2. Department of Zoology and Entomology, Faculty of Science, Helwan University, Cairo 11795, Egypt ;
3. Department of Laboratory Sciences, College of Science & Arts, Al-Rass, Qassim University, AL-Qaseam 51921, Saudi Arabia ;
4. Molecular Drug Evaluation Department, National Organization for Drug Control & Research (NODCAR), Giza 12553, Egypt
Objective In this study, the ameliorative effects of gold nanoparticles (gold NP) on the renal tissue damage in Schistosoma mansoni (S. mansoni)-infected mice was investigated.
Methods High-resolution transmission electron microscopy was used for the characterization of NP. The gold NP at concentrations of 250, 500, and 1000 μg/kg body weight were inoculated into S. mansoni-infected mice.
Results The parasite caused alterations in the histological architecture. Furthermore, it induced a significant reduction in the renal glutathione levels; however, the levels of nitric oxide and malondialdehyde were significantly elevated. The parasite also managed to downregulate KIM-1, NGAL, MCP-1, and TGF-β mRNA expression in infected animals. Notably, gold NP treatment in mice reduced the extent of histological impairment and renal oxidative damage. Gold NP were able to regulate gene expression impaired by S. Mansoni infection.
Conclusion The curative effect of gold NP against renal toxicity in S. mansoni-infected mice is associated with their role as free radical scavengers.
Schistosomiasis is considered one of the most important water-based tropical diseases that affects the world's poorest people[1]. It is a chronic inflammatory disorder caused by flatworms (or flukes) belonging to the genus Schistosoma. This disease threatens about 800 million people worldwide and more than 200 million are infected. The suggested global burden of schistosomiasis is up to 4.5 million disability-adjusted life years[2]. In addition, the general health, growth, cognitive development, and future reproductive health of approximately 60% of African children are adversely affected by the disease[3].
El-Sokkary et al.[3] and Mutapi et al.[4] observed that Schistosoma mansoni (S. mansoni) infection causes oxidative stress in the kidney, liver, and spleen[4-5]. In addition, epidemiological studies have shown that schistosomiasis is associated with renal injury and renal parenchymal changes, which are mediated by immune complexes[6].
The treatment and control of schistosomiasis rely almost exclusively on praziquantel (PZQ)[7-8]. However, drug resistance has been an ongoing threat because of the large-scale administration of PZQ[9-10]. Although PZQ has a broad spectrum of activity, its activity is becoming inadequate, showing only moderate activity against immature worms[7]. Therefore, the discovery and development of novel antischistosomal drugs is crucial.
The discovery of new nanosized particles could improve health care in humans[11]. Within the last few years, the Food and Drug Administration has approved the use of nanotechnology-based drugs and its development by several pharmacological companies. Gold nanoparticles (NP) exert therapeutic effects against disorders such as chronic inflammation, rheumatoid arthritis, pathological neovascularization, and neoplasia[12].
Notably, the use of NP has been extended to the control of various pathogens, such as parasites, viruses, and bacteria. Shamaila et al.[13] concluded that gold NP that are 6-40 nm in size exhibited high antibacterial activity, and the antibacterial activity was dependent on size and dose. Tu[14] discovered that plant-borne molecules could be used to synthesize less toxic, effective mosquitocidal NP against young Aedes, Ochlerotatus, Anopheles, and Culexinstars. Green-synthesized NP have been used as mosquitocides against the vectors of malaria[15-16] and Zika virus[17]. Low doses of lemongrass-synthesized gold NP were observed to enhance the predation potential of freshwater copepod Mesocyclops aspericornis (Daday, 1906) on early instar mosquito larvae. This process might help to control the vectors of malaria and dengue[18]. In addition, artificial titanium dioxide NP have shown safer mosquitocidal potential[19].
In our previous studies on the protective effects of NP against hepatic and neural dysfunctions induced by S. mansoni infection in mice, gold NP exhibited a dose-dependent curative effect in the infected animals[20-21]. Therefore, the aim of the present study was to evaluate the therapeutic effect of gold NP on renal damage induced by S. mansoni infection in mice.
MATERIALS AND METHODS Experimental AnimalsSeventy-two male Swiss albino mice, aged 9-11 weeks, and weighing 20-25 g were maintained under specific pathogen-free conditions, fed a standard diet, and provided with water ad libitum.All reagents below were purchased from Sigma-Aldrich (St Louis, MO, USA) unless otherwise specified.
Gold NanoparticlesGold NP were prepared by the chemical reduction method[22]. Chloroauric acid (HAuCl4) was used as the Au3+ ion precursor, while sodium citrate was used as both reducing and stabilizing agent. The color of the solution slowly turned into faint pink color, indicating the reduction of Au3+ ions to Au nanoparticles.
