2. Hongzhe County Disease Control and Prevention, Huai'an 223199, Jiangsu, China
Objective To trace the source of human H7N9 cases in Huai'an and elucidate the genetic characterization of Huai'an strains associated with both humans and birds in live poultry market.
Methods An enhanced surveillance was implemented when the first human H7N9 case was confirmed in Huai'an. Clinical specimens, cloacal swabs, and fecal samples were collected and screened by real-time reverse transcription-polymerase chain reaction (RT-PCR) for H7N9 virus. The positive samples were subjected to further RT-PCR and genome sequencing. The phylodynamic patterns of H7N9 virus within and separated from Huai'an and evolutionary dynamics of the virus were analyzed.
Results Six patients with H7N9 infection were previously exposed to live poultry market and presented symptoms such as fever (>38.0℃) and headaches.Results of this study support the hypothesis that live poultry markets were the source of human H7N9 exposure. Phylogenetic analysis revealed that all novel H7N9 viruses, including Huai'an strains, could be classified into two distinct clades, A and B. Additionally, the diversified H7N9 virus circulated in live poultry markets in Huai'an. Interestingly, the common ancestors of the Huai'an H7N9 virus existed in January 2012. The mean nucleotide substitution rates for each gene segment of the H7N9 virus were (3.09-7.26)×10-3 substitutions/site per year (95% HPD:1.72×10-3 to 1.16×10-2).
Conclusion Overall, the source of exposure of human H7N9 cases in Huai'an was live poultry market, and our study highlights the presence of divergent genetic lineage of H7N9 virus in both humans and poultry specimens in Huai'an.
In March 2013,a novel avian influenza A (H7N9) virus was isolated from patients in Shanghai,China[1-2]. Human infections were detected in or in proximity to the Yangtze River Delta region,and the virus further spread to northern and southern China[3-4]. As of July 20,2016,802 reported cases have been confirmed,among which 300 deaths were mostly reported from mainland China[5]. Most of the affected areas are along the East Asian/Australian migratory flyway,which contains a high density of poultry markets and farms,in addition to a growing human population; this could potentiate the occurrence of reassortant viruses[6-8]. These regions were considered the most likely hotspots for the emergence of novel H7N9 viruses[8]. Although tracing H7N9 back to its origin is more complicated,several lines of evidence have supported the theory of an avian origin[6, 9-10]. Preliminary sequencing analyses have revealed that the H7N9 virus causing the 2013 outbreak in China is a reassortant virus,in which all the genes have been derived from avian origin and contain six internal protein genes from avian influenza A (H9N2) virus[6]. Gao et al. described that H7N9 might have reassorted with H7N3 HA gene in the Zhejiang duck,with H7N9 NA gene in the Korean wild bird,and with six internal protein genes of H9N2 in the Beijing brambling[1]. Kageyama et al. demonstrated that HA and NA genes of the H7N9 virus most likely originated from Eurasian avian influenza viruses,which is in agreement with the reassortant model proposed by Gao et al. One exception with the last hypothesis is that the NA gene segment showed the greatest homology with an H11N9 virus isolated from a mallard in the Czech Republic[11]. However,Peng et al. drew a controversial conclusion that the H7N9 virus originated from the HA gene of H7N1 in Baer’s Pochard found in the Hunan Province in 2010 and also from the NA gene of the duck influenza virus from Nanchang city in 2000. This is an evolutionarily dynamic model for its human-to-human transmission via mutations[12]. Recent studies have reported that H7N9 viruses have their own genetic diversity where at least two distinct HAs and NAs have been found to be mutated in the amino acid sequence[9, 13].
Huai’an is located at 32°43’00”-34°06’00” N and 118°12’00”-119°36’30” E in the northern part of Jiangsu Province of China,which is in Qinling Mountains-Huaihe River line as a north-south geographical boundary mark. The first case of avian influenza A (H7N9) virus infection was reported on January 28,2014,and as of July 20,2016,six reported cases have been confirmed in Huai’an. Genetic characterization of the influenza A (H7N9) virus circulating in Huai’an remains unknown,and knowledge of the evolutionary relationship between H7N9 viruses within Huai’an as well as out of that region is limited. In this study,we confirmed the first human case of avian influenza A (H7N9) infection reported on January 28,[2014] Later,we identified the H7N9 virus from live poultry markets including those visited by the patients. The phylodynamic patterns of the H7N9 virus within and separated from Huai’an were also analyzed,along with a detailed discussion on the evolutionary dynamics of the virus.
METHODS Ethics StatementThe surveillance and sampling protocols were reviewed and approved by the ethics committee of the Institute for Communicable Disease Control and Prevention of the Huai’an CDC. All throat swab specimens were obtained from hospitalized patients,upon approval from the Human Research Ethics Committee of Huai’an City,and written informed consent was obtained from each patient. The protocol of collecting the cloacal swab specimens of poultry in this study was approved by the Ethics Committee of Huai’an Centers for Disease Control and Prevention and the Commercial Bureau of Huai’an City. Sampling of feces of wild birds was approved by the Forestry Bureau of Huai’an City. All efforts for collecting poultry cloacal swabs were performed in a minimally invasive manner.
