2. Department of Hematology-oncology, Chongqing Cancer Institute/Hospital, Chongqing 400030, China;
3. Department of Pathology, Chongqing Cancer Institute/Hospital, Chongqing 400030, China;
4. Institute of Toxicology, School of Preventive Medicine, The Third Military Medical University, Chongqing 400038, China
Objective To investigate the risk stratification of aggressive B cell lymphoma using the immune microenvironment and clinical factors.
Methods A total of 127 patients with aggressive B cell lymphoma between 2014 and 2015 were enrolled in this study.CD4, Foxp3, CD8, CD68, CD163, PD-1, and PD-L1 expression levels were evaluated in paraffin-embedded lymphoma tissues to identify their roles in the risk stratification.Eleven factors were identified for further evaluation using analysis of variance, chi-square, and multinomial logistic regression analysis.
Results Significant differences in 11 factors (age, Ann Arbor stage, B symptom, ECOG performance status, infiltrating CD8+ T cells, PD-L1 expression, absolute blood monocyte count, serum lactate dehydrogenase, serum iron, serum albumin, and serum β2-microglobulin) were observed among patient groups stratified by at least two risk stratification methods[International Prognostic Index (IPI), revised IPI, and NCCN-IPI models](P < 0.05).Concordance rates were high (81.4%-100.0%) when these factors were used for the risk stratification.No difference in the risk stratification results was observed with or without the Ann Arbor stage data.
Conclusion We developed a convenient and inexpensive tool for use in risk stratification of aggressive B cell lymphomas, although further studies on the role of immune microenvironmental factors are needed.
Non-Hodgkin's lymphoma (NHL) remains an important cause of morbidity and mortality in cancer patients worldwide. More than half of patients with NHL were diagnosed with aggressive B cell lymphomas[1-2], which include diffuse large B cell lymphoma (DLBCL), lymphoblastic B cell lymphoma, Burkitt lymphoma, mantle cell lymphoma, and other lymphoma entities. DLBCL, the largest subgroup of aggressive B cell lymphomas, can be further classified into two subtypes, such as germinal center B cell like subtype and non-germinal center B cell subtype, based on gene expression profiles or immune phenotypes[3-4]. The clinical causes and prognosis of different subtypes of DLBCL and other aggressive B cell lymphomas are quite different due to complex intrinsic mechanisms[5-7]. In recent years, the combination of chemotherapy, antibody-based target therapy, and radiation therapy has significantly improved the survival of patients with aggressive B cell lymphomas, but relapse, refractory diseases, and poor clinical outcomes remain major treatment challenges[8-10].
Multiple risk stratification systems have been developed to identify the cancer patients' prognosis[11-15]. The International Prognostic Index (IPI), based on clinical parameters, such as age, lactate dehydrogenase (LDH), number of extranodal sites, Ann Arbor stage, and ECOG performance status, was one of the most commonly used tools for predicting therapeutic response and prognosis of patients with aggressive NHL in the pre-rituximab era. New versions of the IPI, including the revised IPI (R-IPI) and the NCCN-IPI, are reportedly superior to the previous standard IPI scoring system after the combination of rituximab and chemotherapy was introduced. The lymphoma tumor microenvironment plays an important role in tumor development and progression through interactions between tumor cells and the immune system and influences tumor prognosis and the treatment response[16-24]. Immune checkpoint pathways, particularly the PD-1/PD-L1 pathway, have been reported to mediate exhaustion of tumor-infiltrating cytotoxic T lymphocytes. Therefore, inhibiting the PD-1/PD-L1 pathway is believed to be a highly promising method for treating various advanced tumors, including lymphomas[25-28]. In addition, the components of the differential white blood cell count (WBC-DC), systematic inflammatory and metabolic markers, such as LDH, β2-microglobulin (β2MG), and iron, in peripheral blood are useful inexpensive parameters to categorize the prognoses of malignant diseases including solid and hematological malignancies[29-36]. However, the relationship between the tumor cell microenvironment and risk based on the commonly used score models (IPI, R-IPI, and NCCN-IPI) remains unknown. In this study, we evaluated the densities of CD4, Foxp3, CD8, CD68, and CD163 positive immune cells, the expression of PD-1/PD-L1, counted the systemic immune-inflammatory cells, and measured serum levels of LDH and β2MG in 127 patients with aggressive B cell lymphomas, to analyze the correlation between these immune related factors and the risk stratification in patients with aggressive B cell lymphomas. Our aim was to investigate the relationship between the tumor immune microenvironment as well as systematic inflammatory and metabolic factors and the prognosis of patients with aggressive B cell lymphomas.