The size and morphology of gold NP were determined by transmission electron microscopy (TEM). The samples for TEM were prepared using a clear solution of NP. The prepared solution was loaded onto a Formvar-coated grid, on which a drop of the sample solution (containing dispersed NP) was placed and allowed to air-dry. Electron micrographs were obtained using a JEOL JEM 2000 EX microscope (JEOL Ltd., Tokyo, Janpan).
Infection of MiceThe mice were injected subcutaneously with 100±10 S. mansoni cercariae[23], which were obtained from Schistosome Biological Supply Center, Theodor Bilharz Research Institute (TBRI), Imbaba, Giza, Egypt.
Experimental DesignThe animals were allocated to six groups, each comprising 12 mice. The mice in one group were uninfected and received water (100 μL water/mouse) by oral gavage for 10 d. The remaining mice were infected with 100±10 S. mansoni cercariae. The infected animals were divided into five groups, 46 d post infection (p.i.). One group was infected and left untreated, while three of the remaining four infected groups received an intraperitoneal (IP) injection of gold NP (100 μL) at 250, 500, and 1000 μg/kg body weight, respectively, on days 46 and 49 p.i. On day 46 p.i., the infected mice in the fifth group were orally administered 100 μL of PZQ (6x105 μg/kg body weight) every 24 h for 2 d. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. The investigation was conducted in accordance with the legal and ethical guidelines of the Medical Ethics Committee of TBRI, Giza, Egypt (Approval No. 4018/2011).
Histological StudiesOn day 56 p.i., the animals were killed by rapid decapitation. The right kidney of each subject was immediately removed, fixed in 10% formalin, dehydrated, and embedded in paraffin. The kidney sections (5 μm thick) were stained with Mayer's hematoxylin and 1% aqueous eosin for histopathological analysis[24].
Biochemical StudiesThe left kidney was also removed, weighed, and immediately homogenized in an ice-cold medium[25] containing 50 mmol/L Tris-HCl and 300 mmol/L sucrose at pH 7.4 to yield a 50% (w/v) homogenate. The homogenate was centrifuged at 500 g for 10 min at 4 ℃. The supernatant (10%) was used for the estimation of various biochemical parameters [glutathione (GSH), nitrite/nitrate, and malondialdehyde (MDA)].
The GSH levels in the kidney homogenate were determined by the method outlined by Ellman[26], and the nitrite/nitrate and MDA levels were deter-mined according to the methods of Green et al.[27] and Ohkawa et al.[28].
Quantitative Real-time PCRA cDNA reverse transcription kit (Life Technologies, Darmstadt, Germany) was used to prepare the cDNA template, according to the manufacturer's instructions. We used the ABI Prism 7900HT Sequence Detection System (Applied Biosystems, Darmstadt, Germany), with the TaqMan Gene Expression Master Mix (Life Technologies) to carry out quantitative real-time PCR (qRT-PCR). Table 1 summarizes the TaqMan assays (Life Technologies) that were used. All mRNA levels were normalized to those of GAPDH mRNA. The fold changes in mRNA expression were determined using the 2-ΔΔCT method[29].
|
|
Table 1 Primer Assays Used for qRT-PCR Analysis |
Data are presented as means±standard deviation, and a one-way ANOVA was used. Statistical comparisons among the groups were performed using Duncan's new multiple range test and a statistical package program (SPSS version 17.0, SPSS Inc., Chicago, IL, USA). P≤0.05 was considered significant.
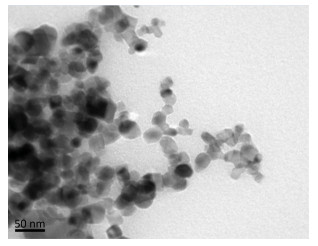
RESULTSThe gold NP were observed to be spherical in shape, with an average diameter of 20±5 nm using electron microscopy (Figure 1). The histological analysis of the renal tissues infected with S. mansoni showed marked congestion, intertubular hemorrhage with marked degenerative changes, and disrupted epithelia with cytoplasmic vacuolation. Schistosomiasis also caused marked inflammation, hemolysis, and glomerular injury (Table 2 and Figure 2). In contrast, the renal tissue morphology was restored following treatment with gold NP (250, 500, and 1000 μg/kg) and PZQ. In addition, the histological analysis of these groups revealed fewer signs of inflammation and hemolysis (Figure 2).
