2. Beijing Tuberculosis and Thoracic Tumor Research Institute, Beijing 101149, China;
3. Administration Office, Clinical Center on Tuberculosis Control, Chinese Center for Disease Control and Prevention, Beijing 101149, China;
4. Public Health Monitor and Information Service Center, Chinese Center for Disease Control and Prevention, Beijing 102206, China;
5. Department of Tuberculosis, Prevention and Treatment Hospital of Shanxi Province, Xian 710100, Shanxi, China
Tuberculosis (TB) is still a global public health problem, with an estimated 10.4 million new TB cases worldwide and 918, 000 TB cases in China in 2015[1]. The incidence of extrapulmonary TB is about 25%-30%[2]. The diagnosis of extrapulmonary TB is generally more difficult than that of TB. Tuberculous meningitis (TBM) is a type of extrapulmonary TB accounting for approximately 1% of all TB cases[3]. Despite this low proportion of TBM among TB cases, TBM remains the most lethal form of TB, with a fatality rate of up to 50% and 20%-30% of surviving patients having permanent sequelae of the central nervous system[4]. Therefore, rapid and effective diagnosis and early treatment of TBM are particularly critical factors to determine the outcome. However, the clinical manifestations of TBM lack specificity, due to which it is notoriously difficult to diagnose TBM promptly. The detection of acid-fast bacilli (AFB) in cerebrospinal fluid (CSF) smear can directly be used to diagnose TBM, although the detection rate is low[5]. Furthermore, despite a higher sensitivity of the Lowenstein-Jensen (L-J) culture medium, it cannot be used to guide the clinical diagnosis and timely treatment as this test requires a long time to provide a result.
The T-SPOT.TB test that was developed gradually in recent years is a diagnostic method for TB and is based on the detection of IFN-gamma-secreting T cells by specific antigen stimulation; it is primarily used for the diagnosis of TB infection. A previous study has reported a sensitivity and specificity of 84.95% and 85.12%, respectively, for the T-SPOT.TB test in diagnosing active pulmonary TB[6]. Unfortunately, this test has been rarely applied in the diagnosis of TBM. The aim of our study was to assess the diagnostic value of CSF T-SPOT.TB (Oxford Immunotec Ltd., Abingdon, UK) in diagnosing TBM in the Chinese population.
In this retrospective study, a total of 96 HIV-negative patients with suspected meningitis were enrolled from Beijing Chest Hospital during 2010-2013. Informed consent was obtained from all subjects, and the study protocol was approved by the ethics committee of Beijing Chest Hospital. The patients were categorized into two groups based on the diagnostic criteria for TBM, one was TBM group and another was non-TBM group, according to 'a consensus case definition for tuberculous meningitis, ' as follows: definite, probable, possible, or not TBM, respectively. (1) Definite TBM was diagnosed based on microbiological identification or evidence from commercial nucleic acid amplification tests of central nervous system Mycobacterium tuberculosis infection. (2) Probable TBM: when imaging is available a diagnostic score of 12 or above is required, and when imaging is not available, a diagnostic score of 10 or above is required. (3) Possible TBM: when imaging is available a diagnostic score of 6-11 is required, and when imaging is not available, a score of 6-9 is required. The definite, probable, and possible TBM cases belonged to the TBM group. In addition, we classified stages Ⅰ, Ⅱ, and Ⅲ of TBM according to the clinical signs of patients presenting with TBM, which can be easily assessed for severity based on modifications of the Medical Research Council staging system[8]. About 10 mL of CSF was collected for performing T-SPOT.TB testing, adenosine deaminase (ADA) testing, bacteriological testing, and so on; these tests were conducted within 4 h after sampling.
Data analysis was conducted by SAS version 9.1 (SAS Institute Inc., Cary, NC, USA). The characteristics of all subjects were analyzed descriptively; the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were assessed between CSF-ADA testing and CSF T-SPOT.TB testing among patients with TBM and patients with different stages of TBM, respectively. The ROC curve was drawn to assess the diagnostic accuracy, and the Youden's Index (YI) was used to select the optimum cut points on the ROC curve and calculate the areas under the curve (AUCs).