MATERIALS AND METHODS Patients and SamplesA total of 127 patients diagnosed with aggressive B cell lymphomas at Chongqing Cancer Institute/Hospital between January 2014 and December 2015 were enrolled in this retrospective study. Informed consent was obtained from all patients, and the study was approved by the Ethics Committee of Chongqing Cancer Institute/Hospital. All cases were reviewed by two experienced pathologists according to the criteria of the World Health Organization classification. Patients' medical history review, physical examination, WBC-DC, renal and liver function tests, such as serum albumin, globulin, β2MG, and bone marrow biopsy, and imaging examinations (ultrasound and radiological examinations of the brain, chest, abdomen, and pelvis) were carried out, and the results were reviewed. Patients were staged according to the Ann Arbor classification, and a risk stratification was performed according to the IPI, R-IPI, and NCCN-IPI scoring systems[16-18].
Immunohistochemistry for Tumor-infiltrating Immune Cell Subsets and PD-1/PD-L1 ExpressionSlides of formalin-fixed, paraffin-embedded tissues were stained with primary monoclonal antibodies to anti-CD4 (clone SP35, rabbit monoclonal; Abcam, Cambridge, MA, USA), anti-Foxp3 (clone 236A/E7, mouse monoclonal; Abcam), anti-CD8 (clone SP16, rabbit monoclonal; Abcam), anti-CD68 (clone KP-1, mouse monoclonal; Abcam), anti-CD163 (clone 10D6, mouse monoclonal; Abcam), anti-PD-1 (clone MRQ22, mouse monoclonal; ORIGENE, Rockville, MD, USA), and anti-PD-L1 (clone SP142, rabbit monoclonal; ORIGENE) using the GTVision Ⅲ detection system (DAKO, Carpinteria, CA USA) according to the manufacturer's instructions. Tumor-infiltrating immune cell subsets were quantified as total counts of CD3, CD4, CD8, Foxp3, CD68, and CD163 positive cells per high power field (0.2 mm2) by manual inspection of stained sections with at least 10 fields of high staining density. Membranous immunostaining for PD-1 and PD-L1 was considered as positive and scored by a staining density ranging from 0 to 3 (0 = no staining, 1 = weak, 2 = moderate, 3 = strong).
Statistical AnalysisAll data were analyzed with SPSS 18.0 software (IBM Corp., Armonk, NY, USA). Categorical variables were compared using the chi-square test. The difference between continuous variables was assessed using analysis of variance (ANOVA). All P-values < 0.05 were considered significant. A multinomial logistic regression model was developed and probability was estimated in a single model using a maximum likelihood technique. The three scoring models (IPI, R-IPI, and NCCN-IPI) were used as the dependent variable in the multinominal logistic regression. Significant factors selected from the ANOVA and chi-square test were used as control variables. Concordance rates for methods using these factors with or without the Ann Arbor stage data were analyzed with paired t-tests.
The predicted probabilities from the model were calculated with the following Equations:
| $ \begin{array}{l} p1 = p\left( {y \le 1\left| {{x_1}, {x_2}, \cdots {x_{\rm{K}}}} \right.} \right) = \frac{{\exp \left( {{\rm{a + }}{{\rm{b}}_1}{{\rm{x}}_1} + {{\rm{b}}_2}{{\rm{x}}_2} + \cdots {{\rm{b}}_{\rm{k}}}{{\rm{x}}_{\rm{k}}}} \right)}}{{1 + \exp \left( {{{\rm{a}}_1} + {{\rm{b}}_1}{{\rm{x}}_1} + {{\rm{b}}_2}{{\rm{x}}_2} + \cdots + {{\rm{b}}_{\rm{k}}}{{\rm{x}}_{\rm{k}}}} \right)}}\\ p2 = p\left( {y \le 2\left| {{x_1}, {x_2}, \cdots {x_{\rm{K}}}} \right.} \right) = \frac{{\exp \left( {{{\rm{a}}_2}{\rm{ + }}{{\rm{b}}_1}{{\rm{x}}_1} + {{\rm{b}}_2}{{\rm{x}}_2} + \cdots {{\rm{b}}_{\rm{k}}}{{\rm{x}}_{\rm{k}}}} \right)}}{{1 + \exp \left( {{{\rm{a}}_2} + {{\rm{b}}_1}{{\rm{x}}_1} + {{\rm{b}}_2}{{\rm{x}}_2} + \cdots + {{\rm{b}}_{\rm{k}}}{{\rm{x}}_{\rm{k}}}} \right)}}\\ p3 = p\left( {y \le 3\left| {{x_1}, {x_2}, \cdots {x_{\rm{K}}}} \right.} \right) = \frac{{\exp \left( {{{\rm{a}}_3}{\rm{ + }}{{\rm{b}}_1}{{\rm{x}}_1} + {{\rm{b}}_2}{{\rm{x}}_2} + \cdots {{\rm{b}}_{\rm{k}}}{{\rm{x}}_{\rm{k}}}} \right)}}{{1 + \exp \left( {{{\rm{a}}_3} + {{\rm{b}}_1}{{\rm{x}}_1} + {{\rm{b}}_2}{{\rm{x}}_2} + \cdots + {{\rm{b}}_{\rm{k}}}{{\rm{x}}_{\rm{k}}}} \right)}}\\ p4 = p\left( {y \le 4\left| {{x_1}, {x_2}, \cdots {x_{\rm{K}}}} \right.} \right) = 1-p1-p2-p3 \end{array} $ |
Where, p is the predicted probability for different scores, and xnis the control variable selected from the ANOVA or chi-square test.
