2. Ozone Complementary Research Center, Baqiyatallah University of Medical Sciences, Tehran 14359-16471, Iran;
3. Young Researchers and Elite Club, Islamic Azad University, Tehran Medical Sciences Branch, Tehran 19168-93814, Iran;
4. Biotechnology Research Center, Pasteur Institute of Iran, Medical Biotechnology Group, Venom and Toxin Lab, Tehran 13169-43551, Iran;
5. Chemical Injuries Research Center, Baqiyatallah University of Medical Sciences, Tehran 14359-16471, Iran;
6. Department of medical nanotechnology Faculty of Advanced technology in medicine, Iran university of Medical Sciences, Tehran 14496-14535, Iran;
7. Department of Pharmacology and Toxicology, School of Pharmacy, Zanjan University of Medical Sciences, Zanjan 45139-56184, Iran;
8. Zanjan Applied Pharmacology Research Center, Zanjan University of Medical sciences, Zanjan 45139-56184, Iran;
9. Molecular Biology Research Center, Baqiyatallah University of Medical Sciences, Tehran 14359-16471, Iran
Objective Scorpion (Hemiscorpius lepturus) stings are a public health concern in Iran, particularly in south and southwestern regions of Iran. The gold standard for the treatment of a scorpion sting is anti-venom therapy. However, immunotherapy can have serious side effects, such as anaphylactic shock (which can sometimes even lead to death). The aim of the current study was to demonstrate the protective effect of ozone against toxicity induced by Hemiscorpius lepturus (H. lepturus) venom in mice.
Methods Eight hours after the injection of ozone to the experimental design groups, the male mice were decapitated and mitochondria were isolated from five different tissues (liver, kidney, heart, brain, and spinal cord) using differential ultracentrifugation. Then, assessment of mitochondrial parameters including mitochondrial reactive oxidative species (ROS) production, mitochondrial membrane potential (MMP), ATP level, and the release of cytochrome c from the mitochondria was performed.
Results Our results showed that H. lepturus venom-induced oxidative stress is related to ROS production and MMP collapse, which is correlated with cytochrome c release and ATP depletion, indicating the predisposition to the cell death signaling.
Conclusion In general, ozone therapy in moderate dose can be considered as clinically effective for the treatment of H. lepturus sting as a protective and antioxidant agent.
Ozone (O3/O2 mixture) therapy has been used in the treatment of ischemic disorders; however, there are no data from clinical trials yet, and it has not yet been accepted as standard medicine in all countries[1]. It has been suggested that ozone could stimulate antioxidant system, regulate inflammatory responses, improve vascular rheology, and increase blood flow in cerebral arteries and tissue oxygenation in hypoxic tissues[2]. It shows that the therapeutic efficacy of ozone is related to the controlled and moderate oxidative stress as a result of ozone reactions with biological components[2].
The recent published data found that scorpion (Hemiscorpius lepturus) toxin can lead to high toxicity in the kidney, heart, liver, lungs, stomach, and intestines, which is similar to massive histopathological alterations results in kidney and liver. In addition, it seems that the kidney and heart have a higher sensitivity to damage after subcutaneous (SC) envenoming in mice[3-4]. Moreover, it has been reported that the immunotoxicity of H. lepturus venom is related to the over-expression of IL-12, TNF-α, IL-6, and IL-10 in human monocytes[5-9].
An in vivo study in rats showed a significant increase in amino-alanine peptidase (AAP) and N-acetyl-β-D-glucosaminidase (NAG) enzyme activity, which are biomarkers of nephrotoxicity and acute renal failure (ARF), and this was confirmed by histopathological abnormalities. This was associated with severe anemia, thrombocytopenia, pyuria, hematuria, and considerable proteinuria[10-11]. In addition, inhibition of pyrimidine, histidine, and tyrosine metabolism and steroid hormone biosynthesis was observed[12].
H. lepturus venom toxicity is endemic in the southwestern province of Khuzestan and in other parts of western Iran, and this scorpion is responsible for 10% of the reported stings with high mortalities (approximately 19.5 deaths each year), mostly during spring and summer[13-15]. The toxic H. lepturus sting causes severe damage in high perfusion organelles, which are very sensitive to oxidative stress due to high oxygen consumption, high concentrations of polyunsaturated fatty acids, and low levels of some antioxidant enzymes[10, 16].
