[8, 9]. However, the optimal frequency of forest bathing and the duration of the impact on human health, especially for patients with CHF, still remains to be elucidated.
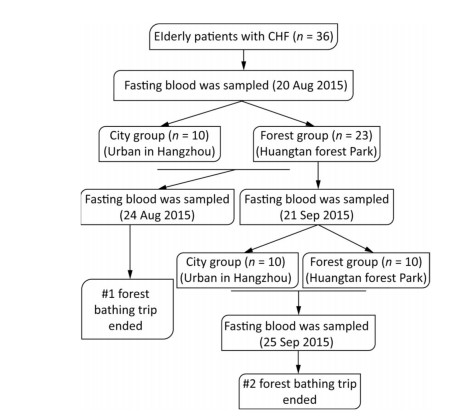
In this study, a total of 45 patients with CHF diagnosed according to the criteria of the American New York Heart Association were recruited as subjects from the urban area in Hangzhou city, China. Based on the inclusion and exclusion criteria described in our previous study[7], a total of 36 patients with CHF were enrolled. The study was conducted in accordance with the Declaration of Helsinki and was approved by the ethics committee of Zhejiang Hospital. This study is registered in the Chinese Clinical Trial Registry (ChiCTR-OOC-15006853). Signed informed consent was obtained from every subject. The experimental procedure and schedule are depicted in Figures S1 & S2 (available at www.besjournal.com), and the details of the study design have been mentioned previously[7]. The study was conducted at two different sites (forest and city) during August 20-24, 2015 (first forest bathing trip), and September 21-25, 2015 (second forest bathing trip). The two experimental sites have been described previously[7], i.e., a forest park named Huangtan located in Pan'an County, Zhejiang Province, as the forest site and an urban site located in the downtown area of Hangzhou that was set as the control. Before the start of the first experiment, all the 36 patients with CHF were randomly categorized at a ratio of 1:2 into two groups consisting of 12 patients in the city control group and 24 patients in the forest group. One participant in the forest group and two in the city group quit during the experiment process, finally resulting in 10 participants in the city control group and 23 participants in the forest group after the first experiment. After 4 weeks, the patients who had experienced the first forest bathing trip were recruited again and 20 of them were enrolled for the second experiment. These 20 patients with CHF were randomly categorized into two groups consisting of 10 patients in each group. During our experiments, drinking water was provided freely to the participants to prevent dehydration, although alcoholic beverages and tea were avoided. The subjects were also accompanied by a nurse and a doctor during their outside walk as a consideration for their safety. Fasting blood samples from each participant were collected before and after each experiment. The levels of brain natriuretic peptide (BNP), interleukin-6 (IL-6), and tumor necrosis factor α (TNF-α) were analyzed using commercially available enzyme-linked immunosorbent assay kits (CUSABIO, Wuhan, China). The activities of serum total SOD (T-SOD) and lipid peroxidation reflected by malondialdehyde (MDA) levels were evaluated using standard assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
|
Download:
|
| Figure S1 The experimental procedure for the twice forest bathing study. | |

|
Download:
|
| Figure S2 The experimental protocol for subjects exposed to the forest or city environment. | |
Results were expressed as mean ± standard deviations SPSS (19.0 version) was used for statistical analysis. Student's t-test or a nonparametric test (Mann-Whitney U test or Wilcoxon signed rank test) was used for two independent or related samples according to the samples' normality and homogeneity of variances initially evaluated by the Kolmogorov-Smirnov test and the Levene's test, respectively. The Kruskal-Wallis test was conducted for analyzing the multigroup comparisons, and a Dunn-Bonferroni test was used for post hoc comparisons. Chi-square test was used to compare proportions. A P value of < 0.05 was considered to be statistically significant.
No statistically significant differences were observed in the clinical characteristics of the enrolled participants between the two groups (Tables S1 & S2, available at www.besjournal.com). None of the participants reported any adverse events during the two experimental sessions.