|
Download:
|
| Figure 1 Transmission electron microscopy image of gold nanoparticles illustrates their shape and size. | |
|
|
Table 2 Histological Score for the Kidney of Non-infected and Infected Mice with S. mansoni and Treated with Gold NP |

|
Download:
|
| Figure 2 Histological changes in the renal tissue of uninfected as well as untreated and treated mice infected with Schistosoma mansoni on day 56 post infection. (A) Uninfected kidney with normal architecture. (B) Renal tissue of mice in S. mansoni-infected group showing a severe inflammatory response, indicated by inflammatory cellular infiltration (white arrow), and degeneration of glomeruli (black arrow). (C, D, E) Renal tissue of mice in S. mansoni-infected groups treated with 250, 500, and 1000 μg gold nanoparticles (NP)/kg, respectively, exhibiting less tissue damage. (F) Kidney of mice in infected group treated with praziquantel, showing fewer lesions. The sections were stained with hematoxylin and eosin; scale bar=25 μm, n=6. | |
The infected mice exhibited significantly reduced renal GSH levels (Table 3). However, treatment with gold NP (250, 500, and 1000 μg/kg) and PZQ resulted in significantly increased GSH levels.
|
|
Table 3 Gold NP Induced Changes in the Level of Renal Glutathione (GSH), Malondialdehyde (MDA) and Nitrite/nitrate of Mice Infected with S. mansoni |
Schistosomiasis significantly increased the levels of both nitrite/nitrate and MDA in the renal tissues. However, the administration of gold NP to schistosome-infected mice significantly reduced the nitrite/nitrate and MDA levels. In addition, our results showed that gold NP treatment was more effective than PZQ treatment (Table 3).
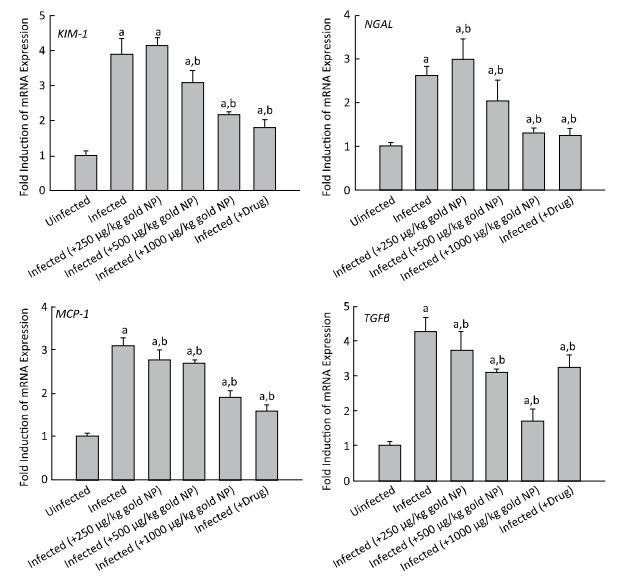
Moreover, significant upregulation was observed in the mRNA expression of kidney injury molecule-1 (KIM-1), neutrophil gelatinase-associated lipocalin (NGAL), monocyte chemoattractant protein-1 (MCP-1), and transforming growth factor beta (TGF-β) in the renal tissue of S. mansoni-infected mice (Figure 3). When compared to the infected untreated group, the group treated with gold NP (at a dose of 250 μg/kg) showed non-significant and significant upregulation of KIM-1 and NGAL mRNA expression, respectively, whereas the mRNA expression of MCP-1 and TGF-β was significantly downregulated.
|
Download:
|
| Figure 3 Gold nanoparticle (NP)-induced alterations in the gene expression of mice kidneys infected with Schistosoma mansoni. Expression of KIM-1, NGAL, MCP-1, and TGF-β mRNA in the kidney tissues was analyzed by quantitative RT-PCR in uninfected mice and S. mansoni-infected mice on day 56 post-infection, with and without gold NP and PZQ treatment. The relative expression is indicated as fold increase compared to that of the uninfected control mice. The values represent means±standard error (SE). (a) Significantly different from uninfected (-gold NP) group at P≤0.05. (b) Significantly different from infected (-gold NP) group at P≤0.05, n=6. | |
In addition, the groups treated with gold NP at doses of 500 and 1000 μg/kg, and PZQ showed significant downregulation in the mRNA expression of KIM-1, NGAL, MCP-1, and TGF-β compared to the infected untreated group (Figure 3).
DISCUSSIONThe use of gold NP for controlling protozoan parasites, such as those that cause malaria and the associated vectors of these diseases, has been the focus of some studies. Small particles of green-synthesized gold NP can pass through the cuticle and the cells of mosquitoes, thereby affecting their molting and other metabolic processes, which might, in turn, lead to death[18]. Low doses of gold NP have effectively reduced motility in Aedes aegypti and Anopheles stephensi larvae, thereby increasing the chances of predation by their natural copepod predator, M. aspericornis, and consequently, controlling the mosquito vector of malaria[18, 30].