Unfortunately, the lack of basic information on clinical manifestations and specific treatments has led to problems in designing clinical trials. Mitochondria are the important organelles during oxidative stress induction due to their poor antioxidant system and high lipid content[2]. Taking into consideration the limited data on the possible mechanisms of H. lepturus venom toxicity, we planned to study the protective effect of ozone against H. lepturus venom–induced oxidative stress in different tissues through precise measurements using various multi-parametric assays.
MATERIALS AND METHODS Scorpion VenomH. lepturus venom from Khuzestan (Iran) was collected by the veterinarian service of the RAZI Vaccine Development and Serum Research Institute of Iran and stored under refrigeration conditions (-20 ℃) until use.
MedozoneMedozone (HAB company 2015, Ozone generator, UMDNS-Nr.12899, power supply = 230/115V-50/Hz, Power input = 240 VA) was used for the generation of the oxygen/ozone mixture (O3/O2 ratio = 99.95%/0.05%) for medical application. The ozone concentrations were adjusted to 5, 30, and 80 µg per mouse to determine the optimal effective dose. The ozone concentration was regulated by dosimeter with flow rate of 0.8 L/min, and the total ozone volume in the injection was 8 mL/kg.
AnimalsOverall, 48 male BaLb/c mice (28 ± 2 g) were purchased from Pasteur Institute of Iran to be used in the current study. The mice were housed in an air-conditioned room with a controlled temperature of 25 ± 2 ℃ and maintained on a 12:12 h light cycle with free access to food and water. The experiments were performed in the same lab in accordance with the institutional guidelines for animal care and use. Each mouse, in all experimental groups, was injected a 100 µL volume of the venom solution. The approximate median lethal dose (LD50) of the crude venom was found to be 5.6 mg/kg after IP injection in Balb/c mice, which is equal to 140 μg per mouse. Then, the effective lethal dose, which can cause death in 3-5 min after a single IP injection of venom in the mice, was determined to be equal to 1 mg of venom per mouse.
Experimental DesignAfter a period of acclimatization, the animals were divided into eight groups, which were treated as follows:
Group 1: the mice received only normal saline (NS) and served as the control group.
Group 2: the mice received an effective dose of crude venom (1 mg per mouse) through IP injection.
Groups 3, 4, and 5: the mice received 5, 30, and 80 µg ozone concentrations per mouse, respectively.
Groups 6, 7, and 8: the mice received 5, 30, and 80 µg ozone concentrations per mouse at 90-120 s after the IP injection of 1 mg crude venom per mouse.
Mitochondrial PreparationThe mice were decapitated, and the desired tissues were dissected on an ice-cold (4 ℃) surface and immediately immersed in liquid nitrogen. Tissue homogenization was performed with a cold mannitol solution, and the mitochondria were isolated by differential centrifugation, as described in a previous report[17]. To ensure uniformity of the experimental conditions, the mitochondrial samples (200 µg/mL mitochondrial protein) were used for all experiments. Therefore, Bradford method was used for the determination of mitochondrial protein concentration using BSA as a standard[18].
Quantification of Mitochondrial Reactive Oxidative Species (ROS)The mitochondrial ROS level was measured using a DCFH-DA reagent with a HITACHI fluorescence spectrophotometer at excitation and emission wavelengths of 495 and 520 nm, respectively. Mitochondrial suspensions were incubated in respiration buffer containing 0.32 mmol/L sucrose, 10 mmol/L Tris-HCl, 20 mmol/L MOPS, 50 μmol/L EGTA, 0.5 mmol/L MgCl2, 0.1 mmol/L KH2PO4, and 5 mmol/L sodium succinate. Then, DCFH-DA was added (final concentration of 10 μmol/L) to the mitochondrial suspension, which was then incubated for 20 min. The results were recorded at 30 and 60 min intervals and reported as relative fluorescence intensity. The mitochondrial ROS level was measured by HITACHI fluorescence spectrophotometer according to our previous work[19]. ROS formation was calculated as a percent value based on the formula × 100:
| $\frac{{{\rm{Fluorescence intensity in treated groups - Fluorescence intensity in control groups}}}}{{{\rm{ Fluorescence}}\,{\rm{intensity in control - Fluorescence intensity of DCF in media without mitochondria}}}}$ | (1) |
MMP was measured by fluorescence spectrophotometer using rhodamine123 (Rh123) as the fluorescent probe at excitation and emission wavelengths of 490 nm and 535 nm, respectively[20]. The capacity of the mitochondria to uptake Rh123 was calculated as the difference (between control and treated mitochondria) in rhodamine123 fluorescence. MMP collapse (%ΔΨm) in all treated (test) was calculated similar to ROS formation percentage formula by replacing Rh123 fluorescence intensity instead of DCF.