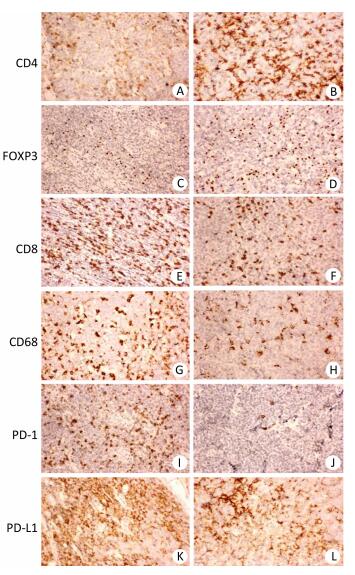
RESULTSThe clinicopathological characteristics of all 127 patients with aggressive B cell lymphomas are summarized in Supplementary Tables 1-3 (available in www.besjournal.com). Figure 1 demonstrated immunohistochemical staining of tumor-infiltrating immune cell subsets, PD1 and PD-L1 in DLBCL and mantle cell lymphoma, in which CD4, CD8 presented membrane positivity, and Foxp3 showed nuclear positivity in T lymphocytes, while CD68 showed membrane positivity in macrophage cells, PD-1 and PDL1 also showed membrane positivity both in tumor cells and microenvironment immune cells. As shown in Supplementary Table 1 (available in www.besjournal.com), no significant difference was found in 18 factors, including sex, histological classification, sites of involvement, absolute blood lymphocyte count, absolute blood lymphocyte count score, absolute blood neutrophil count score, absolute blood neutrophil count, platelet count, platelet count score, neutrophil-to-lymphocyte ratio (NLR), NLR score, monocyte-to-lymphocyte ratio (MLR), platelet-to-lymphocyte ratio (PLR), PLR score, lymphoma infiltrating CD4-positive T cells, lymphoma infiltrating Foxp3-positive T cells, lymphoma infiltrating CD68-positive macrophages, lymphoma infiltrating CD163-positive macrophages, or PD-1 score. The other 13 factors, including age, Ann Arbor stage, B symptoms, ECOG performance status, absolute blood monocyte count, CD8+ T cells, LDH, iron, albumin, and β2MG were significantly different among the four groups. The baseline characteristics with stratification using the revised IPI (Supplementary Table 2, available in www.besjournal.com) and NCCN-IPI (Supplementary Table 3, available in www.besjournal.com) showed similar results as those stratified using the IPI (Supplementary Table 1, available in www.besjournal.com). Table 1 summarizes the important factors from the three scoring systems and shows that there were differences in some of the factors, including blood lymphocyte score, blood monocytes, platelet count, NLR, MLR, and PLR among all three scoring systems. Only factors that were significantly different in the two scoring systems were chosen for further analysis to ensure the accuracy of further statistical analysis (Table 2).