Sample CollectionBeginning on January 28,2014,enhanced surveillance was implemented for suspected cases of H7N9 virus infection among hospitalized patients with severe pneumonia of unexplained origin and birds. Cloacal swabs and fecal samples of live domestic birds were collected from bird markets,and fecal samples of wild birds were collected from migratory areas around Huai’an City. All the samples were collected in sterile 3-mL virus sampling screw-cap tubes and stored at -80 °C immediately.
RNA Extraction,Real-time RT-PCR,cDNA Synthesis,and SequencingViral RNA was extracted from the clinical specimens,cloacal swabs,and fecal samples of wild birds and live poultry using QIAamp Viral Mini Kit (Qiagen,Inc.,Shanghai,China),according to the manufacturers’ instructions. Real-time PCR was performed using influenza A,H1,H1pdm09,H3,H5N1,and H7N9 detection kit (Shanghai Zhijiang Biotechnology,Shanghai,China),following the manufacturers’ instructions,on ABI-viia7 Real-time PCR systems (Applied Biosystems,Foster City,CA). One-step RT-PCR was performed on Bio-Rad T100TM Thermal Cycler (Bio-Rad,Hercules,CA) using Invitrogen SuperscriptTm One-step RT-PCR,following the manufacturer’s instructions.
All eight segment sequences of H7N9 for RT-PCR have been described previously[2, 14]. PCR products were separated on a 1% agarose gel by electrophoresis and purified using Qiagen gel extraction kit (Qiagen,Inc.,Valencia,CA). Amplicons of appropriate sizes were subjected to sequencing with an ABI Prism dye termination sequencing kit and an ABI 373-A genetic analyzer.
Phylogenetic AnalysisThe HA (n=209),NA (n=201),PA (n=174),PB2 (n=174),PB1 (n=164),NP (n=167),M (n=182),and NS (n=174) gene sequences of H7N9 virus strains were aligned using the Molecular Evolutionary Genetics Analysis (MEGA) v6.05 software[15]. Subsequently,the identical sequences were removed to avoid duplications in the successive analyses using the DAMBE program (version 5.3.109). Their nucleotide identities and divergences were calculated using the Lasergene package (version 7.1). The best-fit phylogenetic model was determined by model test v2.1.[4] General time-reversible (GTR) with a gamma distribution model of site rate heterogeneity and a proportion of invariable sites (GTR + Γ4) were found to be the best model for gene segments[16]. The Bayesian MCMC method in BEAST v 1.75 was used to perform phylogenetic analyses[17]. The data were run for 30,000,000 generations,sampling every 1000 generations,with 10% burn-in. The maximum clade credibility tree (MCC) was generated with uncertainly in all estimates reflected in the 95% highest posterior density (95% HPD) intervals and visualized using FigTree v1.4.
Estimating Rates of Nucleotide SubstitutionAfter a comprehensive evolutionary analysis,the H7N9 strains were isolated from all host species (all dataset,n=209,201,174,174,164,167,182,and 174 for HA,NA,PA,PB2,PB1,NP,M,and NS,respectively) and a dataset including only Huai’an isolates (HN dataset,n=10 for all segment sequences of H7N9 virus). In addition to the sequence datasets,the A dataset was further partitioned into the dataset containing human H7N9 virus (H dataset,n=108 for HA; n=110 for NA; n=76 for PA; n=85 for PB2: n=79 for PB1: n=76 for NP; n=85 for M; n=84 for NS) and bird-H7N9 virus (B dataset,n=101 for HA; n=91 for NA; n=98 for PA; n=89 for PB2: n=85 for PB1: n=91 for NP; n=97 for M; n=90 for NS). Meanwhile,the evolutionary rates of nucleotide substitutions and time-scale analyses for the datasets of H7N9 virus were estimated using an uncorrelated lognormal-distribution relaxed molecular clock model and extended Bayesian Skyline tree prior to it being available in the BEAST v 1.75 package[17]. The 95% HPD values were obtained to ascertain the uncertainty in the parameter estimates. The datasets were based on the ORF of eight segment sequences of H7N9 virus for which the year of sampling was known (dates ranged from 2013 to 2015).