Determination of Mitochondrial SwellingThe change in mitochondrial volume, due to the colloidal osmotic effects of solute flux into and out of the mitochondrial matrix, was measured by monitoring the absorbance at 540 nm (A540) by ELISA reader after incubation of desired isolated mitochondria in swelling buffer (70 mmol/L sucrose, 230 mmol/L mannitol, 3 mmol/L HEPES, 2 mmol/L tris-phosphate, 5 mmol/L succinate, and 1 µmol/L of rotenone)[19].
ATP Level AssayATP level was measured by luciferase enzyme, based on the bioluminescence intensity, using the Sirius tube luminometer (Berthold Detection System, Germany)[21]. The results were expressed compared with control groups.
Cytochrome C Release AssayThe amount of released cytochrome C was assayed in a microplate reader at 450 nm using the Quantikine® Rat/Mouse Cytochrome C Immunoassay kit (Minneapolis, MN) in a manner similar to our previous study[22].
Statistical AnalysisThe results are presented as mean ± SD. All statistical analyses were performed using the SPSS software, version 17. The assays were performed in triplicates, and the mean was used for statistical analysis. The statistical significance was determined using the one-way ANOVA test, which was followed by the post-hoc Tukey test. The statistical significance was set at P < 0.05.
RESULTS Effects of Different Treatments on the Survival of MiceBased on a pilot study, the effective H. lepturus venom dose that caused death in 3-5 min was determined to be approximately to 1 mg per mouse. We then tried to determine whether different concentrations of ozone (5, 30, and 80 μg per mouse) could help the mice survive over 8 h (cut-off time = 24 h). Animals were individually observed after injection at least six times at 5 min intervals (30 min) and then checking periodically every 1 h (6 h) and final checking 24 h after injection. Our results showed that maximum protection was conferred by 80 μg ozone for the optimal time ( > 8 h) in mice which received H. lepturus crude venom. Our results showed that the administration of an optimal concentration of ozone (30 μg per mouse) to mice that had received an effective dose of crude venom (1 mg per mouse) through IP injection led to a significant increase in survival time (P < 0.05). This data indicated that the venom had a reciprocal protective effect against the mitochondrial damages induced by the ozone. The results of survival time listed in Table 1.
|
|
Table 1 The Rate of Animal Death, Percentage and Survival Time in Different Experimental Groups |
As shown in Table 2, the rate of ROS formation in the isolated mitochondria (obtained from the different tissues including liver, kidney, heart, muscle, spinal cord, and brain) in mice envenomed with H. lepturus venom significantly increased compared with the control groups in a time-dependent manner, after 60 min of exposure. However, in comparison with only ozone treated group (5 µg), ROS generation did not significantly increase until 60 min after exposure, as compared with the control mitochondria (P > 0.05; data not shown). A more substantial increase in ROS formation was observed in the brain mitochondria than in the mitochondria isolated from kidney, muscle, liver, or spinal cord. The level of ROS formation was higher in the H. lepturus (1 mg), ozone (80 µg), and H. lepturus (1 mg) + ozone (80 µg)-treated groups than those were in the control groups (Table 2). There was also a significant amelioration in ROS production in the H. lepturus (1 mg) + ozone (30 µg)-treated groups.
|
|
Table 2 Effect of O2/O3 Mixture (Ozone) on the ROS Formation after H. lepturus Envenoming in Isolated Mitochondria |
As shown in Figure 1, there was a significant difference in MMP collapse between the control and H. lepturus venom-treated groups (1 mg), which was similar to the groups treated with only ozone (80 µg) (P < 0.001). Based on our results, there was no significant difference in MMP collapse between the control-and ozone (5 and 30 µg)-treated groups (1 mg) (P > 0.05). Furthermore, a drastically protection against H. lepturus venom was seen in 30 µg ozone 1 mg H. lepturus venom treated groups. However, a more substantial increase in MMP collapse was reported in the ozone (80 µg) + H. lepturus venom (1 mg)-treated groups. The highest MMP collapse was seen in the kidney and heart mitochondria (Figure 1).