|
|
Supplementary Table 1 Baseline Characteristics of Patients by IPI Stratification |
|
|
Supplementary Table 2 Baseline Characteristics of Patients with Stratification of Revised IPI |
|
|
Supplementary Table 3 Baseline Characteristics of Patients with Stratification of NCCN-IPI |
|
|
Table 1 Results of ANOVA and Chi-Square Tests in Different Risk Stratification Models |
|
|
Table 2 Likelihood Ratio Tests of Various Factors in Estimating the Risk Stratification by Multinomial Logistic Regression |
|
Download:
|
| Figure 1 Immunohistochemistry of CD4, Foxp3, CD8, CD68, PD-1, and PD-L1 in aggressive B cell lymphoma. (A, C, E, G, I, K) Representative case of diffuse large B cell lymphoma (DLBCL) stained with CD4, FOXP3, CD8, CD68, PD-1, and PD-L1 antibodies. (B, D, F, H, J, L) Representative case of mantle cell lymphoma (MCL) stained with CD4, Foxp3, CD8, CD68, PD-1, and PD-L1 antibodies. Original magnification, × 200. | |
Multinomial logistic regression models were developed for the IPI, R-IPI, and NCCN-IPI subgroups to explore the probability of risk estimates for patients with aggressive B cell lymphomas using a combination of the factors selected from the ANOVA and chi-square test results (Tables 3, 4). As shown in Table 3, when the Ann Arbor stage data were considered, the concordance rates of the predicted risk stratification using multinomial logistic regression were 85.1%-100.0%. The concordance rates between the actual and predicted stratification were both 100.0% in the NCCN-IPI group. In comparison, when the Ann Arbor stage data were not considered (Table 4), the concordance rates of the predicted risk stratification decreased slightly to 81.4%-100.0%. To further investigate the difference in the estimated risk stratification using the above clinicopathological factors with or without the Ann Arbor stage data, a paired t-test was performed to compare the results of Tables 3 and 4. As shown in Table 5, no significant difference in the estimated risk stratification was detected using the above clinicopathological factors with or without the Ann Arbor stage data.
|
|
Table 3 Concordance Rate of Predicted Risk Stratification Using Multinomial Logistic Regression (Influence of Ann Arbor Stages Considered) |
|
|
Table 4 Concordance Rate of Predicted Risk Stratification by Multinomial Logistic Regression (influence of Ann Arbor stages not considered) |
|
|
Table 5 Paired t Test on the Concordance Rates of Three Predicted Stratification Models with and without Ann Arbor Stage Data |
The IPI is a powerful tool that has been widely used clinically for predicting outcomes of patients with aggressive NHL[16]. However, use of the IPI scoring system to identify high-risk patients has been challenged in the rituximab era because the combination of monoclonal anti-CD20 antibody, cyclophosphamide, adriamycin, vincristine, and prednisone (CHOP) has improved overall survival for all risk groups of DLBCLs[37-38]. Modifications to the standard IPI scoring model were proposed to resolve the discrepancies between the risk scores and clinical outcomes. Two modified IPI based scoring models, the R-IPI and NCCN-IPI, are reported to be better than the standard IPI for evaluating the clinical prognosis of aggressive B cell lymphomas[17-18]. In recent years, large-scale studies have demonstrated that the molecular and cellular nature of the tumor immune microenvironment, including immune cells and receptors/ligands expressed in immune cells is important for tumor growth and clinical prognosis[26-27, 39-42]. However, the association between the immune microenvironment and the IPI-based risk stratification for aggressive B cell lymphomas remains largely unknown. In our study, a significant difference in the distribution of CD8+ T lymphocytes and PD-L1 expression status was observed between the low-risk and high-risk groups stratified either by the IPI, R-IPI, or NCCN-IPI (Table 1). Such a difference in the distribution indicates that immune markers, such as tumor-infiltrating T lymphocytes and the PD-1/PD-L1 pathway, might play important roles in the prognosis of aggressive B cell lymphomas.
Since the link between inflammation and cancer was first reported by Rudolf Virchow in 1863, extensive evidence has demonstrated that immune cells, including T cells, macrophages (TAMs), evasion[23-24, 43-45]. Immune suppression induced by tumor cells results in decreased cytotoxicity of CD8+ T cells, increased immune-suppressive T cells, such as regulatory T cells (Tregs), higher expression of inhibitory modulatory molecules, such as CTLA-4, PD-1, and PD-L1, in the microenvironment, and a poor prognosis. For this reason, monitoring immunomodulatory factors and immune status in the tumor microenvironment, such as tumor-infiltrating T cells, could be used to predict the prognosis of solid malignancies including lymphomas[31-32, 46-50]. To test this hypothesis, we evaluated immune cell distribution and expression of PD-L1 and PD-1 on tissue sections from 127 patients with aggressive B cell lymphomas. Our research demonstrated that immune microenvi-ronmental factors, including tumor-infiltrating T cells, such as CD8+ T lymphocytes, and immune checkpoint molecules, such as PD-L1, can be used to predict prognosis of patients with aggressive B cell lymphomas.
