2. School of Basic Medicine, Shenzhen University Health Sciences Center, Shenzhen 518060, Guangdong, China ;
3. Department of Epidemiology and Health Statistics, College of Public Health, Zhengzhou University, Zhengzhou 450003, Henan, China
Objective This meta-analysis was performed to summarize the association of the ADIPOQ rs2241766 and rs266729 polymorphisms with metabolic syndrome (MS) in the Chinese population.
Methods We searched for articles in MEDLINE via PubMed, EMBASE, HuGE Navigator, CNKI, and Wanfang databases and calculated odds ratios (ORs) with 95% confidence intervals (CIs) to determine the strength of associations in fixed-or random-effects models.
Results We included 21 articles in the meta-analysis:17 reports of ADIPOQ rs2241766 with 3628 cases and 3000 controls and 8 of rs266729 with 2021 cases and 2226 controls. We found an increased risk of MS with the ADIPOQ rs2241766 polymorphism in some genetic models (allele model:OR=1.12, 95% CI:1.03-1.21; dominant model:OR=1.15, 95% CI:1.04-1.28; homozygote model:OR=1.22, 95% CI:1.00-1.49) but no association with the ADIPOQ rs266729 polymorphism (allele model:OR=0.98, 95% CI:0.82-1.17; dominant model:OR=0.90, 95% CI:0.79-1.02; recessive model:OR=1.09, 95% CI:0.85-1.39; homozygote model:OR=1.03, 95% CI:0.80-1.33).
Conclusion The results of this meta-analysis suggest an association between the ADIPOQ rs2241766 polymorphism and MS in the Chinese population. G allele of ADIPOQ rs2241766 increases the risk of MS. Better designed studies with different ethnic populations and larger sample sizes are needed for assessing the relationship between ADIPOQ rs2241766 and rs266729 polymorphisms and MS in the future.
Metabolic syndrome (MS) is a cluster of physiological and biochemical abnormalities, including abdominal obesity, dyslipidemia, hypertension and hyperglycemia[1]. Insulin resistance and central obesity are the core features of MS[1]. The prevalence of MS is increasing and is a public health problem worldwide. A previous study indicated that the adjusted prevalence of MS in middle-aged Chinese people in 2004-2005 had significantly increased since 1998 (10.0% vs. 7.2%, P < 0.05)[2]. Data from the International Diabetes Federation (IDF) in 2005 showed that the prevalence of MS ranged from 10% to 50% among different populations with respect to ethnicity, nationality, sex, and age[3].
Previous studies suggested an association between hereditary factors and MS[4-5]. A large number of studies have reported MS-related candidate genes[6]. The adiponectin gene (ADIPOQ), located on chromosome 3q27, has been evaluated as a susceptibility locus for MS and its components[7-8]. Studies of a T/G mutation at position 45 in exon 2 and a C/G mutation at position-11377 in the promoter region in ADIPOQ were inconsistent. The +45T/G (rs2241766) sites were found closely related to obesity[9], type 2 diabetes[10], and MS[11]. However, other studies also found no significant association between rs2241766 polymorphism and obesity[12], MS[13], or hypertension[14]. A family-based study demonstrated that the ADIPOQ promoter variant rs266729 was associated with obesity and its traits in an Arabic population[15]. Other studies consistently revealed that the G allele of rs266729 might be a risk factor for type 2 diabetes[16-17]. However, Li et al.[11] found no significant association between the rs266729 polymorphism and MS.
Therefore, we conducted a meta-analysis to summarize the association between the ADIPOQ rs2241766 and rs266729 polymorphisms and MS in the Chinese population.
MATERIALS AND METHODS Study SelectionWe performed a systematic search of MEDLINE via PubMed, EMBASE, HuGE Navigator, CNKI, and Wanfang databases for articles evaluating the association between ADIPOQ polymorphisms and MS in the Chinese population published in English or Chinese. Moreover, we searched the reference lists and contacted the corresponding authors of all identified relevant publications. The search involved the following keyword strings:‘adiponectin’, ‘ADIPOQ’, ‘adiponectin gene polymorphism’, ‘AMP1’, ‘metabolic syndrome’, ‘MS’, ‘metabolic syndrome X’, ‘syndrome X’, ‘genetic variability’, ‘allele’, ‘SNP’, ‘polymorphism’, and‘Chinese’. All documents were updated to July 28, 2015.
Articles were included if they were a case-control or cross-sectional study published as an original study evaluating the association of the ADIPOQ rs2241766 and rs266729 polymorphisms with MS, if the outcome was MS defined by standard criteria, if the article provided sufficient data for evaluating odds ratios (ORs) with their 95% confidence intervals (95% CIs) directly or indirectly, and if control participants satisfied the Hardy-Weinberg equilibrium (HWE). The major exclusion criteria were articles in which genotype frequencies or alleles could not be ascertained, reviews or abstracts and studies which not studying on ADIPOQ polymorphisms or MS.