RESULTS Epidemiological Features of Human Confirmed CasesAfter the first laboratory-confirmed human infection of H7N9 in Huai’an,surveillance was reinforced for hospitalized patients with pneumonia of unexplained origin. As of May 28,2015,a total of 22 hospitalized patients had been reported,and six cases of H7N9 virus infection were identified in Huai'an. The epidemiological and clinical characteristics of patients are summarized in Table 1; all six H7N9 cases had visited a live poultry market 4-5 days before illness onset. Case 2 (ID 065) and case 6 (ID 002) also had direct contact with poultry. The interval from illness onset to hospitalization ranged from 2 to 8 days. Two of the six cases (ID062 and 002) died on days 6 and 8 after illness onset. As summarized in Table 1,the median age of the confirmed cases was 61 years (range 42-83 years). Four confirmed cases occurred in males (66.7%) and 83.3% of the case patients were urban residents. Occupations of those with confirmed cases included one retired,one factory worker,one cook,one poultry worker,and two housekeepers who had recently been exposed to live poultry. Only 33.3% of the confirmed cases had direct contact with poultry. The clinical history revealed that all the patients had suffered from fevers (>38.0 °C),headaches,and other common clinical symptoms,including arthralgia (33.3%),shortness of breath (66.7%),cough (83.3%),and sore throat (83.3%).
|
|
Table 1 Epidemiological and Clinical Characteristics of Patients with H7N9 Virus Infection in Huai’an |
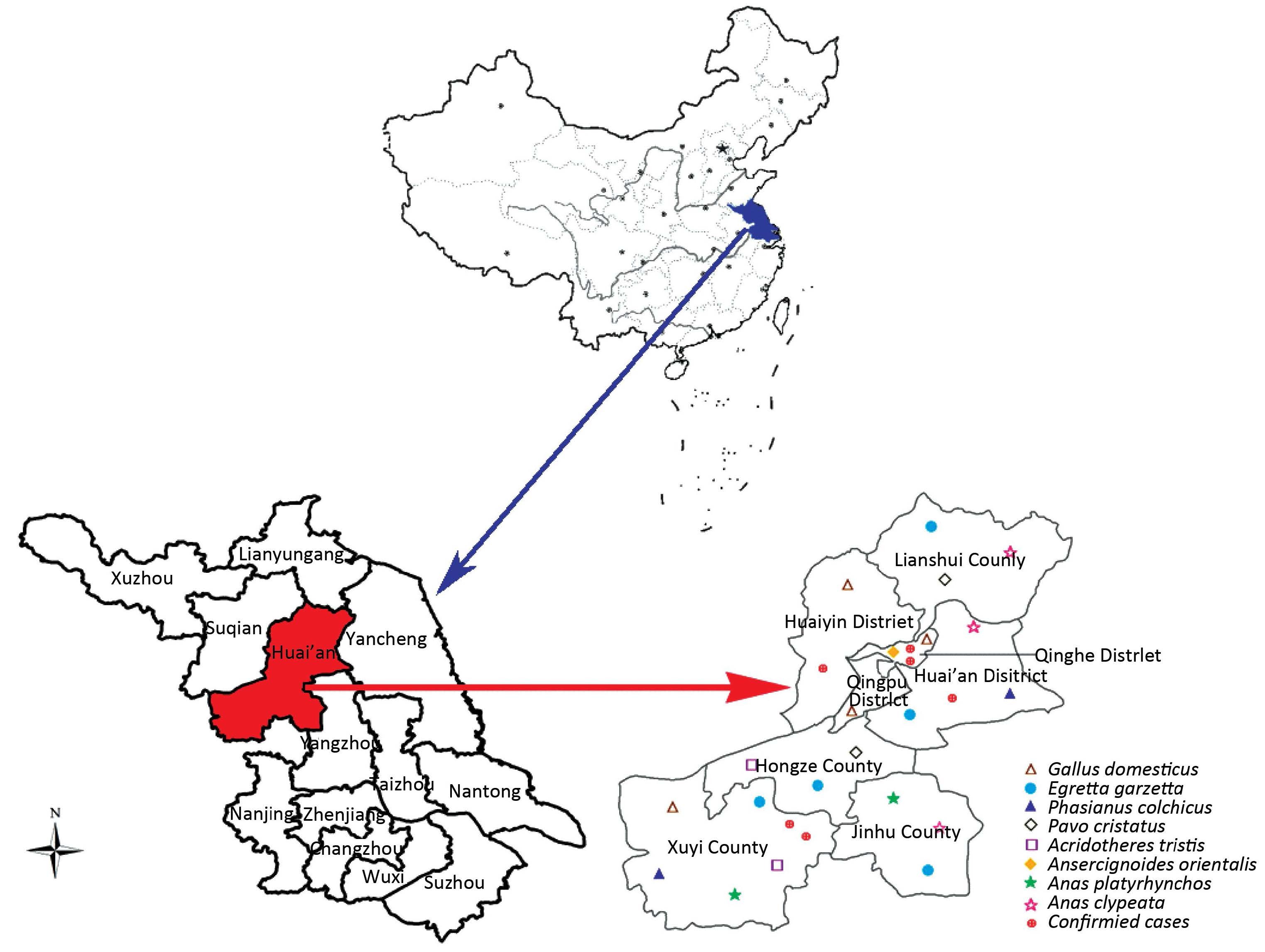
To gain a better insight on H7N9 circulating in Huai’an wild and domestic birds,cloacal swabs and fecal samples were collected from residential habitats of migratory birds and from live poultry markets beginning on January 28,[2014] Finally,a total of 496 samples belonging to eight species were collected (Table 2). The majority of samples (75.20%) were cloacal swabs collected from live poultry markets in four counties,Lianshui,Hongze,Jinghu,and Xuyi,and in four districts,Qinghe,Qingpu,Huaiyin,and Huai’an. The remaining samples (24.80%) were feces of Egretta garzetta collected in residential habitats in Lianshui,Hongze,Jinghu,and Xuyi counties and in the district of Huai’an. Among 496 samples,6 (1.21%) were found to be positive for H7N9 virus,verified by real-time RT-PCR,including three Gallus domesticus, one Anas platyrhynchos,and two Anas clypeata. Interestingly,H7N9 virus was not identified in the feces of the migratory bird E. garzetta. Detailed features including the species and geographical location of the wild and domestic birds in Huai’an are summarized in Table 2 and Figure 1.