The clinical behavior and prognosis of malignant tumors depend on the interactions between tumors and their microenvironment. For example, TAMs are required for tumor cell migration, invasion, and formation of metastases, and high levels of TAMs are associated with a poor prognosis in many cancers, including breast cancer, melanoma, hepatocarcinoma, prostate cancer, and lymphomas[45, 51-54]. However, activation of tumor-specific cytotoxic CD8+ T lymphocytes plays a vital role in the anti-tumor immune response, while suppressive immune cells, including CD4+Foxp3+ Tregs, and MDSCs, and other inhibitory factors, such as the newly recognized immune checkpoint molecules PD-1/PD-L1, can lower the function of CD8+ T lymphocytes. Discordant results on the correlation between immune cells and molecules involved in the tumor microenvironment and tumor prognosis have been identified in various malignant tumors[55-57].
Because of the association between chronic inflammation and cancer, inflammatory and nutritional biomarkers in the blood, such as WBC-DC, serum LDH, albumin, β2MG, and iron, have become important indicators of patients' immune and nutritional status when evaluating tumor prognosis and the treatment responses of patients with malignant tumors, such as solid cancers and lymphohematopoietic malignancies[16, 32, 40, 58-59]. As expected, large differences in serum LDH, albumin, β2MG, and iron expression levels were observed among the subgroups stratified by the IPI, R-IPI, or NCCN-IPI, whereas only circulatory monocytes were significantly different among the subgroups stratified by the IPI and R-IPI.
An accurate pathological diagnosis and risk stratification are crucial for effective treatment of lymphomas[2]. Ultrasound, X-ray, computed tomography, magnetic resonance imaging, and other sophisticated techniques, such as positron emission-computed tomography, are traditionally used to provide information on prognosis stage and risk stratification of lymphomas during routine clinical practice. Our results demonstrate that immune microenvironmental biomarkers and systematic inflammatory/nutritional biomarkers in the blood provide an inexpensive and more convenient way to stratify lymphoma risk. By analyzing the correlation between the tumor microenvironment (CD8+T lymphocytes and PD-L1 expression), WBC-DC, serum LDH, albumin, β2MG, iron, and ECOG performance status without the Ann Arbor stage data, we reached satisfactory concordance rates similar to the estimates with the above factors in addition to the Ann Arbor stage data. Our study might lead to the development of a new approach for risk stratification of aggressive B cell lymphomas. In addition, these biomarkers could be used to evaluate the efficacy and safety of anti-lymphoma drugs on the tumor-microenvironment-on-chip platform[60].
The limitations of this study include the small sample size and lack of sufficient data for a survival curve analysis because of the loss to follow-up for most lymphoma patients before 2015. In addition, the fixation state of the tissue samples and the areas analyzed in tissue sections may have diminished the quantification of immune cells and the expression of protein biomarkers in this study. However, this study did demonstrate for the first time that parameters in the tumor immune microenvironment, as well as systematic inflammatory and metabolic markers can be used for risk estimates of aggressive B cell lymphoma.
In conclusion, the association between the tumor immune microenvironment, systematic inflammatory and metabolic biomarkers, and clinical characteristics of aggressive B cell lymphoma paves the way to develop an economical and simple risk stratification method for routine clinical practice. Large cohorts of retrospective and prospective studies are required to further validate this practice.