Silver nanoparticles (Ag NP) are also found to be good examples of antimicrobial agents[31-33]. Ag NP have shown antifungal activity against plant diseases[34]. The antiparasitic activities of Ag NP were detected against protozoans such as Leishmania donovani[35], Giardia lamblia[36], and Plasmodium falciparum[37]. In addition, Ag NP have shown potent scolicidal activity in metazoan parasites such as Echinococcus granulosus[38]. The antimicrobial activities of metal NP against those pathogens were owing to different mechanisms such as the destruction of cell membrane integrity and the inhibition of protein synthesis and DNA replication in microbial cells[34, 39-40].
In 1970, renal glomerular disease was linked to hepatosplenic schistosomiasis, and researchers proposed that portal-systemic shunting might lead to the development of glomerular lesions, which were manifested by histological changes[41]. Since then, a few studies from Brazil[42] and Egypt[43-44] have highlighted about schistosomal glomerulopathy. The present study focused on the therapeutic effect of gold NP against the renal pathological changes induced by schistosomal infection. We found that schistosomiasis induced significant histological alterations in the renal tissue, accompanied by marked inflammation, hemorrhage, and glomerular degradation. However, treatment with gold NP significantly improved the histological architecture of the renal tissue. These results are consistent with those of van Velthuysen and Florquin[45] and Diab et al.[46].
Severe histological alterations in the renal tissue, including shrunken glomeruli and vacuolated tubules, which were induced by S. mansoni infection, have been reported by Diab et al.[46]. Glomerular lesions are mainly associated with hepatosplenic schistosomiasis, and portosystemic shunts might play a role in the development of glomerular damage. Membranoproliferative glomerulonephritis is a major sign of schistosomiasis. Focal and segmental glomerulosclerosis often appear late in chronic renal disease. Crescentic glomerulonephritis and membranous glomerulopathy have been rarely observed[45].
The kidney is a unique target organ for NP because of its ability to filter particles that are smaller than 10 nm in diameter rapidly[47]. This ability can be attributed to the presence of glomerular filtration unit, basement membrane, and interdigitating podocytes. The application of small quantities of gold NP at low concentrations in the medical field does not create serious hazards[48]. It has been reported that particles larger than 10 nm can not be filtered by the glomerulus[49].
Choi et al.[50] demonstrated that the NP that are 80-100 nm in diameter target the mesangium of the kidney, whereas the smaller particles are only evident within the peritubular capillaries. Patra et al.[51] investigated the toxicity of gold NP in three different cell lines (one of which was baby hamster kidney) in vitro, and reported that gold NP exerted no adverse effects on the cell morphology or viability. Silver NP also play a significant role in the treatment of kidney diseases[47].
The renal GSH level was significantly reduced in S. mansoni-infected mice, whereas the nitrite/nitrate and MDA levels were significantly increased. Our results are consistent with those of previous studies in which gold NP treatment caused a significant increase in GSH levels and a significant reduction in the nitrite/nitrate and MDA levels[20, 46, 52-53].
A marked redox imbalance was evident in the kidneys of S. mansoni-infected mice because the activities of renal superoxide dismutase and catalase are modulated in different ways[52]. A previous study reported that during schistosome infestation, the parasite tends to switch from the Krebs cycle to anaerobic glycolysis within the host. This results in a surplus of O2, which subjects the infected host to a state of oxidative stress owing to the increased generation of free radicals[53].
Dkhil et al.[20] concluded that schistosomiasis disrupts the antioxidant levels and increases the free radical levels in the brain tissue. However, administration of gold NP (250, 500, and 1000 μg/kg) increases the GSH levels and decreases the nitrite/nitrate and MDA levels in the brain tissue. Moreover, Khan et al.[54] reported that a microscopic analysis suggested that gold NPs are not cytotoxic because they suppress reactive oxygen species (ROS) generation, and they do not promote the release of proinflammatory cytokines (TNF-α and IL1-β) in the renal and hepatic tissues of rats. These properties make them suitable candidates for nanomedicine applications.
The overexpression of NGAL and KIM-1 might occur in chronic renal disease[55-56]. In addition, MCP-1 is a key mediator in acute ischemic and toxic renal injuries. Moreover, the levels of tubular KIM-1 in healthy kidneys are significantly increased following kidney injury[47-48]. It has been reported that in animals, the overproduction of KIM-1 protein in the affected segments of the proximal tubule can occur when toxins or the pathophysiological state results in the dedifferentiation of epithelial cells[55-59]. Dedifferentiation is a very early sign of epithelial response to an injury[60].
The marked upregulation of NGAL mRNA appears in the early post-ischemic kidney of mice and in the regions of colonic epithelium affected by inflammation or neoplasia[56]. NGAL production might result from the interactions between the inflammatory cells and epithelial lining. Furthermore, overexpression of NGAL has been observed in both neutrophils and epithelial cells[60-61]. Mishra et al.[56] reported that injured epithelial cells might upregulate NGAL to induce apoptosis of the infiltrating neutrophils, thereby stimulating the resident cells to survive the damage of inflammatory response. Similarly, TGF-β mediates renal fibrosis. The inhibition of renal TGF-β expression and activation may be a critical mechanism against renal fibrosis[56].