A total of 96 HIV-negative patients were evaluated during the 3-year study period. The proportion of patients with TBM and that of non-TBM cases were 54.2% (52/96) and 45.8% (44/96), respectively. Of the TBM patients, 12 patients were confirmed as having TBM by bacteriological, or polymerase chain reaction (PCR) tests. Of the 52 patients with TBM, 12, 26, and 14 patients had definite, probable, and possible TBM, respectively. The median age was 32 years (range: 23.00-44.25 years), 61.5% (32/52) of females was higher compared to male, and patients with stages Ⅰ, Ⅱ, and Ⅲ of TBM accounted for 23.1% (12/52), 57.7% (30/52), and 19.2% (10/52), respectively.
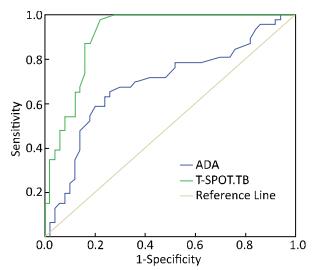
The comparison between the T-SPOT.TB test and the ADA test for the diagnostic accuracy is illustrated in Table 1 and Figure 1. The sensitivity (97.8%) and specificity (78.0%) of the T-SPOT.TB test were higher than those of the ADA test (63.0% and 74.0%, respectively); meanwhile, the AUC for the T-SPOT.TB test was 0.910, whereas it was only for 0.689 for the ADA test.
|
|
Table 1 ROC Curve Results of T-SPOT.TB and ADA Tests for Diagnosing TBM |
|
Download:
|
| Figure 1 ROC curve analysis of T-SPOT.TB and ADA tests for diagnosing TBM. | |
We further compared the T-SPOT.TB and ADA tests for different stages of TBM, and the results showed that the sensitivity and specificity of the T-SPOT.TB test for stage Ⅰ (96.7% and 77.8%), stage Ⅱ (97.2% and 77.9%), and stage Ⅲ (98.9% and 78.1%) were, respectively, significantly higher than those for the ADA test. In addition, the sensitivity and specificity of the T-SPOT.TB test for stage Ⅲ of TBM were higher than those for stages Ⅰ and Ⅱ, as shown in Table 2.
|
|
Table 2 Comparison of T-SPOT.TB and ADA Tests for Different Stages of TBM |
Currently, the diagnosis of TBM is a global issue and has become increasingly important. The conventional methods of culturing on solid and liquid media are the gold standard for diagnosis, though most of the cases show negative results, which usually causes difficulty in visualization of AFB in direct smears or in cultures of CSF for TBM. Thakur et al. found that smear positivity for AFB was 7.9%, while the automated Bactec MGIT 960 system displayed a higher rate of recovery of MTB (27.4%) than that by L-J media (10.9%)[9]. In our study, AFB were not found in any patient, but 12 patients (23.1%) had a positive result of culture or PCR of CSF. Thus, the low sensitivity of bacteriological examination of CSF cannot fulfill the diagnostic criteria of TBM.
In our study, we showed that the sensitivity of the CSF T-SPOT.TB test was 97.8%, which was significantly higher than those reported from South Korea (59%)[10] and by a meta-analysis (77%)[11], indicating that the T-SPOT.TB test was useful in diagnosing TBM. In recent years the CSF-ADA test was reported to have considerable value in the diagnosis of TBM, with a sensitivity of 92% and a specificity of 90.47%[12], whereas, in the present study, the sensitivity (63.0%) and specificity (74.0%) of the ADA test were lower. We also found the ADA test had 69.0% PPV and 68.5% NPV, which were lower than those for the CSF T-SPOT.TB test (80.3% and 97.5%, respectively). We also used ROC curves to compare the T-SPOT.TB test and the ADA test, which showed that the AUCs for the two diagnostic methods were 0.910 and 0.689, respectively. Thus, our data suggest that the T-SPOT.TB test is a rapid and accurate diagnostic method for TBM in China, and the diagnostic value of ADA test for TBM was unfavorable.