|
|
Table 2 Prevalence of H7N9 Virus in Various Bird Species and Locations in Huai’an |
|
Download:
|
| Figure 1 Geographic distribution of H7N9 virus-affected cases and sampling sites in Huai’an (in red color) and the location of Jiangsu Province in China (in blue color). | |
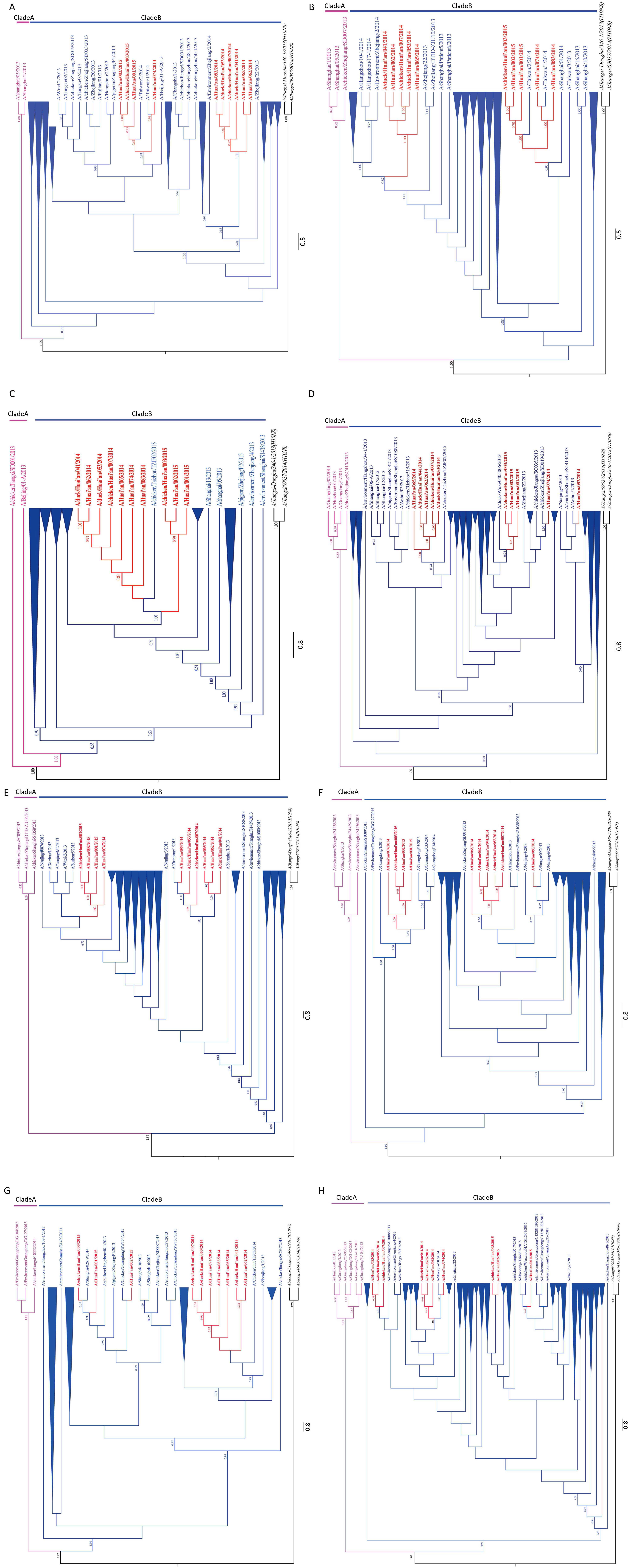
To investigate the evolutionary relationships between H7N9 viruses circulating within and around Huai’an,phylogenetic trees were reconstructed by using Bayesian methods. This was based on sequences of each segment of the H7N9 virus recovered in the present study (Table 3),as well as those retrieved from the GISAID. The sequences of representative H7N9 viruses sampled during 2013-2015 from different geographical regions of China originated from avian,environmental,and human specimens. The phylogenetic trees generated with eight gene segment sequences of H7N9 virus revealed the same topology (Figure 2). On the phylogenetic tree,these viruses formed two distinct genetic clades A and B. Clade A represented the H7N9 virus,which was located most closely to the common ancestor. Specifically,the H7N9 viruses originated from the Yangtze River Delta region and formed an independent clade (clade A) in the HA,NA,PA,and NP trees,while the H7N9 viruses circulated mostly in Guangdong and formed clade A in the PB1,MP,and NS trees. Notably,in the PB2 tree,A/chicken/Jiangxi/SD001/2013 and A/Beijing/01-A/ 2013 located most closely to the common ancestor formed clade A. Clade B,representing a large number of isolates from geographically diverse provinces of China,was divided into several further lineages,which included all ten Huai’an isolates. Notably,Huai’an isolates formed two distinct lineages. Strains CH007,DU041,DU053,HU062,and HU065 clustered together and formed a distinct genetic lineage (CH007 lineage),while isolates HU001,HU002,CH003,and HU074 formed another distinct genetic lineage (HU001 lineage) with a close evolutionary relationship with A/Taiwan/1/2014 and A/Taiwan/2/2014 in the surface HA and NA trees. Interestingly,the strain HU083 was found to be closely related to the CH007 lineage based on the HA gene sequence,while it was close to HU001 within the other lineage based on the NA gene. The HU001 lineage and CH007 lineage of internal gene (NP,PA) trees were primarily consistent with HA tree,and PB1 gene tree was similar to NA tree. In addition,strains CH007,DU041,DU053,HU062,HU065,HU074,and HU083 fell into the CH007 lineage in the PB2,NS,and MP trees,while strains HU001,HU002,and CH003 formed the HU001 lineage.
|
|
Table 3 The Sequences Recovered from Patients and Poultry in the Study |
|
Download:
|