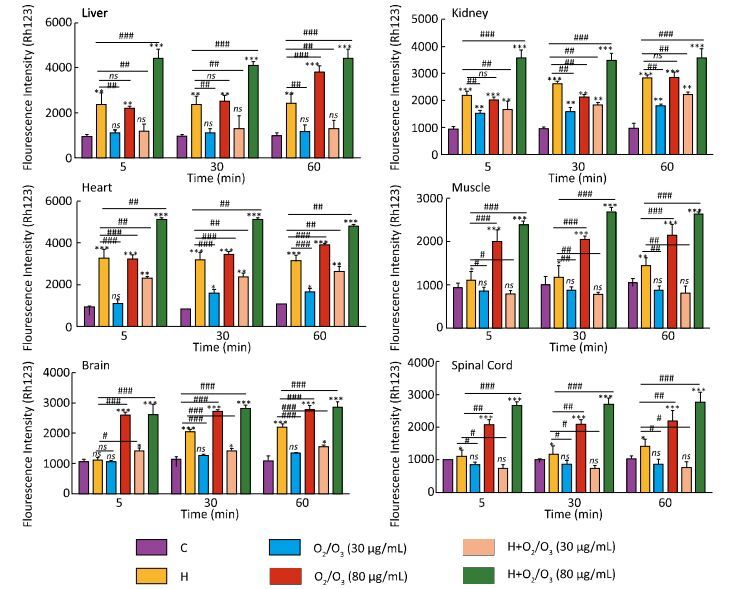
|
Download:
|
| Figure 1 Effect of O2/O3 mixture (Ozone) on mitochondrial membrane potential (MMP) collapse (ΔΨ%) in isolated mitochondria after H. lepturus envenoming. Mitochondria membrane potential collapse (ΔΨ%) was measured by Rhodamine123 as described in Materials and Methods. The effect of Ozone concentration (5, 30, and 80 µg per mouse) on the mitochondrial membrane potential in isolated mitochondria was evaluated. The values are expressed as mean ± SD (n = 3). Values represented as mean ± SD (n = 3). *P < 0.05; **P < 0.01; and ***P < 0.001 compared with control mitochondria. [C: Control; Ozone: O2/O3; H: H. lepturus (1 mg); H + Ozone: H. lepturus (1 mg) + Ozone (O2/O3)]. | |
Mitochondrial swelling was measured by the decrease in absorbance at 540 nm (A540), and this was an indicator of mitochondrial membrane permeability collapse in three groups of mice: H. lepturus venom (1 mg)-, ozone (80 µg)-, and ozone (80 µg) + H. lepturus venom (1 mg)-treated groups (Figure 2). The administration of ozone (30 µg) prevented H. lepturus venom-induced swelling because there was no significant increase in mitochondrial swelling at this concentration. The order of protection of the above-mentioned treatments against H. lepturus venom-induced mitochondrial swelling following incubation was as follows: muscle/kidney > spinal cord > brain > heart > liver. This data indicated not only the protective effect of ozone (30 µg) against mitochondrial damages induced by H. lepturus venom (1 mg) but also an interesting reciprocal protective effect provided by the venom against the mitochondrial damages induced by the ozone.