Data ExtractionStudy selection and data extraction were conducted by two researchers (Zhou JM and Zhang M) independently. Data extracted included the author’s last name, year of publication, ethnicity of subjects, study type, definition of MS, number of cases and controls, genotype frequencies in both groups and genotyping method, and the P value for HWE in control participants. Any disagreements were resolved by discussion or by consulting a third investigator (Hu DS).
Data AnalysisThe association between ADIPOQ polymorphism and MS was estimated by ORs and 95% CIs in allele, dominant, recessive, and homozygote genetic models using Stata version 12.0 (STATA Corp., College Station, Texas, USA). A Z test was used to evaluate the statistical significance of the pooled ORs, with P < 0.05 considered statistically significant. We used the inconsistency index I2 to assess the heterogeneity of studies. Substantial heterogeneity was considered when Phet < 0.05 or I2 > 50%, and the random-effects model was applied for assessing the pooled ORs and 95% CIs. The fixed-effects model was used in case of no substantial heterogeneity. We performed subgroup analyses by ethnicity; population region, divided as south and north by the Yangtze River; and criteria of MS defined by the Chinese Diabetes Society (CDS), IDF, Adult Treatment Panel Ⅲ of the National Cholesterol Education Program (NCEP ATP Ⅲ), World Health Organization (WHO), or De Ferranti et al.[18]. Sensitivity analysis, conducted by removing one study at a time and calculating the pooled ORs for the remaining studies, was performed to assess the stability of the results. We used Egger’s test (reported as t and P values) to estimate publication bias. P < 0.05 was considered statistically significant.
Quality Score Assessment for the Included StudiesThe quality of the included studies was assessed using the Ottawa-Newcastle system (NOS)[19]. The NOS uses a star rating system to judge quality based on three broad perspectives: selection of study groups, comparability of categories, and ascertainment of exposure. A maximum of one star is given for each numbered item within the selection and exposure categories. A maximum of two stars is given for comparability. Studies with a total score≥7 were considered high quality.
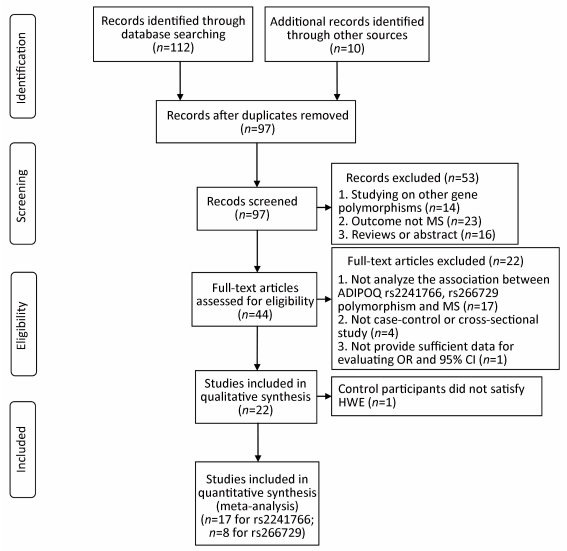
RESULTS Characteristics of StudiesOur literature search yielded 112 articles identified by searching databases and an additional 10 articles identified by searching the reference lists and contacting the corresponding authors of all identified related publications (Figure 1). We preliminarily included 22 articles[13, 20-40] and tested genotype frequencies for HWE in control participants to confirm eligibility. For studies evaluating the rs2241766 polymorphism and MS, withα=0.003 (0.05/18≈0.003) used to measure HWE, controls deviated from HWE in one paper[40]. We included 17 publications for the association between rs2241766 polymorphism and MS (Table 1, Figure 1). For studies evaluating the association between rs266729 polymorphism and MS, withα=0.006 (0.05/8≈0.006) used to test HWE, all control groups satisfied HWE. Finally, we included only 8 articles on the association between rs266729 polymorphism and MS (Table 1, Figure 1). Four articles described both polymorphisms. Most studies had a case-control design. The median quality score for included studies was 6.95 (range 6-8); 71.4% were considered a relatively high quality.