| Figure 2 Phylogenetic trees of avian influenza A (H7N9) viruses isolated in and outside Huai’an. The Bayesian tree for each segment,including HA (A),NA (B),PB2 (C),PB1 (D),PA (E),NP (F),MP (G),and NS (H) of H7N9 viruses is shown. Posterior node probabilities (>0.50) are shown in above branches. H10N8 strains (A/Jiangxi-Donghu/346-1/2013,A/Jiangxi/09037/2014) in italic font were used as the outgroups. The H7N9 strains in clade A and B are indicated in pink and blue,respectively. The sequences recovered in this study are shown in red with bold fonts. | |
Genetic analyses of these sequences indicated that the variation between the clades showed diversity at the nucleotide level up to 1.0% based on the surface HA and NA genes,with little difference being observed between clades A and B,based on internal gene sequences. The interclade nucleotide diversity was above 0.9%. Comparison of the gene sequences of HU001 and CH007 lineages revealed nucleotide divergences up to 1.7% (HA),2.5% (NA),0.4% (PB2),0.8% (PB1),2.4% (PA),3.7% (NP),0.8% (MP),and 1.2% (NS) (Table S1,see the www. besjournal.com). The nucleotide divergences within the HU001 lineage were less than 0.8% for HA gene,1.2% for NA,and 0.7% for internal protein genes. The strains in the CH007 lineage were found to have higher diversity than those in the HU001 lineage,with <2.0% nucleotide divergence for the surface HA and NA genes and <2.7% for internal protein genes.
Signature Amino Acids of Huai’an Strains Associated with Virulence,Host Adaptation,and Drug ResistanceAll Huai’an strains presented molecular markers for mammalian adaptation and virulence in HA (N174S,G186V,T221P,Q226L),NA (69-73,a deletion),PB1 (I368V),and PA (K356R,S409N) but showed an avian signature,specifically glutamic acid (E) at residue 627 and aspartic acid (D) at residue 701 in the PB2 proteins[1, 18]. Like most isolated H7N9 viruses,all of the strains sequenced in Huai’an lacked the H274Y,R292K mutation in the NA gene and carried the S31N mutation in the M2 gene,which indicates that they are probably sensitive to oseltamivir and resistant to amantadine[18-20]. In addition,28 site mutations were identified in the surface HA and NA proteins of Huai’an strains (Table S2,in the supplementary material see the www.besjournal.com). Among these amino acid substitutions,there were nine site mutations shared by at least two isolates (G18S,E78K,N84D,V241I,S247P,A401T) in the NA gene. A previous study has reported that E119V,R152K mutations in the surface NA protein were related to resistance to oseltamivir[1, 18]. Notably,the strain CH007 and DU53 in the present study carried two novel site mutations Y and L at residues 119 and 152 in the NA gene,respectively (Table 4).