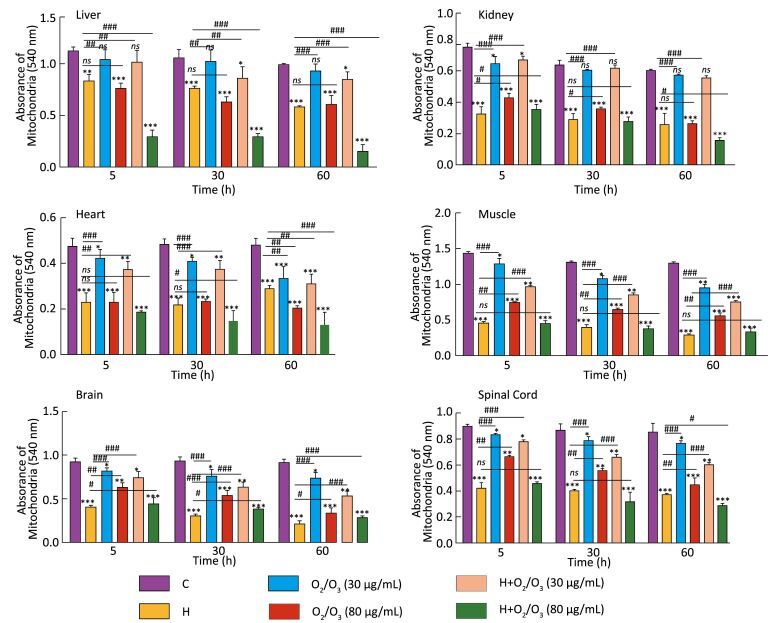
|
Download:
|
| Figure 2 Effect of O2/O3 mixture (Ozone) on the mitochondrial swelling after H. lepturus envenoming. Mitochondrial swelling was measured by determination of absorbance at 540 nm as described in Materials and Methods. Values represented as mean ± SD (n = 4). *P < 0.05; **P < 0.01; and ***P < 0.001; compared with control mitochondria. [C: Control; Ozone: O2/O3; H: H. lepturus (1 mg); H + Ozone: H. lepturus (1 mg) + Ozone (O2/O3)]. | |
As the electron transfer chain is required for ATP production in mitochondria, measuring the ATP levels in isolated mitochondria is the main evaluation for mitochondrial functionality. As shown in Table 3, there was a significant difference in ATP levels among the control, the H. lepturus venom (1 mg) only groups, and the ozone (80 µg) + H. lepturus venom (1 mg) group (P < 0.001). This reduction in the mitochondrial ATP level indicated mitochondrial dysfunction. In addition, 30 µg ozone treatment was significantly reversed the ATP level in post-treatment of H. lepturus venom (1 mg) in all the tissues compared with the control mitochondria. Post-treatment with ozone (80 µg) and H. lepturus (1 mg) significantly decreased ATP levels in the isolated mitochondria of different tissues. The highest and lowest amelioration of ATP levels following incubation with H. lepturus venom (1 mg) was seen in the kidney and brain, respectively.
|
|
Table 3 Effect of O2/O3 Mixture (Ozone) on Mitochondrial ATP Level after H. lepturusEnvenoming in Isolated Mitochondria |
As shown in Table 4, there was a significant difference in the release of cytochrome c among the control, H. lepturus venom (1 mg) groups, and the ozone (80 µg) groups (P < 0.001), whereas this was not observed in the ozone (30 µg) + H. lepturus venom (1 mg) group. The present data indicated that there is not only a protective effect provided by the ozone (30 µg) against the mitochondrial damages induced by the H. lepturus venom (1 mg) but also an interesting reciprocal protective effect provided by the venom against the mitochondrial damages induced by the ozone.
|
|
Table 4 Effect of O2/O3 Mixture (Ozone) on Mitochondrial on Cytochrome C Release after H. lepturus Envenoming in Isolated Mitochondria |
H. lepturus, one of the most venomous scorpions in tropical and sub-tropical areas, poses health problems in many parts of the world, including Iran[13]. Envenomation is characterized by sweating, fever, hypertension, and neurological disorders[23]. The use of anti-venom derived from a polyclonal immune response raised in horses is the common therapy for envenomation treatment[23-24]. Unfortunately, immunotherapy can have serious side effects, such as anaphylactic shock, which can sometimes lead to death[4]. Based on these facts; we planned to study the protective effects of ozone against H. lepturus venom in mitochondria isolated from vital organs by precisely measuring different oxidative stress endpoints in order to determine novel therapeutic agents for the future. Furthermore, our present data indicates that there is not only a protective effect provided by the ozone against the mitochondrial damages induced by the scorpion venom, but also an interesting reciprocal protective effect provided by the venom against the mitochondrial damages induced by the ozone.