| 1. | Shankland K, Armitage JO, Hancock BW. Non-Hodgkin lymphoma. Lancet, 2012, 380: 848–57. doi:10.1016/S0140-6736(12)60605-9 |
| 2. | Ansell SM. Non-Hodgkin Lymphoma:Diagnosis and Treatment. Mayo Clin Proc, 2015, 90: 1152–63. doi:10.1016/j.mayocp.2015.04.025 |
| 3. | Martelli M, Ferreri AJ, Agostinelli C, et al. Diffuse large B-cell lymphoma. Crit Rev Oncol Hematol, 2013, 87: 146–71. doi:10.1016/j.critrevonc.2012.12.009 |
| 4. | Pasqualucci L, Dalla-Favera R. The genetic landscape of diffuse large B-cell lymphoma. Semin Hematol, 2015, 52: 67–76. doi:10.1053/j.seminhematol.2015.01.005 |
| 5. | Sandoval-Sus JD, Chavez J, Dalia S. A new therapeutic era in GCB and ABC Diffuse large B-cell lymphoma molecular subtypes:a cell of origin driven review. Curr Cancer Drug Targets, 2016, 16: 305–22. doi:10.2174/1568009615666151030102539 |
| 6. | Sweetenham JW. Following aggressive B-cell lymphoma. Blood, 2015, 125: 3673–74. doi:10.1182/blood-2015-04-641738 |
| 7. | Dunleavy K. Aggressive B cell Lymphoma:Optimal Therapy for MYC-positive, Double-Hit, and Triple-Hit DLBCL. Curr Treat Options Oncol, 2015, 16: 58. doi:10.1007/s11864-015-0374-0 |
| 8. | Iioka F, Izumi K, Kamoda Y, et al. Outcomes of very elderly patients with aggressive B-cell non-Hodgkin lymphoma treated with reduced-dose chemotherapy. Int J Clin Oncol, 2016, 21: 498–505. doi:10.1007/s10147-015-0912-6 |
| 9. | Lyman GH, Crawford J, Tomita D, et al. Changing patterns of chemotherapy relative dose intensity and supportive care for aggressive B-cell non-Hodgkin lymphoma. Leuk Lymphoma, 2015: 1–8. |
| 10. | Nannya Y, Goto N, Shimizu M, et al. Efficacy of rituximab maintenance therapy for aggressive B-cell lymphoma depends on use of rituximab in induction therapy:a meta-analysis of randomized controlled trials. Haematologica, 2015, 100: e519–20. doi:10.3324/haematol.2015.136622 |
| 11. | Omry-Orbach G. Risk Stratification in Differentiated Thyroid Cancer:An Ongoing Process. Rambam Maimonides Med J, 2016, 7. |
| 12. | Roy P, Chan SM, Ng V, et al. Risk Stratification of Patients With Early Breast Cancer. Clin Breast Cancer, 2014, 14: 68–73. doi:10.1016/j.clbc.2013.09.005 |
| 13. | De Donk NWCJV, Sonneveld P. Diagnosis and Risk Stratification in Multiple Myeloma. Hematol Oncol Clin North Am, 2014, 28: 791. doi:10.1016/j.hoc.2014.06.007 |
| 14. | Tuomi T, Pasanen A, Luomaranta A, et al. Risk-stratification of endometrial carcinomas revisited:a combined preoperative and intraoperative scoring system for a reliable prediction of an advanced disease. Gynecol Oncol, 2015, 137: 23–7. |
| 15. | Vose JM. Mantle cell lymphoma:2015 update on diagnosis, risk-stratification, and clinical management. Am J Hematol, 2015, 90: 739–45. doi:10.1002/ajh.v90.8 |
| 16. | Factors A. A predictive model for aggressive non-Hodgkin's lymphoma. The International Non-Hodgkin's Lymphoma Prognostic Factors Project. N Engl J Med, 1993, 329: 987–94. doi:10.1056/NEJM199309303291402 |
| 17. | Sehn LH, Berry B, Chhanabhai M, et al. The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood, 2007, 109: 1857–61. doi:10.1182/blood-2006-08-038257 |
| 18. | Zhou Z, Sehn LH, Rademaker AW, et al. An enhanced International Prognostic Index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era. Blood, 2014, 123: 837–42. doi:10.1182/blood-2013-09-524108 |
| 19. | Melchardt T, Troppan K, Weiss L, et al. A modified scoring of the NCCN-IPI is more accurate in the elderly and is improved by albumin and beta2 -microglobulin. Br J Haematol, 2015, 168: 239–45. doi:10.1111/bjh.13116 |
| 20. | Troppan KT, Melchardt T, Deutsch A, et al. The significance of pretreatment anemia in the era of R-IPI and NCCN-IPI prognostic risk assessment tools:a dual-center study in diffuse large B-cell lymphoma patients. Eur J Haematol, 2015, 95: 538–44. doi:10.1111/ejh.12529 |
