2. School of Public Health, Physiotherapy & Sports Science, University College Dublin, Dublin D04 N2E5, Ireland;
3. Children's Hospital Affiliated to Capital Institute of Pediatrics, Beijing 100020, China;
4. Beijing Centers for Disease Control and Prevention, Beijing 100013, China
Botulism is a severe flaccid-paralytic disease caused by the botulinum neurotoxins (BoNTs) produced by Clostridium botulinum, as well as some strains of Clostridium butyricum and Clostridium baratii[1-2]. Human botulism is a rare but life-threatening disease, mainly caused by the ingestion of food contaminated with BoNTs (foodborne botulism). It may also arise following contamination of a wound with C. botulinum spores (wound botulism), or in infants by intestinal colonization and subsequent toxin production (infant botulism, IB)[3]. IB often occurs in children under the age of 1 year, which reflects their susceptibility to gut colonization by BoNT-producing clostridia. Positive confirmation of IB is normally established when BoNT and/or isolates of BoNT-producing clostridia are detected in the stool. The aim of this study was to conduct a retrospective epidemiological investigation of the etiology of a suspected case of IB.
BoNT poisoning was suspected in a three-month-old infant. The 11 samples collected for this investigation included a sample of leftover PIF that had been ingested by the infant, a 5-mL enema sample, and nine environmental swabs taken from the infant's family environment (one from the indoor and one from the outdoor window sill, three desks and one table, and each from the inside of the infant's feeding bottle, water cup, and bottle nipple). Both animal and human fecal specimens were processed in accordance with the protocols approved by Ethics Committee of China National Center for Food Safety Risk Assessment (CFSA, permit no. 2014004) and written informed consent from the patient's parent.
C. botulinum was isolated and BoNT was detected according to the China National Food Safety Standard (GB/T4789.12-2003) and the US Food and Drug Administration[4-5]. Presumptive C. botulinum colonies were selected for Gram staining, microscopic examination, and API 20A and VITEK2 biochemical test-based identification in accordance with the recommended procedures. In addition, 16S rRNA gene sequencing, detection of the bont genes encoding neurotoxin types A, B, E, and F, and BoNT production assays were carried out according to previously published methods[5-6]. PFGE typing was performed on C. botulinum and C. sporogenes cultured from the samples described above according to a previously published method[7].
Neither Clostridium species nor lethal BoNT were isolated or detected in the pre-culture obtained from the PIF sample. However, the culture supernatants prepared from cooked meat medium (CMM) cultures taken from the environmental swabs (including a desk, window sill, and table) and the enema sample resulted in the death of laboratory mice within 10 min after intraperitoneal injection. Signs of poisoning and the nature of the death of the mice were consistent with type Ⅰ poisoning by the positive type strain of C. sporogenes. Mice injected with the same cultures that had been either boiled at 100 ℃ for 10 min or trypsinized also died (Table 1). Mice injected intraperitoneally with the CMM-and tripticasopeptone glucose broth (TPGY)-cultured PIF sample and the tripticasopetone glucose broth TPGY-cultured enema sample exhibited type Ⅱ poisoning symptoms and death. The typical signs of type Ⅱ poisoning were noted in the first 24 h, and included ruffling of the fur followed in sequence by labored breathing ('wasp waist'), limb weakness, and finally total paralysis, during which the mice gasped for breath before they died. Signs of poisoning prior to death were also observed in mice injected intraperitoneally with trypsin-treated cultures. However, neither symptoms nor death occurred in mice injected intraperitoneally with the above cultures but that had been heated at 100 ℃ for 10 min (Table 1). The poisoning symptoms and deaths of the mice were consistent with those observed in type A BoNT positive control mice. Moreover, 1:10, 1:100, and 1:1, 000 dilutions of the above supernatants resulted in similar symptoms of poisoning and death in mice.
|
|
Table 1 Death Caused by Inoculation with Prepared Supernatants Recovered from Study Isolates |
The TPGY cultures isolated from the enema sample and the PIF were incubated at 37 ℃ for 45 min, together with monovalent anti-BoNT against types A, B, E, and F toxins, and polyvalent anti-BoNT (including against types A, B, C, D, E, and F). Both sets of antitoxins were injected separately into the mice intraperitoneally. Mice injected with monovalent anti-BoNT against toxin types A, E, and F presented with poisoning symptoms and eventually died, while those injected with monovalent anti-BoNT against type B toxin and with polyvalent anti-BoNT survived.