Overexpression of KIM-1[58, 63], NGAL[61], MCP-1[64-65], and TGF-β[66] proteins is suggestive of renal epithelial injury and inflammatory responses due to the ongoing release of ROS resulting from the parasitic infection. Antioxidants play a protective role against the upregulation of MCP-1[67] and TGF-β[68]. Notably, gold NP treatment is capable of reducing oxidative damage and improving the suppressed antioxidant responses observed in S. mansoni infection[20-21]. To the best of our knowledge, these results have demonstrated for the first time that gold NP could significantly downregulate KIM-1, NGAL, and MCP-1 mRNA in the renal tissue of S. mansoni-infected mice. Barathmanikanth et al.[69] and Tedesco et al.[70] reported that nanogold could act as a potent antioxidant by inhibiting the formation of ROS.
In the present study, we revealed the novel therapeutic potential of gold NP against renal disorders induced by S. mansoni infection. Gold NP reduced the levels of oxidative stress and histological disruption in the kidney. In addition, they restored the expression of the affected renal genes. The curative effect of gold NP is associated with their role as free radical scavengers. Further studies are required to determine the therapeutic potential and efficacy of a drug delivery system, such as the loading of antischistosomal drugs onto nanocarriers.
ACKNOWLEDGMENTThe authors extend appreciation to the Deanship of Scientific Research at King Saud University for funding the study through the research group project No. RG-198.
| 1. | Steinmann P, Keiser J, Bos R, et al. Schistosomiasis and water resources development:systematic review, meta-analysis, and estimates of people at risk[J]. Lancet Infect Dis , 2006, 6 :411–25. doi:10.1016/S1473-3099(06)70521-7 |
| 2. | King CH, Dickman K, Tisch DJ. Reassessment of the cost of chronic helmintic infection:a meta-analysis of disability-related outcomes in endemic schistosomiasis[J]. Lancet , 2005, 365 :1561–9. doi:10.1016/S0140-6736(05)66457-4 |
| 3. | El-Sokkary GH, Omar HM, Hassanein AF, et al. Melatonin reduces oxidative damage and increases survival of mice infected with Schistosoma mansoni[J]. Free Radic Biol Med , 2002, 32 :319–32. doi:10.1016/S0891-5849(01)00753-5 |
| 4. | Mutapi F. Changing policy and practice in the control of pediatric schistosomiasis[J]. Pediatrics , 2015, 135 :536–44. doi:10.1542/peds.2014-3189 |
| 5. | Facundo HT, Brandt CT, Owen JS, et al. Elevated levels of erythrocyte-conjugated dienes indicate increased lipid peroxidation in schistosomiasis mansoni patients[J]. Braz J Med Biol Res , 2004, 37 :957–62. |
| 6. | Rodrigues VL, Otoni A, Voieta I, et al. Glomerulonephritis in schistosomiasis mansoni:a time to reappraise[J]. Rev Soc Bras Med Trop , 2010, 43 :638–42. doi:10.1590/S0037-86822010000600007 |
| 7. | Doenhoff MJ, Cioli D, Utzinger J. Praziquantel:mechanisms of action, resistance and new derivatives for schistosomiasis[J]. Curr Opin Infect Dis , 2008, 21 :659–67. doi:10.1097/QCO.0b013e328318978f |
| 8. | Utzinger J, Keiser J. Schistosomiasis and soil-transmitted helminthiasis:common drugs for treatment and control[J]. Expert Opin Pharmacother , 2004, 5 :263–85. doi:10.1517/14656566.5.2.263 |
| 9. | Botros SS, Bennett JL. Praziquantel resistance[J]. Expert Opin Drug Discov , 2007, 2 :S35–40. doi:10.1517/17460441.2.S1.S35 |
| 10. | Caffrey CR. Chemotherapy of schistosomiasis:present and future[J]. Curr Opin Chem Biol , 2007, 11 :433–9. doi:10.1016/j.cbpa.2007.05.031 |
| 11. | Shrivastava S, Dash D. Applying Nanotechnology to Human Health:Revolution in Biomedical Sciences[J]. J Nanotechnol , 2009, 2009 :1–14. |
| 12. | Mukherjee P, Bhattacharya R, Wang P, et al. Antiangiogenic properties of gold nanoparticles[J]. Clin Cancer Res , 2005, 11 :3530–4. doi:10.1158/1078-0432.CCR-04-2482 |