According to the available reports, H. lepturus venom is primarily a cytotoxic agent with heart, kidney, liver, blood, and lungs as the major target organs[25-26]. These organs are known to be very sensitive to the shortage of energy produced in mitochondria to maintain cell homeostasis[27]. Some studies have proposed that the major mechanism of H. lepturus venom–induced cytotoxicity is the increase in ROS production, as these are molecules to initiate cell death signaling and induce oxidative stress[5, 10]. Our results confirmed that H. lepturus toxicity is associated with increased ROS generation in the mitochondria. In addition, our current study showed that ozone (30 μg) not only prevented H. lepturus toxicity but also inhibited ROS formation in isolated mitochondria. Our results also showed that the ozone (30 μg) was more effective against H. lepturus-induced ROS formation in the spinal cord, brain, and liver than in the other tissues (P < 0.001). Interestingly, a higher concentration of ozone (80 μg) showed higher toxicity and no protection when compared with ozone (30 μg) (Table 1). Thus, the antioxidant activities of ozone (30 μg) in moderate concentrations could protect against H. lepturus induced oxidative stress markers in different sub-cellular processes, including ROS formation, mitochondrial permeability transition (MPT) pore opening followed by the collapse of the MMP, mitochondrial swelling, and cytochrome c release. Our results are in line with other studies that established that the ROS production in pathologic states substantially increases the obi-semiquinone pool by inhibiting the electron transfer, and oxidation of the GSH pool is a critical marker to ascertain increased ROS formation in isolated mitochondria and oxidative injury[27-30].
Since the oxidation of thiol in the MPT significantly (P < 0.05) increases the unlimited proton movement across the inner mitochondrial membrane and induces mitochondrial swelling, MMP disruption, and the uncoupling of oxidative phosphorylation[33], it is therefore suggested that ozone (30 μg) can cross the inner mitochondrial membrane and ameliorate the rate of intracellular ROS formation and block MPT pore opening in the event of mitochondrial dysfunction. Another suggestion is that the high concentration of unsaturated fatty acids in the inner mitochondrial membrane is the main factor in the disruption of mitochondrial and oxidative stress markers[31].
Our data supports this hypothesis, as the decline in MMP and the cytochrome c release into cytosol significantly increased following H. lepturus venom-induced ROS formation in the isolated mitochondria. It is assumed that the damage to the mitochondria is a consequence of ROS formation, considering the fact that MPT pore opening is one of the main steps in H. lepturus venom-induced mitochondrial dysfunction and ozone (30 μg) acts to prevent H. lepturus venom-induced toxicity. Furthermore, decrease in the ATP level reflects the impairment of the mitochondrial respiratory chain and a significant expulsion of cytochrome c, which suggests the involvement of oxidative stress in MPT pore opening and cell death signaling. It is therefore concluded that there is not only a protective effect provided by the ozone against the mitochondrial damages induced by the scorpion venom, but also an interesting reciprocal protective effect provided by the venom against the mitochondrial damages induced by the ozone.
CONCLUSIONIn this study, the mice injected with 1 mg H. lepturus venom died after 3-5 min, while the mice injected with 1 mg H. lepturus venom and 30 µg ozone survived over 8 h. However, our results showed that a higher concentration of ozone (80 µg) caused electron transfer chain impairment, which leads to increased ROS production, MMP collapse, the amelioration of ATP levels, and cytochrome c release. Our study suggests that mitochondrial dysfunction and the uncoupling of oxidative phosphorylation may play key roles in the etiology of H. lepturus venom toxicity. In general, the present study results confirmed that a moderate concentration of ozone (30 µg) can be considered as a protective and antioxidant agent for the treatment of H. lepturus envenoming.