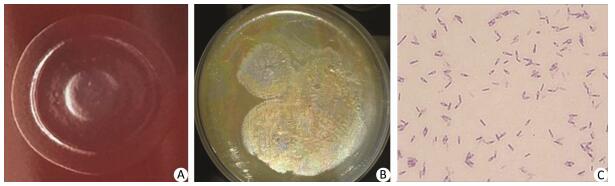
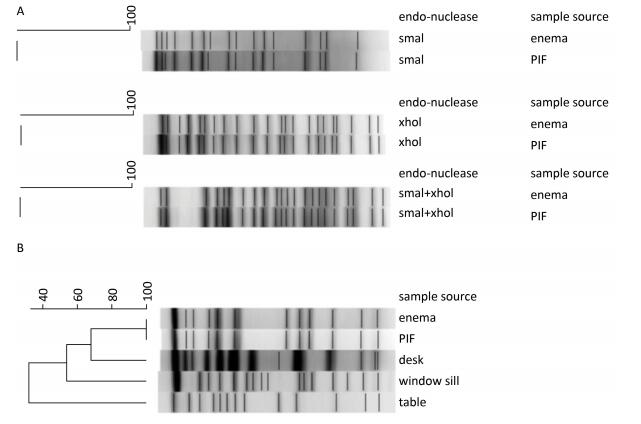
Two types of Clostridium isolates were cultured from the enema sample, the PIF sample, and the environmental swabs. The type suspected to be C. botulinum was cultured from the enema and PIF samples. Colonies of this isolate were circular, semi-transparent, and centrally raised or flat when grown on Columbia blood agar. The colonies had limited spreading on the plate, and had regular and smooth edges (Figure 1A). On egg yolk medium, the colonies exhibited surface iridescence when examined by oblique light (Figure 1B). This luster zone extended beyond and followed the irregular contour of the colony. Gram-stained isolates examined microscopically revealed oval-shaped spores that were wider than the bacterium and seemed to be sub-terminally localized, with a shape similar to that of a tennis racket (Figure 1C). All biochemical results, including API 20A and VITECK 2 analyses, confirmed the isolates as C. botulinum. 16S rRNA gene sequencing identified the isolates as C. botulinum or C. sporogenes, and PCR assays identified both as positive for bont/b genes. A total of five isolates of another type of Clostridium were detected in the enema sample, PIF, and environmental swabs of the table, window sill, and desk from the infant's home. Biochemical testing and 16S rRNA gene sequencing confirmed this isolate as C. sporogenes. PFGE analysis indicated that the two C. botulinum isolates from the enema sample and PIF had the same pulsotype (Figure 2A), and that the two C. sporogenes isolates from the enema sample and PIF were the same (Figure 2B), while the three C. sporogenes isolates taken from swabs of the desk, window sill and table were different (Figure 2B).