|
|
Table S1 Clinical Characteristics of the Participants |
|
|
Table S2 The Gender Ratio and Distribution of New York Heart Association Class of the Participants for the Second Experiment |
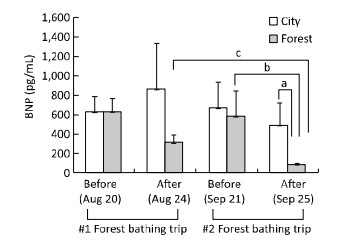
Circulating levels of BNP have been reported to be directly associated with cardiac function and are identified as a marker of CHF[10]. As shown in Figure 1, the BNP levels were similar between the two groups before the first experiment. Later, patients who experienced the 4-day forest bathing trip (on August 24, 2015) showed a significantly lower BNP level compared with that in patients in the city group or the baseline level (on August 20, 2015). The BNP levels of the control group remained statistically unchanged. However, after a 4-week interval (on September 21, 2015), the BNP levels of the participants who experienced the first forest bathing trip reversed to their initial baseline levels (on August 20, 2015). Interestingly, a further decline in the BNP level was observed in the subjects after the second forest bathing trip (on September 25, 2015). Nevertheless, the BNP levels remained unchanged among those who experienced the first forest bathing trip and stayed in the control urban area during the second experiment (control for the second experiment). Blood pressure was monitored simultaneously during the two experiments, which revealed a declining trend of systolic blood pressure and diastolic blood pressure in the participants after the forest bathing trips, although the change was not statistically significant (Figure S3, available at www.besjournal.com). This result may be because some of the patients with CHF did not have hypertension. They had a normal level of blood pressure and showed little response to the forest bathing experiments in terms of lowering the blood pressure. Therefore, this result indicates a narrow effect of forest bathing on hypertension in the current experiments.
|
Download:
|
| Figure 1 Effect of twice forest bathing trips on circulating levels of brain natriuretic peptide (BNP) in elderly patients with chronic heart failure (CHF). For the first experiment, n = 10 in the City group and n = 23 in the Forest group. After the first forest bathing trip, the 23 subjects from the first forest group was recruited after four weeks and 20 enrolled patients were divided randomly into two groups (n = 10 in City; n = 10 in Forest) for a second time of the four-day forest bathing trip. aP < 0.05, analyzed by Mann-Whitney U test; bP < 0.05, analyzed by Wilcoxon Signed Ranks test; cP < 0.01, analyzed by the Kruskal-Wallis test, followed by the Dunn-Bonferroni test. | |
|
Download:
|
| Figure S3 Effect of forest bathing on blood pressure indicators in CHF subjects. After the first four-day forest bathing trip (n = 10 for City; n = 23 for Forest), 20 patients experienced the first forest bathing trip were divided randomly into two groups for a second experiment (n = 10 in City; n = 10 in Forest) four weeks later. The systolic blood pressure (SBP, A) and diastolic blood pressure (DBP, B) was measured via a mercury sphygmomanometer. | |
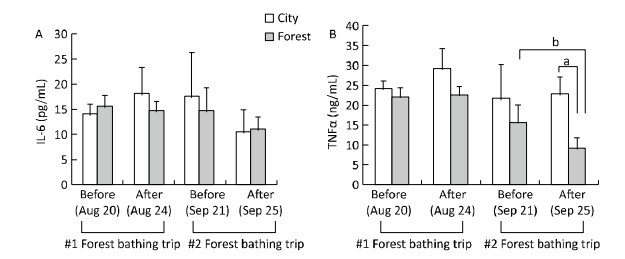
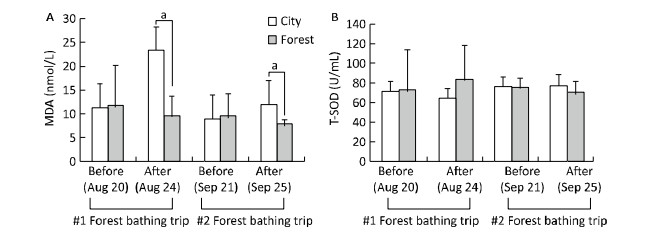
The pathogenesis of CVD, including heart failure, has been reported to be associated with a high inflammatory response and oxidative stress[11]. In the present study, the levels of the inflammatory cytokines IL-6 and TNF-α were found to be evaluated. After the second forest bathing trip (on September 25, 2015), the participants of the forest group showed a significant decrement in TNF-α levels (Figure 2). In addition, a decreased trend of IL-6 was observed in both the two groups after the second experiment, although the change was not statistically different, which may be due to the small sample size. The activities of serum T-SOD and MDA levels were selected as oxidative indicators (Figure 3), and the results showed that the T-SOD levels remained statistically unchanged after the second experiment in both the two groups. However, a lower level of MDA was observed in the forest group compared with that in the control group after the second experiment, showing a similar trend to that of the first experiment.