| 1. | Filippo CD, Luongo M, Marfella R, et al. Oxygen/ozone protects the heart from acute myocardial infarction through local increase of eNOS activity and endothelial progenitor cells recruitment. Naunyn Schmiedebergs Arch Pharmacol, 2010, 382: 287–91. doi:10.1007/s00210-010-0545-2 |
| 2. | Sagai M, Bocci V. Mechanisms of Action Involved in Ozone Therapy:Is healing induced via a mild oxidative stress?. Med Gas Res, 2011, 1: 29–47. doi:10.1186/2045-9912-1-29 |
| 3. | Pipelzadeh MH, Dezfulianb AR, Jalalic MT, et al. In vitro and in vivo studies on some toxic effects of the venom from Hemiscorpiuslepturus scorpion. Toxicon, 2006, 48: 93–103. doi:10.1016/j.toxicon.2006.04.017 |
| 4. | Heidarpour M, Ennaifer E, Ahari H, et al. Histopathological changes induced by Hemiscorpiuslepturus scorpion venom in mice. Toxicon, 2012, 59: 373–8. doi:10.1016/j.toxicon.2011.12.011 |
| 5. | Hadaddezfuli R, Khodadadi A, Assarehzadegan MA, et al. Hemiscorpiuslepturus venom induces expression and production of interluckin-12 in human monocytes. Toxicon, 2015, 100: 27–31. doi:10.1016/j.toxicon.2015.04.007 |
| 6. | Borchani L, Sassi A, Ben Gharsa H, et al. The pathological effects of Heminecrolysin, a dermonecrotic toxin from Hemiscorpiuslepturus scorpion venom are mediated through its lysophospholipase D activity. Toxicon, 2013, 68: 30–9. doi:10.1016/j.toxicon.2013.03.012 |
| 7. | Khanbashi S, Khodadadi A, Assarehzadegan MA, et al. Assessment of immunogenic characteristics of Hemiscorpiuslepturus venom and its cross-reactivity with venoms from Androctonuscrassicauda and Mesobuthuseupeus. J Immunotoxicol, 2015, 12: 217–22. doi:10.3109/1547691X.2014.927542 |
| 8. | Jalali A, Bavarsad-Omidian N, Babaei M, et al. The pharmacokinetics of Hemiscorpiuslepturus scorpion venom and Raziantivenom following intramuscular administration in rat. J Venom Res, 2012, 3: 1–6. |
| 9. | Valavi E, Ahmadzadeh A, Amoori P, et al. High frequency of acquired ADAMTS13 deficiency after hemolysis in HemiscorpiusLepturus (scorpion) stung children. Indian J Pediatr, 2014, 81: 665–9. doi:10.1007/s12098-013-1089-5 |
| 10. | Pipelzadeh MH, Jalali A, Dezfulian AR, et al. A forward to optimization of antivenom therapy:An in vivo study upon the effectiveness of the antivenom against early and delayed nephrotoxicity induced by the venom of the Iranian scorpion Hemiscorpiuslepturus in rat. Toxicon, 2015, 100: 13–9. doi:10.1016/j.toxicon.2015.03.023 |
| 11. | Gadalean F, Kaycsa A, Gluhovschi G, et al. Is the urinary biomarkers assessment a non-invasive approach to tubular lesions of the solitary kidney?. Ren Fail, 2013, 35: 1358–64. doi:10.3109/0886022X.2013.828367 |
| 12. | Arjmand M, Akbari Z, Taghizadeh N, et al. NMR-based metabonomics survey in rats envenomed by Hemiscorpiuslepturus venom. Toxicon, 2015, 94: 16–22. doi:10.1016/j.toxicon.2014.12.003 |
| 13. | Vazirianzadeh B, Farhadpour F, Hosseinzadeh M, et al. An epidemiological and clinical study on scorpionism in hospitalized children in khuzestan, Iran. J Arthropod Borne Dis, 2012, 6: 62–9. |
| 14. | Dehghani R, Fathi B. Scorpion sting in Iran:a review. Toxicon, 2012, 60: 919–33. doi:10.1016/j.toxicon.2012.06.002 |
| 15. | Jalali A, Rahim F. Epidemiological review of scorpion envenomation in Iran. Iran J Pharm Res, 2014, 13: 743–56. |