In addition, in the current study, we compared the diagnostic performance of the T-SPOT.TB and ADA tests in patients with different stages of TBM, and the results showed that the sensitivities of the T-SPOT.TB test for stages Ⅰ, Ⅱ, and Ⅲ of TBM were significantly higher than those of the ADA test, and the corresponding specificities of the T-SPOT.TB test were also slightly higher. Furthermore, the sensitivity and specificity of the T-SPOT.TB test were the highest for stage Ⅲ of TBM, whereas no similar studies on T-SPOT.TB test for different stages of TBM have been reported yet. Our study indicated that the T-SPOT.TB test may currently be the most effective method for diagnosing TBM in China.
Several limitations exist in our study. First, the number of enrolled cases was small, due to which we could not further analyze the definite, probable, and possible TBM cases. A large sample size is needed to determine the diagnostic value of CSF T-SPOT.TB for TBM in the future. The second limitation is the bias in patient selection. All the patients in our study were recruited from one hospital near the northeast of China, which cannot represent the status of entire China. Despite these limitations, our study still has several strengths. To the best of our knowledge, this is the first study to investigate the diagnostic value of CSF T-SPOT.TB test for different stages of TBM. Our data confirmed that the CSF T-SPOT.TB test showed a favorable diagnostic performance in patients with different stages of TBM, and we also found that the CSF T-SPOT.TB test had higher sensitivity and specificity for diagnosing TBM. These results can probably be used as a reference to apply CSF T-SPOT.TB as a routine laboratory test for the diagnosis of TBM.
AcknowledgmentsWe would like to express our gratitude to the participants for their cooperation in this study.
| 1. | World Health Organization. G Global Tuberculosis Report 2016, WHO, Geneva, Switzerland, 2016. |
| 2. | Sharma SK, Mohan A. Extra-pulmonary tuberculosis. Indian J Med Res, 2004, 120: 316–53. |
| 3. | Thwaites GE, van Toorn R, Schoeman J. Tuberculous meningitis:more questions, still too few answers. Lancet Neurol, 2013, 12: 999–1010. doi:10.1016/S1474-4422(13)70168-6 |
| 4. | Joosten AA, van der Valk PD, Geelen JA, et al. Tuberculous meningitis:pitfalls in diagnosis. ActaNeurol Scand, 2000, 102: 388–94. |
| 5. | Mekonnen A. Smear-positive pulmonary tuberculosis and AFB examination practices according to the standard checklist of WHO's tuberculosis laboratory assessment tool in three governmental hospitals, Eastern Ethiopia. BMC Res Notes, 2014, 7: 295. doi:10.1186/1756-0500-7-295 |
| 6. | Wang L, Yu Y, Chen W, et al. Evaluation of the characteristics of the enzyme-linked immunospot assay for diagnosis of active tuberculosis in China. Clin Vaccine Immunol, 2015, 22: 510–5. doi:10.1128/CVI.00023-15 |
| 7. | Marais S, Thwaites G, Schoeman JF, et al. Tuberculous meningitis:a uniform case definition for use in clinical research. Lancet Infect Dis, 2010, 10: 803–12. doi:10.1016/S1473-3099(10)70138-9 |
| 8. | Medical Research Council, Streptomycin in Tuberculosis Trials Committee. Streptomycin treatment of tuberculous meningitis. Lancet, 1948, 251: 582–96. |
| 9. | Thakur R, Goyal R, Sarma S. Laboratory diagnosis of tuberculous meningitis-Is there a scope for further improvement?. J Lab Physicians, 2010, 2: 21–4. doi:10.4103/0974-2727.66705 |
| 10. | Kim SH, Cho OH, Park SJ, et al. Rapid diagnosis of tuberculous meningitis by T cell-based assays on peripheral blood and cerebrospinal fluid mononuclear cells. Clin Infect Dis, 2010, 50: 1349–58. doi:10.1086/649517 |
| 11. | Yu J, Wang ZJ, Chen LH, et al. Diagnostic accuracy of interferon-gamma release assays for tuberculous meningitis:a meta-analysis. Int J Tuberc Lung Dis, 2016, 20: 494–9. doi:10.5588/ijtld.15.0600 |
| 12. | Gupta BK, Bharat A, Debapriya B, et al. Adenosine Deaminase Levels in CSF of Tuberculous Meningitis Patients. J Clin Med Res, 2010, 2: 220–4. |
 2017, Vol. 30
2017, Vol. 30