| 13. | Shamaila Sh, Zafar N, Riaz S, et al. Gold Nanoparticles:An Efficient Antimicrobial Agent against Enteric Bacterial Human Pathogen[J]. Nanomat , 2016, 6, 71 :1–10. |
| 14. | Tu Y. The discovery of artemisinin (qinghaosu) and gifts from Chinese medicine[J]. Nat Med , 2011, 17 :1217–20. doi:10.1038/nm.2471 |
| 15. | Benelli G. Research in mosquito control:current challenges for a brighter future[J]. Parasitol Res , 2015, 114 :2801–5. doi:10.1007/s00436-015-4586-9 |
| 16. | Benelli G. Plant-mediated biosynthesis of nanoparticles as an emerging tool against mosquitoes of medical and veterinary importance:a review[J]. Parasitol Res , 2016, 115 :23–34. doi:10.1007/s00436-015-4800-9 |
| 17. | Benelli G, Mehlhorn H. Declining malaria, rising dengue and Zika virus:insights for mosquito vector control[J]. Parasitol Res , 2016, 115 :1747–54. doi:10.1007/s00436-016-4971-z |
| 18. | Murugan K, Benelli G, Panneerselvam C, et al. Cymbopogon citratus-synthesized gold nanoparticles boost the predation efficiency of copepod Mesocyclops aspericornis against malaria and dengue mosquitoes[J]. Exp Parasitol , 2015, 153 :129–38. doi:10.1016/j.exppara.2015.03.017 |
| 19. | Murugan K, Dinesh D, Kavithaa K, et al. Hydrothermal synthesis of titanium dioxide nanoparticles:mosquitocidal potential and anticancer activity on human breast cancer cells (MCF-7)[J]. Parasitol Res , 2016, 115 :1085–96. doi:10.1007/s00436-015-4838-8 |
| 20. | Dkhil MA, Bauomy AA, Diab MS, et al. Antioxidant and hepatoprotective role of gold nanoparticles against murine hepatic schistosomiasis[J]. Int J Nanomedicine , 2015, 10 :7467–75. |
| 21. | Dkhil MA, Bauomy AA, Diab MS, et al. Impact of gold nanoparticles on brain of mice infected with Schistosoma mansoni[J]. Parasitol Res , 2015, 114 :3711–9. doi:10.1007/s00436-015-4600-2 |
| 22. | Turkevich J, Stevenson PC, Hillier J. A study of the nucleation and growth processes in the synthesis of colloidal gold[J]. Faraday Soc , 1951, 11 :55–75. doi:10.1039/df9511100055 |
| 23. | Oliver L, Stirewalt MA. An efficient method for the exposure of mice to cercaria of Schistosoma mansoni[J]. J Parasitol , 1952, 38 :19–23. doi:10.2307/3274166 |
| 24. | Drury RA, Wallington EA. Carleton's Histological Technique, 5th edn[J]. Oxford University Press, Oxford , 1980 :139–42. |
| 25. | Tsakiris S, Schulpis KH, Marinou K, et al. Protective effect of L-cysteine and glutathione on the modulated suckling rat brain Na+, K+, -ATPase and Mg2+ -ATPase activities induced by the in vitro galactosaemia[J]. Pharmacol Res , 2004, 49 :475–9. doi:10.1016/j.phrs.2003.11.006 |
| 26. | Ellman GL. Tissue sulfhydryl groups[J]. Arch Biochem Biophys , 1959, 82 :70–7. doi:10.1016/0003-9861(59)90090-6 |
| 27. | Green LC, Wagner DA, Glogowski J, et al. Analysis of nitrate, nitrite, and[15N] nitrate in biological fluids[J]. Anal Biochem , 1982, 126 :131–8. doi:10.1016/0003-2697(82)90118-X |
| 28. | Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction[J]. Anal Biochem , 1979, 95 :351–8. doi:10.1016/0003-2697(79)90738-3 |
| 29. | Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C (T)) Method[J]. Methods , 2001, 25 :402–8. doi:10.1006/meth.2001.1262 |
| 30. | Subramaniam J, Murugan K, Panneerselvam C, et al. Multipurpose effectiveness of Couroupita guianensis-synthesized gold nanoparticles:high antiplasmodial potential, field efficacy against malaria vectors and synergy with Aplocheilus lineatuspredators[J]. Environ Sci Pollut Res , 2016, 10 :1–16. |
| 31. | Yuan X, Setyawati MI, Tan AS, et al. Highly luminescent silver nanoclusters with tunable emissions:cyclic reduction-decomposition synthesis and antimicrobial properties[J]. NPG Asia Mater , 2013, 5 :e39. doi:10.1038/am.2013.3 |