|
|
Table 4 The molecular markers of Huai’an strains associated with virulence, host adaptation,and drug resistance |
To estimate the population evolutionary time-scale for the H7N9 virus,we analyzed known year-of-sampling datasets with an uncorrelated lognormal-distribution-relaxed molecular clock model and an extended Bayesian Skyline model for eight gene segments of H7N9. The Bayesian Markov Chain Monte Carlo (MCMC) analysis indicated that the parameter values estimated from the segment sequence data deviated substantially from those of the random dataset,with no overlapping 95% HPD,suggesting that there was a significant temporal signal in these data[17]. The population evolutionary rates of the H7N9 virus analysis showed that the mean nucleotide substitution rates (MNSR) for each gene segments were (3.09-7.26)×10-3 substitutions/ site per year,with a 95% HPD that ranged from 1.72×10-3 to 1.16×10-2 substitutions/site per year (Table 5). The mean values of the time to the most recent common ancestor (TMRCA) calculated for all datasets based on sampled genetic diversity were between 4.56 and 8.71 years ago (95% HPD=3.39-15.99 years). Notably,the same mean values estimated for the divergence for the B dataset were found. In the case of HN dataset,the TMRCA of H7N9 genes existing were 2.36-2.86 years ago or around January 2012. We also found that the spread of novel H7N9 virus to Huai’an from other provinces of China began around January 2012. Accordingly,it then took approximately 2 years before the first infected case was confirmed.
|
|
Table 5 Summary of evolution rates in the eight gene segments of H7N9 virus in and out of Huai’an |
Since the emergence of the novel H7N9 influenza A virus in China in 2013,it has circulated mostly throughout mainland China. Along with severe infection and a high mortality rate (-27%),it has persisted for more than 2 years,therefore creating two epidemic peaks[20-22]. With the number of human infections increasing,H7N9 virus has become a significant global threat to public health[23]. In the present study,the first human infection with H7N9 virus was confirmed in Huai’an. We confirmed that the infection source in these cases was epidemiologically linked with live poultry markets in Huai’an. All cases had a history of exposure to live poultry markets and making direct or indirect contact with poultry before the onset of illness. Notably,the eight gene segments of CH003 isolated from chicken in a live poultry market showed the highest similarity with the human virus (99.3%-100.0% identity with HU002). HU002 visited the live poultry market,when CH003 was isolated from the poultry 6 days before illness onset (Table S1,see the www.besjournal.com). In addition,eight gene segments of HU062 isolated from case 1 and DU041 isolated from duck in another live poultry market,which case 1 visited,shared 98.9%-99.9% identity. Thus,the epidemiological and genetic data indicated that contaminated live poultry markets were the origin of the H7N9 virus that infected humans. Therefore,it is essential to enhance both epidemiological and laboratory-based influenza surveillance for chicken,duck,and other avian species,specifically in live poultry markets[24-25].
In particular,H7N9 virus was not detected in the migratory bird species E. garzetta,which is the most common migratory bird in Huai’an. Previous studies demonstrated that the novel H7N9 virus was reassorted from duck (HA gene segment),migratory birds (NA gene segment),and the avian influenza H9N2 virus (six internal protein genes)[1, 8]. However,the H7N9 virus has not been identified in migratory birds[7, 25]. These studies suggest that migratory birds may only be a donor for the surface NA gene[7, 25-26]. The domestic duck may act as a key intermediate host for viral reassortment in the early stages and ultimately generate the novel H7N9 virus. The duck transferring H7N9 into its environment could potentially lead to infection of domestic poultry via the fecal-oral route,in which the duck may inhabit the same general area[27].
Earlier phylogeny studies on HA and NA genes suggest that H7N9 could form a single phylogenetic group or clade on the phylogenetic tree[1-2, 23]. This is also demonstrated in SH05,which clustered with A/Shanghai/1/2013 on the HA gene tree or possibly together with A/Shanghai/1/2013 (SH1) and A/Zhejiang/SD007/2013 on the NA gene tree. In accordance with these results,almost all H7N9 viruses are genetically homogeneous. However,SH05 and SH1 isolated in Shanghai showed 1.8% divergence compared to the other H7N9 viral HA and NA gene sequences (data not shown). In our opinion,our results point in the direction that all of the current H7N9 viruses may have originated in the Yangtze River delta region and Guangdong and then spread to other provinces of China. Furthermore,we suggest that all H7N9 viruses formed distinct clades (A and B) on the surface HA and NA gene trees in accordance with internal gene trees. Particularly,the Huai’an isolates within clade B showed high genetic diversity and therefore divided into two lineages. The topology of the H7N9 phylogenetic tree revealed that the virus in Huai’an has undergone viral fitness after two distinct viral introductions (i.e.,Huai’an isolates may be from diverse geographical origins or divided into two lineages originated from a single area). This phenomenon may be explained by the trade of poultry between Huai’an and other provinces in China,which may have led to increased prevalence of H7N9 from varied geographical origins. Backyard poultry breeding habitats and the ubiquitous live market system where poultry are kept together in large numbers may also contribute to H7N9 transmis sion[27]. Notably,the topology of the HU083 and HU074 surface HA and NA gene trees in this study was found to be completely inconsistent when compared with the internal gene trees. Although the evolutionary mechanism of HU83 and HU074 could not be accurately elucidated,virus reassortment and quasi-evolution incidents occurring in patients should remain under close attention[26-28].