|
Download:
|
| Figure 2 Effect of twice forest bathing trip on serum pro-inflammatory cytokines in the participants. Interleukin-6 (IL-6, A), and tumor necrosis factor α (TNF-α, B) were evaluated. After the first four-day forest bathing trip (n = 10 for City; n = 23 for Forest), 20 patients experienced the first forest bathing trip were divided randomly into two groups for a second experiment (n = 10 in City; n = 10 in Forest) four weeks later. aP < 0.05, analyzed by Mann-Whitney U test; bP < 0.05, analyzed by Wilcoxon Signed Ranks test. | |
|
Download:
|
| Figure 3 Effect of twice forest bathing on oxidative stress alteration reflected by malondialdehyde (MDA, A) activity and total superoxide dismutase (T-SOD, B) level in the experimental subjects. Four weeks after the first four-day forest bathing trip (n = 10 for City; n = 23 for Forest), 20 patients experienced the first forest bathing trip were divided randomly into two groups for a second experiment (n = 10 in City; n = 10 in Forest). aP < 0.05, analyzed by Mann-Whitney U test. | |
The intake of cardiovascular drugs in each subject was also considered in this study due to their potential effects on the heart function. The proportion of drug intake, including angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, calcium channel blockers, beta-receptor blockers, and antiplatelet agents (clopidogrel or aspirin), was not significantly different between the city and forest groups of the first and second forest bathing experiments according to the chi-square analysis (Table S3 available at www.besjournal.com). In addition, because the individual administration of drugs for each participant was carried out in the usual manner during the two 4-day experimental periods, the intake of drugs may not have affected the comparison between the pre-experimental levels and post-experimental levels for each group.
|
|
Table S3 The Medication Information of the Participants |
In conclusion, despite the small sample size, our exploratory study concerning the health impact duration of forest bathing on elderly patients with CHF provides supportive evidence that two 4-day forest bathing trips with a 4-week interval could offer additive benefits as reflected by the decline in BNP levels and the attenuated inflammatory response and oxidative stress. These preliminary results are encouraging, and the details for the optimal frequency of multiple forest bathing trips will be considered in our future work, which will further pave the way for analyzing the effects of such intervention in patients with CVDs.
Acknowledgment We appreciate the assistance of Mr. CHEN Xiu Yuan from the Forestry Bureau of Pan'an County for his service as a guide at the forest experimental site.
| 1. | Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol, 2011, 8: 30–41. doi:10.1038/nrcardio.2010.165 |
| 2. | Shah AS, Langrish JP, Nair H, et al. Global association of air pollution and heart failure:a systematic review and meta-analysis. Lancet, 2013, 382: 1039–48. doi:10.1016/S0140-6736(13)60898-3 |
| 3. | Li Q, Morimoto K, Nakadai A, et al. Forest bathing enhances human natural killer activity and expression of anti-cancer proteins. Int J Immunopathol Pharmaco, 2007, 20: 3–8. doi:10.1177/03946320070200S202 |
| 4. | Mao GX, Lan XG, Cao YB, et al. Effects of short-term forest bathing on human health in a broad-leaved evergreen forest in Zhejiang Province, China. Biomed Environ Sci, 2012, 25: 317–24. |
| 5. | Mao GX, Cao YB, Lan XG, et al. Therapeutic effect of forest bathing on human hypertension in the elderly. J Cardiol, 2012, 60: 495–502. doi:10.1016/j.jjcc.2012.08.003 |
| 6. | Jia BB, Yang ZX, Mao GX, et al. Health Effect of Forest Bathing Trip on Elderly Patients with Chronic Obstructive Pulmonary Disease, Biomed Environ Sci, 2016; 29, 212-8. |
| 7. | Mao GX, Cao YB, Wang BZ, et al. The salutary influence of Forest bathing on elderly patients with chronic heart failure. Int J Environ Res Public Health, 2017, 14: E368. doi:10.3390/ijerph14040368 |
| 8. | Ideno Y, Hayashi K, Abe Y, et al. Blood pressure-lowering effect of Shinrin-yoku (Forest bathing):a systematic review and meta-analysis. BMC Complement Altern Med, 2017, 17: 409. doi:10.1186/s12906-017-1912-z |
| 9. | Hansen MM, Jones R, Tocchini K. Shinrin-Yoku (Forest Bathing) and Nature Therapy:A State-of-the-Art Review. Int J Environ Res Public Health, 2017, 14: E851. doi:10.3390/ijerph14080851 |
| 10. | Latini R, Masson S, Anand I, et al. The comparative prognostic value of plasma neurohormones at baseline in patients withheart failure enrolled in Val-HeFT. Eur Heart J, 2004, 25: 292–9. doi:10.1016/j.ehj.2003.10.030 |
| 11. | Gutierrez A, Van Wagoner DR. Oxidant and inflammatory mechanisms and targeted therapy in atrial fibrillation:An update. J Cardiovasc Pharmacol, 2015, 66: 523–9. doi:10.1097/FJC.0000000000000313 |
 2018, Vol. 31
2018, Vol. 31