| 21. | Kiyasu J, Miyoshi H, Hirata A, et al. Expression of programmed cell death ligand 1 is associated with poor overall survival in patients with diffuse large B-cell lymphoma. Blood, 2015, 126: 2193–201. doi:10.1182/blood-2015-02-629600 |
| 22. | Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol, 2013, 14: 1014–22. doi:10.1038/ni.2703 |
| 23. | Hanahan D, Weinberg RA. Hallmarks of cancer:the next generation. Cell, 2011, 144: 646–74. doi:10.1016/j.cell.2011.02.013 |
| 24. | Carbone A, Tripodo C, Carlo-Stella C, et al. The role of inflammation in lymphoma. Adv Exp Med Biol, 2014, 816: 315–33. doi:10.1007/978-3-0348-0837-8 |
| 25. | Bachy E, Coiffier B. Anti-PD1 antibody:a new approach to treatment of lymphomas. Lancet Oncol, 2014, 15: 7–8. doi:10.1016/S1470-2045(13)70587-4 |
| 26. | Andorsky DJ, Yamada RE, Said J, et al. Programmed death ligand 1 is expressed by non-hodgkin lymphomas and inhibits the activity of tumor-associated T cells. Clin Cancer Res, 2011, 17: 4232–44. doi:10.1158/1078-0432.CCR-10-2660 |
| 27. | Janakiram M, Pareek V, Cheng H, et al. Immune checkpoint blockade in human cancer therapy:lung cancer and hematologic malignancies. Immunotherapy, 2016, 8: 809–19. doi:10.2217/imt-2016-0001 |
| 28. | Mahoney KM, Freeman GJ, McDermott DF. The Next Immune-Checkpoint Inhibitors:PD-1/PD-L1 Blockade in Melanoma. Clin Ther, 2015, 37: 764–82. doi:10.1016/j.clinthera.2015.02.018 |
| 29. | Galdiero MR, Bonavita E, Barajon I, et al. Tumor associated macrophages and neutrophils in cancer. Immunobiology, 2013, 218: 1402–10. doi:10.1016/j.imbio.2013.06.003 |
| 30. | Liang W, Ferrara N. The Complex Role of Neutrophils in Tumor Angiogenesis and Metastasis. Cancer Immunol Res, 2016, 4: 83–91. doi:10.1158/2326-6066.CIR-15-0313 |
| 31. | Powell DR, Huttenlocher A. Neutrophils in the Tumor Microenvironment. Trends Immunol, 2016, 37: 41–52. doi:10.1016/j.it.2015.11.008 |
| 32. | Yan M, Jurasz P. The role of platelets in the tumor microenvironment:From solid tumors to leukemia. Biochim Biophys Acta, 2016, 1863: 392–400. doi:10.1016/j.bbamcr.2015.07.008 |
| 33. | Noble F, Hopkins J, Curtis N, et al. The role of systemic inflammatory and nutritional blood-borne markers in predicting response to neoadjuvant chemotherapy and survival in oesophagogastric cancer. Med Oncol, 2013, 30: 596. doi:10.1007/s12032-013-0596-6 |
| 34. | Fiala O, Pesek M, Finek J, et al. Change in Serum Lactate Dehydrogenase Is Associated with Outcome of Patients with Advanced-stage NSCLC Treated with Erlotinib. Anticancer Res, 2016, 36: 2459–65. |
| 35. | Wang XL, Wang XL, He S, et al. Association of beta2-microglobulin with the prognosis of non-Hodgkin's lymphoma:a meta analysis. Int J Clin Exp Med, 2015, 8: 3992–9. |
| 36. | Kwok JC, Richardson DR. The iron metabolism of neoplastic cells:alterations that facilitate proliferation?. Crit Rev Oncol Hematol, 2002, 42: 65–78. doi:10.1016/S1040-8428(01)00213-X |
| 37. | Plosker GL, Figgitt DP. Rituximab:a review of its use in non-Hodgkin's lymphoma and chronic lymphocytic leukaemia. Drugs, 2003, 63: 803–43. doi:10.2165/00003495-200363080-00005 |
| 38. | Habermann TM, Weller EA, Morrison VA, et al. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol, 2006, 24: 3121–7. doi:10.1200/JCO.2005.05.1003 |
| 39. | Keane C, Vari F, Hertzberg M, et al. Ratios of T-cell immune effectors and checkpoint molecules as prognostic biomarkers in diffuse large B-cell lymphoma:a population-based study. Lancet Haematol, 2015, 2: e445–55. doi:10.1016/S2352-3026(15)00150-7 |
| 40. | Ho CL, Lu CS, Chen JH, et al. Neutrophil/Lymphocyte Ratio, Lymphocyte/Monocyte Ratio, and Absolute Lymphocyte Count/Absolute Monocyte Count Prognostic Score in Diffuse Large B-Cell Lymphoma:Useful Prognostic Tools in the Rituximab Era. Medicine, 2015, 94: e993. doi:10.1097/MD.0000000000000993 |
| 41. | Westin JR, Fayad LE. Beyond R-CHOP and the IPI in large-cell lymphoma:molecular markers as an opportunity for stratification. Curr Hematol Malig Rep, 2009, 4: 218–24. doi:10.1007/s11899-009-0029-y |