In this study,an evolutionary dynamics analysis of H7N9 virus was performed on gene sequences in the H7N9 virus isolated from infected humans and poultry,in addition to collection of environmental samples contaminated by poultry between 2013 and 2015. The MNSR of H7N9 virus was (3.09-7.26)×10-3 substitutions/site per year,although substitution rates of (4.81-5.21)×10-3 have been reported in another study[10]. The MNSR was estimated on the host population,gene,and coalescent parameters[16]. As previously established,a Bayesian method was employed for calculating the TMRCA. Results revealed that the internal genes experienced a longer latency time than did the surface genes before they evolved into the novel H7N9 viruses that began circulating in poultry (Table 5). Other TMRCA estimations for the HA and NA genes have been conducted employing most of the H7 strains and N9 strains or only the novel H7N9 isolates,but with limitations[6]. These studies reveal a broad range of viral divergence somewhere between January 2012 and July 2012 for the H7N9 virus circulating in Huai’an,depending upon demographic and clock models[6,10.12].
As the H7N9 virus is a low-pathogenicity avian influenza virus,it can infect poultry and then circulate in humans with pandemic potential[29]. The viral evolution and transmission route were very laborious to investigate. Thus,implementation of enhanced surveillance and control measures in live poultry markets can aid in reducing the transmission of this avian influenza virus to humans.
CONCLUSIONSIn summary,to the best of our knowledge,we confirm that the first human infection with H7N9 virus in Huai’an was sourced at a live poultry market. We found that H7N9 virus circulating in humans and poultry across China clustered into two phylogenetic groups,and these classifications were not related to the specific host. Furthermore,our findings indicate that the diversified H7N9 virus circulated within the poultry in Huai’an. Meanwhile,this study also indicates that two lineages of the H7N9 virus within one clade are prevalent with their special site mutations in Huai’an and also that the virus may be continuing to circulate in Huai’an.
|
|
Table S1 Nucleotide Identity and Divergence of Each Segment of H7N9 Virus Isolated from Huai’an with Each Other |
|
|
Table S2 Substitutions in the Surface Proteins of H7N9 Viruses in the Study |
ACKNOWLEDGMENTS
We would like to thank Dr. Kaylyn E. Germ from Texas Tech University and Dr. TANG S from University of Saskatchewan who read this paper in an earlier draft form and offered insightful comments. We thank HU KX,YAO LS,ZHANG LP,and MA XZ of Chinese Academy of Inspection and Quarantine,Institute of Health Quarantine,ZHEN W of National Institute for Viral Disease Control and Prevention,Chinese Center for Disease Control and Prevention,ZHANG DT of Beijing Center for Disease Prevention and Control,and CHENG Y of Hongzhe County Disease Control and Prevention,FAN W,LIU L,HOU HY,SU Q,SHI YJ,and LI JY of Huai’an Disease Control and Prevention for their help. We also acknowledge the authors who submitted the sequences used in this study to GeneBank and GISAID.
| 1. | Gao R, Cao B, Hu Y, et al. Human infection with a novel avian-origin influenza A (H7N9) virus[J]. N Engl J Med , 2013, 368 :1888–97. doi:10.1056/NEJMoa1304459 |
| 2. | Li Q, Zhou L, Zhou M, et al. Epidemiology of human infections with avian influenza A(H7N9) virus in China[J]. N Engl J Med , 2014, 370 :520–32. doi:10.1056/NEJMoa1304617 |
| 3. | Wang XY, Chai CL, Li FD, et al. Epidemiology of human infections with avian influenza A(H7N9) virus in the two waves before and after October 2013 in Zhejiang province, China[J]. Epidemiol Infect , 2015, 143 :1833–38. doi:10.1017/S0950268814002866 |
| 4. | Kadota J. Emergence of a new strain of the avian influenza A (H7N9) virus[J]. Respir Investig , 2013, 51 :49. doi:10.1016/j.resinv.2013.05.001 |
| 5. | Wu P, Peng Z, Fang VJ, et al. Human Infection with Influenza A (H7N9) Virus during 3 Major Epidemic Waves, China, 2013-2015[J]. Emerg Infect Dis , 2016, 22 :964–72. doi:10.3201/eid2206.151752 |
| 6. | Liu D, Shi W, Shi Y, et al. Origin and diversity of novel avian influenza A H7N9 viruses causing human infection:phylogenetic, structural, and coalescent analyses[J]. Lancet , 2013, 381 :1926–32. doi:10.1016/S0140-6736(13)60938-1 |