|
Download:
|
| Figure 1 Morphology of C. botulinum on different bacterial culture media and Gram staining. (A) Columbia blood agar. (B) Egg yolk agar. (C) Gram staining as viewed under a microscope. | |
|
Download:
|
| Figure 2 A 1% agarose gel showing the pulsotypes of (A) two C. botulinum isolates using different restriction endonucleases and (B) five C. sporogenes isolates digested with Sma I restriction endonuclease. | |
In IB, poisoning is mainly the result of progressive functional injury to nerve endings caused by the heat-labile neurotoxin elaborated by C. botulinum. This bacterium was ingested and germinated, allowing it to colonize the affected infant's intestine. It has been reported that 95% of IB cases develop in infants aged 1.5 to 6 months, with the incidence peaking between 2 and 4 months[8]. IB is likely to be misdiagnosed due to its sporadic nature and features, its atypical clinical manifestations, the lengthy and complicated laboratory analysis that is required for its detection, and the animal experiments required for toxin verification and typing. IB cases have been reported on all continents except Africa[8]. C. botulinum is the predominant etiologic agent of all botulism cases reported, except for the few cases in which C. butyricum and C. baratii were the causative species. IB can be contracted from contaminated food, soil, and close contact with infected caregivers and pets. Compared with the number of adult botulism cases, only a few cases of IB have been reported in China. The incidence of IB has been somewhat underestimated in China for a number of reasons, including the under-developed National Foodborne Disease Reporting System, which was established only in 2010, poor reporting awareness, inadequate clinical expertise, a limited laboratory testing capacity amongst the local CDCs, and a lack of clinicians especially in remote rural areas. In the present study, C. botulinum was isolated from the infant's enema sample and PIF. In vivo experiments in mice identified type B BoNT-producing strains in both samples and indistinguishable pulsotypes. PCR assays confirmed the presence of the bont/b gene. In addition, two isolates of C. sporogenes of the same pulsotype were contemporaneously isolated from the infant's enema sample and residual PIF, which suggested contamination of the PIF by both C. botulinum and C. sporogenes. Whether this was related to intrinsic or extrinsic modes of PIF contamination could not be determined. The former would have resulted in a large IB outbreak after the contaminated product was made available for retail sale. However, the absence of similar cases is indicative of contamination during consumption. Recent literature from other jurisdictions reported an IB case caused by a caregiver whose hand was found to be contaminated. However, it was not possible to determine whether this case could be similarly explained due to the absence of hand swabs taken from the infant's caregivers. Therefore, those in close contact with infants must be vigilant regarding personal as well as home hygiene standards. The scope of our study was limited by the relatively small amount of PIF that could be obtained for evaluation. A more extensive study of additional samples could provide additional perspective on whether there is a possible public health hazard from C. botulinum spores that may be present in commercial infant formula in China.
AUTHOR CONTRIBUTIONSDONG Yin Ping was involved in the experiments and the writing of the manuscript, and WANG Wei, JIANG Tao, XU Jin, HAN Chun Hui, and YAN Shao Fei in the experiments. LI Ying, MA Xiao Chen, ZHANG Di, ZHAO Yao, and ZENG Biao participated in the epidemiological investigation and sample collection. Séamus Fanning helped to correct the manuscript. LI Feng Qin took part in the collaboration with the hospital and local CDC as well as in the study design, organization of the experiments, and the manuscript writing. All authors reviewed the manuscript.
| 1. | Kruger M, Shehata AA, Schrodl W, et al. Glyphosate suppresses the antagonistic effect of Enterococcus spp. on Clostridium botulinum. Anaerobe, 2013, 20: 74–8. doi:10.1016/j.anaerobe.2013.01.005 |
| 2. | Peck MW, Smith TJ, Anniballi F, et al. Historical Perspectives and Guidelines for Botulinum Neurotoxin Subtype Nomenclature. Toxins, 2017, 9: 38. doi:10.3390/toxins9010038 |
| 3. | Simon S, Fiebig U, Liu Y, et al. Recommended Immunological Strategies to Screen for Botulinum Neurotoxin-Containing Samples. Toxins, 2015, 7: 5011–34. doi:10.3390/toxins7124860 |
| 4. | China National Food Safety Standard GB/T4789. 12 Microbiological examination of food hygiene: Clostridium botulinum and botulinum toxin test. (2003) |
| 5. | FDA. Bacteriological Analytical Manual (BAM): Chapter 17 Clostridium botulinum. (2001) |
| 6. | Weisburg WG, Barns SM, Pelletier DA, et al. 16S ribosomal DNA amplification for phylogenetic study. Bacteriol, 1991, 173: 697–703. doi:10.1128/jb.173.2.697-703.1991 |
| 7. | Dong YP, Li ZG, LI FQ. Comparison of mouse assay and pulsed field gel electrophoresis of Clostridium sporogenes isolated from China and New Zealand. Chinese Journal of Food Hygiene, 2016, 28: 40–4. |
| 8. | Fenicia L, Anniballi F. Infant botulism. Ann Ist Super Sanita, 2009, 45: 134–46. |
 2017, Vol. 30
2017, Vol. 30