| 16. | Jridi I, Catacchio I, Majdoub H, et al. Hemilipin, a novel Hemiscorpiuslepturus venom heterodimeric phospholipase A2, which inhibits angiogenesis in vitro and in vivo. Toxicon, 2015, 105: 34–44. doi:10.1016/j.toxicon.2015.08.022 |
| 17. | Hosseini MJ, Jafarian I, Farahani S, et al. New mechanistic approach of inorganic palladium toxicity:impairment in mitochondrial electron transfer. Metallomics, 2016, 8: 252–60. doi:10.1039/C5MT00249D |
| 18. | Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem, 1976, 72: 248–54. doi:10.1016/0003-2697(76)90527-3 |
| 19. | Mashayekhi V, Tehrani KH, Hashemzaei M, et al. Mechanistic approach for the toxic effects of perfluorooctanoic acid on isolated rat liver and brain mitochondria. Hum ExpToxicol, 2015, 34: 985–96. doi:10.1177/0960327114565492 |
| 20. | Naserzadeh P, Pourahmad J. Toxicity mechanisms of cigarette smoke on eye and kidney using isolated Mitochondria. Iran J Pharm Res, 2013, 9: 55–62. |
| 21. | Nazari A, Sadr SS, Faghihi M, et al. Vasopressin attenuates ischemia-reperfusion injury via reduction of oxidative stress and inhibition of mitochondrial permeability transition pore opening in rat hearts. Eur J Pharmacol, 2015, 760: 96–102. doi:10.1016/j.ejphar.2015.04.006 |
| 22. | Mashayekhi V, Eskandari MR, Kobarfard F, et al. Induction of mitochondrial permeability transition (MPT) pore opening and ROS formation as a mechanism for methamphetamine-induced mitochondrial toxicity. Naunyn Schmiedebergs Arch Pharmacol, 2014, 387: 47–58. doi:10.1007/s00210-013-0919-3 |
| 23. | Seyedian R, Jalali A, Babaee MH, et al. A biodistribution study of Hemiscorpiuslepturus scorpion venom and available polyclonal antivenom in rats. J Venom Anim Toxins Incl Trop Dis, 2012, 18: 295–303. doi:10.1590/S1678-91992012000300007 |
| 24. | Mohseni A, Vazirianzadeh B, Hossienzadeh M, et al. The roles of some scorpions, Hemiscorpiuslepturus and Androctonuscrassicauda, in a scorpionism focus in Ramhormorz, southwestern Iran. J Insect Sci, 2013, 13: 89–96. |
| 25. | Radmanesh M. Cutaneous manifestations of the Hemiscorpiuslepturus sting:a clinical study. Int J Dermatol, 1998, 37: 500–7. doi:10.1046/j.1365-4362.1998.00386.x |
| 26. | Jalali A, Pipelzadeh MH, Sayedian R, et al. A review of epidemiological, clinical and in vitro physiological studies of envenomation by the scorpion Hemiscorpiuslepturus (Hemiscorpiidae) in Iran. Toxicon, 2010, 55: 173–9. doi:10.1016/j.toxicon.2009.09.012 |
| 27. | Jafarian I, Eskandari MR, Mashayekhi V, et al. Toxicity of valproic acid in isolated rat liver mitochondria. Toxicol Mech Methods, 2013, 23: 617–23. doi:10.3109/15376516.2013.821567 |
| 28. | Naserzadeh P, Hosseini MJ, Asl BM, et al. Toxicity mechanisms of cigarette smoke on mouse fetus mitochondria. Iran J Pharm Res, 2015, 14: 131–8. |
| 29. | Eskandari MR, Mashayekhi V, Aslani M, et al. Toxicity of thallium on isolated rat liver mitochondria:the role of oxidative stress and MPT pore opening. Environ Toxicol, 2015, 30: 232–41. doi:10.1002/tox.v30.2 |
| 30. | Lash LH. Mitochondrial glutathione transport:physiological, pathological and toxicological implications. Chem Biol Interact, 2006, 163: 54–67. doi:10.1016/j.cbi.2006.03.001 |
| 31. | Korotkov SM. Effects of Tl(+) on ion permeability, membrane potential and respiration of isolated rat liver mitochondria. J Bioenerg Biomembr, 2009, 41: 277–87. doi:10.1007/s10863-009-9225-7 |
 2017, Vol. 30
2017, Vol. 30