| 42. | Blank C, Mackensen A. Contribution of the PD-L1/PD-1 pathway to T-cell exhaustion:an update on implications for chronic infections and tumor evasion. Cancer Immunol Immunother, 2007, 56: 739–45. doi:10.1007/s00262-006-0272-1 |
| 43. | Balkwill F, Mantovani A. Inflammation and cancer:back to Virchow?. Lancet, 2001, 357: 539–45. doi:10.1016/S0140-6736(00)04046-0 |
| 44. | Catalano V, Turdo A, Di Franco S, et al. Tumor and its microenvironment:a synergistic interplay. Semin Cancer Biol, 2013, 23: 522–32. doi:10.1016/j.semcancer.2013.08.007 |
| 45. | Kridel R, Steidl C, Gascoyne RD. Tumor-associated macrophages in diffuse large B-cell lymphoma. Haematologica, 2015, 100: 143–5. doi:10.3324/haematol.2015.124008 |
| 46. | Nicholas NS, Apollonio B, Ramsay AG. Tumor microenvironment (TME)-driven immune suppression in B cell malignancy. Biochim Biophys Acta, 2016, 1863: 471–82. doi:10.1016/j.bbamcr.2015.11.003 |
| 47. | Rosenquist R, Davi F, Ghia P. The microenvironment in lymphomas——dissecting the complex crosstalk between tumor cells and 'by-stander' cells. Semin Cancer Biol, 2014, 24: 1–2. doi:10.1016/j.semcancer.2013.12.002 |
| 48. | Wang D, DuBois RN. Immunosuppression associated with chronic inflammation in the tumor microenvironment. Carcinogenesis, 2015, 36: 1085–93. doi:10.1093/carcin/bgv123 |
| 49. | Ribas A, Tumeh PC. The future of cancer therapy:selecting patients likely to respond to PD1/L1 blockade. Clin Cancer Res, 2014, 20: 4982–4. doi:10.1158/1078-0432.CCR-14-0933 |
| 50. | Chen BJ, Chapuy B, Ouyang J, et al. PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clin Cancer Res, 2013, 19: 3462–73. doi:10.1158/1078-0432.CCR-13-0855 |
| 51. | Gwak JM, Jang MH, Kim DI, et al. Prognostic value of tumor-associated macrophages according to histologic locations and hormone receptor status in breast cancer. PloS One, 2015, 10: e0125728. doi:10.1371/journal.pone.0125728 |
| 52. | Jensen TO, Schmidt H, Moller HJ, et al. Macrophage markers in serum and tumor have prognostic impact in American Joint Committee on Cancer stage Ⅰ/Ⅱ melanoma. J Clin Oncol, 2009, 27: 3330–7. doi:10.1200/JCO.2008.19.9919 |
| 53. | Kong LQ, Zhu XD, Xu HX, et al. The clinical significance of the CD163+ and CD68+ macrophages in patients with hepatocellular carcinoma. PloS One, 2013, 8: e59771. doi:10.1371/journal.pone.0059771 |
| 54. | Lissbrant IF, Stattin P, Wikstrom P, et al. Tumor associated macrophages in human prostate cancer:relation to clinicopathological variables and survival. Int J Oncol, 2000, 17: 445–51. |
| 55. | Li Z, Dong P, Ren M, et al. PD-L1 Expression Is Associated with Tumor FOXP3(+) Regulatory T-Cell Infiltration of Breast Cancer and Poor Prognosis of Patient. J Cancer, 2016, 7: 784–93. doi:10.7150/jca.14549 |
| 56. | Saito T, Nishikawa H, Wada H. Two FOXP3(+)CD4(+) T cell subpopulations distinctly control the prognosis of colorectal cancers. Nat Med, 2016, 22: 679–84. doi:10.1038/nm.4086 |
| 57. | Quigley DA, Kristensen V. Predicting prognosis and therapeutic response from interactions between lymphocytes and tumor cells. Mol Oncol, 2015, 9: 2054–62. doi:10.1016/j.molonc.2015.10.003 |
| 58. | Rossi D, Fangazio M, De Paoli L, et al. Beta-2-microglobulin is an independent predictor of progression in asymptomatic multiple myeloma. Cancer, 2010, 116: 2188–200. |
| 59. | Kukulj S, Jaganjac M, Boranic M, et al. Altered iron metabolism, inflammation, transferrin receptors, and ferritin expression in non-small-cell lung cancer. Med Oncol, 2010, 27: 268–77. doi:10.1007/s12032-009-9203-2 |
| 60. | Kwak B, Ozcelikkale A, Shin CS, et al. Simulation of complex transport of nanoparticles around a tumor using tumor-micro-environment-on-chip. J Control Release, 2014, 194: 157–67. doi:10.1016/j.jconrel.2014.08.027 |
 2017, Vol. 30
2017, Vol. 30