| 32. | Zheng KY, Yuan X, Goswami N, et al. Recent advances in the synthesis, characterization, and biomedical applications of ultrasmall thiolated silver nanoclusters[J]. RSC Adv , 2014, 4 :60581–96. doi:10.1039/C4RA12054J |
| 33. | Zheng K, Setyawati MI, Lim TP, et al. antimicrobial cluster bombs:silver nanoclusters packed with daptomycin[J]. ACS Nano , 2016, 23, 10 :7934–42. |
| 34. | Kim SW, Jung JH, Lamsa K, et al. Antifungal effects of silver nanoparticles (AgNPs) against various plant pathogenic fungi[J]. Mycobiology , 2012, 40 :53–35. doi:10.5941/MYCO.2012.40.1.053 |
| 35. | Allahverdiyev AM, Abamor ES, Bagirova M, et al. Antileishmanial effect of silver nanoparticles and their enhanced antiparasitic activity under ultraviolet light[J]. Int J Nanomedicine , 2011, 6 :2705–14. |
| 36. | Said DE, ElSamad LM, Gohar YM. Validity of silver, chitosan, and curcumin nanoparticles as anti-Giardia agents[J]. Parasitol Res , 2012, 111 :545–54. doi:10.1007/s00436-012-2866-1 |
| 37. | Mishra A, Kaushik NK, Sardar M, et al. Evaluation of antiplasmodial activity of green synthesized silver nanoparticles[J]. Colloids Surf B Biointerfaces , 2013, 111 :713–8. doi:10.1016/j.colsurfb.2013.06.036 |
| 38. | Rahimi MT, Ahmadpour E, Esboei BR, et al. Scolicidal activity of biosynthesized silver nanoparticles against Echinococcus granulosus protoscolices[J]. Int J Surg , 2015, 19 :128–33. doi:10.1016/j.ijsu.2015.05.043 |
| 39. | Feng QL, Wu J, Chen GQ, et al. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus[J]. J Biomed Mater Res , 2000, 52 :662–8. doi:10.1002/(ISSN)1097-4636 |
| 40. | Pelczar M, Reid R, Chan ECS. Microbiologia[J]. São Paulo:McGraw-Hill , 1980, 2008 . |
| 41. | Barsoum RS, Bassily S, Baligh OK, et al. Renal disease in hepatosplenic schistosomiasis:a clinicopathological study[J]. Trans R Soc Trop Med Hyg , 1977, 71 :387–91. doi:10.1016/0035-9203(77)90035-9 |
| 42. | Queiroz FP, Brito E, Martinelli R, et al. Nephrotic syndrome in patients with Schistosoma mansoni infections[J]. Am J Trop Med Hyg , 1973, 22 :622–8. |
| 43. | Higashi GL, Farid Z, Bassily S, et al. Nephrotic syndrome in schistosomiasis mansoni complicated by chronic salmonellosis[J]. Am J Trop Med Hyg , 1975, 24 :713–4. |
| 44. | Sabour MS, El-Said W, Abou-Gabal I. A clinical and pathological study of schistosomal nephritis[J]. Bull World Health Organ , 1972, 47 :549–57. |
| 45. | van Velthuysen ML, Florquin S. Glomerulopathy associated with parasitic infections[J]. Clin Microbiol Rev , 2000, 13 :55–66. doi:10.1128/CMR.13.1.55-66.2000 |
| 46. | Diab MSM, Bauomy AA, Dkhil MA, et al. Role of Morus alba in ameliorating Schistosoma mansoni-induced renal and testicular injuries in mice[J]. Pakistan J Zool , 2013, 45 :1367–75. |
| 47. | Brede C, Labhasetwar V. Applications of nanoparticles in the detection and treatment of kidney diseases[J]. Adv Chronic Kidney Dis , 2013, 20 :454–65. doi:10.1053/j.ackd.2013.07.006 |
| 48. | Doudi M, Setorki M. The effect of gold nanoparticle on renal function in rats[J]. Nanomed J , 2014, 1 :171–9. |
| 49. | Choi HS, Liu W, Misra P, et al. Renal clearance of quantum dots[J]. Nat Biotechnol , 2007, 25 :1165–70. doi:10.1038/nbt1340 |
| 50. | Choi CH, Zuckerman JE, Webster P, et al. Targeting kidney mesangium by nanoparticles of defined size[J]. Proc Natl Acad Sci U S A , 2011, 108 :6656–61. doi:10.1073/pnas.1103573108 |
| 51. | Patra HK, Banerjee S, Chaudhuri U, et al. Cell selective response to gold nanoparticles[J]. Nanomedicine , 2007, 3 :111–9. |
| 52. | de Oliveira RB, Senger MR, Vasques LM, et al. Schistosoma mansoni infection causes oxidative stress and alters receptor for advanced glycation endproduct (RAGE) and tau levels in multiple organs in mice[J]. Int J Parasitol , 2013, 43 :371–9. doi:10.1016/j.ijpara.2012.12.006 |