|
Download:
|
| Figure 1 Flow diagram of included studies. | |
|
|
Table 1 Characteristics of the Studies Included in the Meta-analysis |
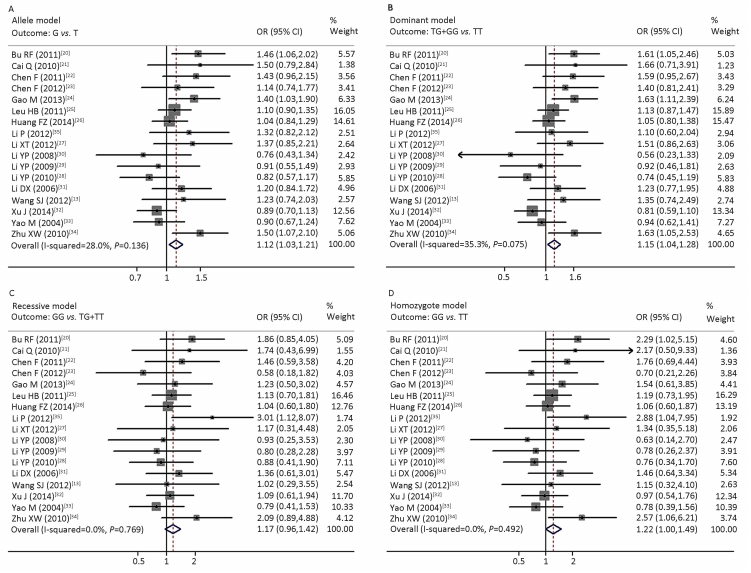
For the association between rs2241766 and MS, 3628 cases and 3000 controls were identified. Figure 2 shows the forest plots of MS risk associated with the ADIPOQ rs2241766 polymorphism in the allele (A), dominant (B), recessive (C), and homozygote (D) models. The fixed-effects model generated a combined allelic OR of 1.12 (95% CI: 1.03-1.21, P=0.006) for the G allele, 1.15 (95% CI: 1.04-1.28, P=0.008) for the TG+GG genotype in the dominant model, 1.17 (95% CI: 0.96-1.42, P=0.114) for the GG genotype in the recessive model, and 1.22 (95% CI: 1.00-1.49, P=0.047) for the GG genotype in the homozygote model (Figure 2, Table 2), for a significant association between the ADIPOQ rs2241766 polymorphism and overall risk of MS except in the recessive model.
|
Download:
|
| Figure 2 Forest plots of the risk of MS associated with the ADIPOQ rs2241766 polymorphism in the allele (A), dominant (B), recessive (C), and homozygote (D) models. The squares and horizontal lines correspond to the study-specific odds ratios (ORs) and 95% confidence intervals (CIs). The area of each square reflects the study-specific weight. The diamonds represent the combined ORs with its 95% CIs. | |
|
|
Table 2 Subgroup Analyses of Associations between the ADIOPQ Polymorphisms and MS by Genetic Model |
Regarding ethnicity, MS risk was increased with the rs2241766 polymorphism in three of the genetic models, the allele model (OR=1.18, 95% CI: 1.05-1.34), dominant model (OR=1.22, 95% CI: 1.04-1.43), and homozygote model (OR=1.40, 95% CI: 1.05-1.88) in all Chinese (not just Han Chinese) but not in Han Chinese alone. MS risk was increased with the rs2241766 polymorphism in the allele model in both the north (OR=1.17, 95% CI: 1.04-1.31) and south (OR=1.13, 95% CI: 1.00-1.29) regions, but in the dominant model only in the north (OR=1.24, 95% CI: 1.07-1.44). Regarding MS definition, MS risk was increased only with the CDS diagnostic criteria in the allele (OR=1.40, 95% CI: 1.18-1.66), and dominant (OR=1.56, 95% CI: 1.25-1.94) models (Table 2).
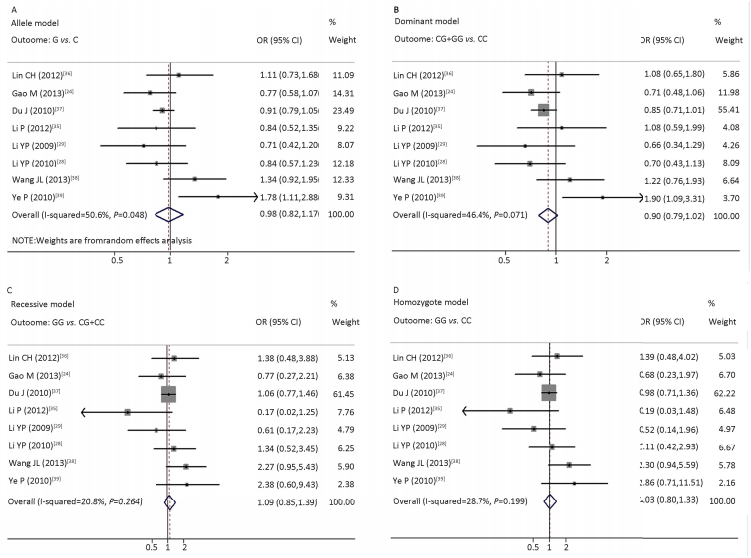
Association between rs266729 and MSIn total, 2021 MS patients and 2226 controls were analyzed for the association between rs266729 and MS. Figure 3 depicts forest plots of MS risk with ADIPOQ rs266729 polymorphism in the allele (A), dominant (B), recessive (C), and homozygote (D) models. The allele model presented significant heterogeneity (I2=50.6%, Phet=0.048). Thus, the random-effects model was used to generate a combined allelic OR of 0.98 (95% CI: 0.82-1.17, P=0.802) for the G allele. We obtained a combined OR of 0.90 (95% CI: 0.79-1.02, P=0.104) for the CG+GG genotype in the dominant model, 1.09 (95% CI: 0.85-1.39, P=0.508) for the GG genotype in the recessive model, and 1.03 (95% CI: 0.80-1.33, P=0.816) for the GG genotype in the homozygote model using the fixed-effects model (Figure 3; Table 2). All these genetic models displayed no association between rs266729 polymorphism and MS in the overall analyses.