| 7. | Liu W, Fan H, Raghwani J, et al. Occurrence and reassortment of avian influenza A (H7N9) viruses derived from coinfected birds in China[J]. J Virol , 2014, 88 :13344–51. doi:10.1128/JVI.01777-14 |
| 8. | Wang D, Yang L, Gao R, et al. Genetic tuning of the novel avian influenza A(H7N9) virus during interspecies transmission,China, 2013[J]. Euro Surveill , 2014, 19 :25. |
| 9. | Wu Y, Bi Y, Vavricka CJ, et al. Characterization of two distinct neuraminidases from avian-origin human-infecting H7N9 influenza viruses[J]. Cell Res , 2013, 23 :1347–55. doi:10.1038/cr.2013.144 |
| 10. | Wang Y, Dai Z, Cheng H, et al. Towards a better understanding of the novel avian-origin H7N9 influenza A virus in China[J]. Sci Rep , 2013, 3 :2318.1–2318.10. |
| 11. | Kageyama T, Fujisaki S, Takashita E, et al. Genetic analysis of novel avian A(H7N9) influenza viruses isolated from patients in China, February to April 2013[J]. Euro Surveill , 2013, 18 :20453. |
| 12. | Peng J, Yang H, Jiang H, et al. The origin of novel avian influenza A (H7N9) and mutation dynamics for its human-to-human transmissible capacity[J]. PloS one , 2014, 9 :e93094. doi:10.1371/journal.pone.0093094 |
| 13. | Farooqui A, Leon AJ, Huang L, et al. Genetic diversity of the 2013-14 human isolates of influenza H7N9 in China[J]. BMC Infect Dis , 2015, 15 :1–8. doi:10.1186/s12879-014-0722-x |
| 14. | Chen F, Li J, Sun B, et al. Isolation and characteristic analysis of a novel strain H7N9 of avian influenza virus A from a patient with influenza-like symptoms in China[J]. Int J Infect Dis , 2015, 33 :130–1. doi:10.1016/j.ijid.2015.01.013 |
| 15. | Koichiro Tamura, Glen Stecher, Daniel Peterson, et al. EGA6:Molecular Evolutionary Genetics Analysis version 6.0[J]. Mol Biol Evo , 2013, 30 :2725–9. doi:10.1093/molbev/mst197 |
| 16. | Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences[J]. J Mol Evol , 1980, 16 :111–20. doi:10.1007/BF01731581 |
| 17. | Drummond AJ, Rambaut A. BEAST:Bayesian evolutionary analysis by sampling trees[J]. BMC Evol Biol , 2007, 7 :214. doi:10.1186/1471-2148-7-214 |
| 18. | Qi L, Lu L, Sun Z, et al. Genomic signature and protein sequence analysis of a novel influenza A(H7N9) virus that causes an outbreak in humans in China[J]. Microbes Infect , 2013, 15 :432–9. doi:10.1016/j.micinf.2013.04.004 |
| 19. | Zhang Q, Shi J, Deng G, et al. H7N9 influenza viruses are transmissible in ferrets by respiratory droplet[J]. Science , 2013, 341 :410–4. doi:10.1126/science.1240532 |
| 20. | Poovorawan Y. Epidemic of avian influenza A (H7N9) virus in China[J]. Pathog Glob Health , 2014, 108 :169–70. doi:10.1179/2047772414Z.000000000206 |
| 21. | Erdem H, Unal S. New global viral threats[J]. Saudi Med J , 2015, 36 :393–8. doi:10.15537/smj.2015.4.10089 |
| 22. | Arunachalam R. Adaptive evolution of a novel avian-origin influenza A/H7N9 virus[J]. Genomics , 2014, 104 :545–53. doi:10.1016/j.ygeno.2014.10.012 |
| 23. | Husain M. Avian influenza A (H7N9) virus infection in humans:epidemiology, evolution, and pathogenesis[J]. Infect Genet Evol , 2014, 28 :304–12. doi:10.1016/j.meegid.2014.10.016 |
| 24. | Zhu W, Shu Y. Genetic tuning of avian influenza A (H7N9) virus promotes viral fitness within different species[J]. Microbes Infect , 2015, 17 :118–22. doi:10.1016/j.micinf.2014.11.010 |
| 25. | Wu P, Jiang H, Wu JT, et al. Poultry market closures and human infection with influenza A(H7N9) virus, China, 2013-14[J]. Emerg Infect Dis , 2014, 20 :1891–4. doi:10.3201/eid2011.140556 |
| 26. | Jones JC, Sonnberg S, Webby RJ, et al. Influenza A(H7N9) Virus Transmission between Finches and Poultry[J]. Emerg Infect Dis , 2015, 21 :619–28. doi:10.3201/eid2104.141703 |
| 27. | Webster RG, Bean WJ, Gorman OT, et al. Evolution and ecology of influenza A viruses[J]. Microbiol Rev , 1992, 56 :152–79. |
| 28. | Pu J, Wang S, Yin Y, et al. Evolution of the H9N2 influenza genotype that facilitated the genesis of the novel H7N9 virus[J]. Proc Natl Acad Sci U S A , 2015, 112 :548–53. doi:10.1073/pnas.1422456112 |
| 29. | Liu J, Xiao H, Wu Y, et al. H7N9:a low pathogenic avian influenza A virus infecting humans[J]. Curr Opin Virol , 2014, 5 :91–7. doi:10.1016/j.coviro.2014.03.001 |
 2016, Vol. 29
2016, Vol. 29