| 53. | Tielens AG. Energy generation in parasitic helminths[J]. Parasitol Today , 1994, 10 :346–52. doi:10.1016/0169-4758(94)90245-3 |
| 54. | Khan HA, Abdelhalim MA, Alhomida AS, et al. Effects of naked gold nanoparticles on proinflammatory cytokines mRNA expression in rat liver and kidney[J]. Biomed Res Int , 2013, 2013 :590730. |
| 55. | Bonventre JV. Kidney injury molecule-1 (KIM-1):a urinary biomarker and much more[J]. Nephrol Dial Transplant , 2009, 24 :3265–8. doi:10.1093/ndt/gfp010 |
| 56. | Mishra J, Ma Q, Prada A, et al. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury[J]. J Am Soc Nephrol , 2003, 14 :2534–43. doi:10.1097/01.ASN.0000088027.54400.C6 |
| 57. | Han WK, Bailly V, Abichandani R, et al. Kidney Injury Molecule-1 (KIM-1):a novel biomarker for human renal proximal tubule injury[J]. Kidney Int , 2002, 62 :237–44. doi:10.1046/j.1523-1755.2002.00433.x |
| 58. | Ichimura T, Bonventre JV, Bailly V, et al. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury[J]. J Biol Chem , 1998, 273 :4135–42. doi:10.1074/jbc.273.7.4135 |
| 59. | Zhou Y, Vaidya VS, Brown RP, et al. Comparison of kidney injury molecule-1 and other nephrotoxicity biomarkers in urine and kidney following acute exposure to gentamicin, mercury, and chromium[J]. Toxicol Sci , 2008, 101 :159–70. |
| 60. | Bonventre JV. Dedifferentiation and proliferation of surviving epithelial cells in acute renal failure[J]. J Am Soc Nephrol , 2003, 14 (Suppl 1) :S55–61. |
| 61. | Betsuyaku T, Nishimura M, Takeyabu K, et al. Neutrophil granule proteins in bronchoalveolar lavage fluid from subjects with subclinical emphysema[J]. Am J Respir Crit Care Med , 1999, 159 :1985–91. doi:10.1164/ajrccm.159.6.9809043 |
| 62. | Carlson M, Raab Y, Seveus L, et al. Human neutrophil lipocalin is a unique marker of neutrophil inflammation in ulcerative colitis and proctitis[J]. Gut , 2002, 50 :501–6. doi:10.1136/gut.50.4.501 |
| 63. | Park KM, Byun JY, Kramers C, et al. Inducible nitric-oxide synthase is an important contributor to prolonged protective effects of ischemic preconditioning in the mouse kidney[J]. J Biol Chem , 2003, 278 :27256–66. doi:10.1074/jbc.M301778200 |
| 64. | Gwinner W, Scheuer H, Haller H, et al. Pivotal role of xanthine oxidase in the initiation of tubulointerstitial renal injury in rats with hyperlipidemia[J]. Kidney Int , 2006, 69 :481–7. doi:10.1038/sj.ki.5000121 |
| 65. | Sung FL, Zhu TY, Au-Yeung KK, et al. Enhanced MCP-1 expression during ischemia/reperfusion injury is mediated by oxidative stress and NF-kappaB[J]. Kidney Int , 2002, 62 :1160–70. doi:10.1111/j.1523-1755.2002.kid577.x |
| 66. | Lodha S, Dani D, Mehta R, et al. Angiotensin II-induced mesangial cell apoptosis:role of oxidative stress[J]. Mol Med , 2002, 8 :830–40. |
| 67. | Chen HC, Guh JY, Shin SJ, et al. Reactive oxygen species enhances endothelin-1 production of diabetic rat glomeruli in vitro and in vivo[J]. J Lab Clin Med , 2000, 135 :309–15. doi:10.1067/mlc.2000.105616 |
| 68. | Park SH, Choi HJ, Lee JH, et al. High glucose inhibits renal proximal tubule cell proliferation and involves PKC, oxidative stress, and TGF-beta 1[J]. Kidney Int , 2001, 59 :1695–705. doi:10.1046/j.1523-1755.2001.0590051695.x |
| 69. | Barathmanikanth S, Kalishwaralal K, Sriram M, et al. Anti-oxidant effect of gold nanoparticles restrains hyperglycemic conditions in diabetic mice[J]. J Nanobiotechnol , 2010, 8 :16. doi:10.1186/1477-3155-8-16 |
| 70. | Tedesco S, Doyle H, Blasco J, et al. Oxidative stress and toxicity of gold nanoparticles in Mytilus edulis[J]. Aquat Toxicol , 2010, 100 :178–86. doi:10.1016/j.aquatox.2010.03.001 |
 2016, Vol. 29
2016, Vol. 29