|
Download:
|
| Figure 3 Forest plots of risk of MS associated with ADIPOQ rs266729 polymorphism in allele (A), dominant (B), recessive (C), homozygote (D) models. The squares and horizontal lines correspond to the study-specific ORs and 95% CIs. The area of each square reflects the study-specific weight. The diamonds represent the combined ORs with its 95% CIs. | |
Regarding the MS definition, MS risk was increased with the CDS, NCEP ATP Ⅲ, and De Ferranti definitions, but not with the IDF definition, in the allele (OR=1.35, 95% CI: 1.06-1.72), recessive (OR=1.94, 95% CI: 1.07-3.54), and homozygote (OR=2.04, 95% CI: 1.11-3.75) models. MS risk was not associated with the rs266729 polymorphism according to ethnicity or region in any model (Table 2).
Heterogeneity AnalysisFor the rs2241766 polymorphism, we found no significant heterogeneity among studies in the overall analyses (allele model: I2=28.0%, Phet=0.136; dominant model: I2=35.3%, Phet=0.075; recessive model: I2=0.0%, Phet=0.769; and homozygote model: I2=0.0%, Phet=0.492) (Table 2). For the rs266729 polymorphism, the allele model showed substantial heterogeneity among studies (I2=50.6%, Phet=0.048), with no evidence of heterogeneity for other models (dominant model: I2=46.4%, Phet=0.071; recessive model: I2=20.8%, Phet=0.264; and homozygote model: I2=28.7%, Phet=0.199) (Table 2).
Sensitivity Analysis and Publication BiasThe sensitivity analysis showed that no individual study affected the overall conclusions. Therefore, our meta-analysis results were stable and reliable. Moreover, we found no publication bias according to the Egger’s test (Table 3).
|
|
Table 3 Results of Egger’s Test for Publication Bias |
In this meta-analysis of the association of ADIPOQ rs2241766 and rs266729 with MS in the Chinese population, MS risk was increased with the rs2241766 polymorphism in the allele, dominant and homozygote models, but the rs266729 polymorphism was not associated with MS in any model. This meta-analysis suggested an association between the ADIPOQ rs2241766 polymorphism and MS in the Chinese population. G allele of ADIPOQ rs2241766 increased the risk of MS.
Adiponectin, a specific protein produced by fat cells, functions as a collagen-like protein hormone with anti-diabetic, anti-inflammatory, anti-atherosclerotic[11] and insulin-sensitizing properties[41]. A meta-analysis of genome-wide association analyses indicated that the ADIPOQ was the only major gene for plasma adiponectin, which explained 6.7% of the phenotypic variance[42]. A previous study showed that synonymous mutation of ADIPOQ locus may affect steady-state mRNA levels by altering RNA splicing or stability[43], suggesting an allele-specific differential expression of adiponectin. Alterations of plasma adiponectin levels due to such differential expression of adiponectin have been linked to MS and its components[44]. A variant of ADIPOQ rs2241766 G/T was reported to be related to low serum levels of adiponectin[45-47]. The ADIPOQ exon 45 G allele point mutation was discovered as a risk factor for type 2 diabetes mellitus in both Chinese[48] and Japanese populations[49]. The ADIPOQ rs266729 polymorphism has been shown to affect circulating adiponectin concentrations and significantly increase the risk of MS among people in Thailand[50] and Croatia[51].
In the current study, we found the rs2241766 variant was significantly associated with MS, which is consistent with another systematic meta-analysis including 2684 cases and 2864 controls in a Chinese population[24]. In contrast, no relationship was found between SNP+45T > G and MS among Asian Indians[52] or Malaysians[53]. We found the rs2241766 polymorphism associated with MS in all Chinese (not just Han Chinese) but not in Han Chinese alone. The inconsistent results among the publications might be due to the different genetic backgrounds of the study populations. The strength of the association between the rs2241766 polymorphism and MS differed by region in China. One reason could be that the link between genetic variation and disease can be modified by different dietary patterns[54-55]. Populations from different geographical regions might have different lifestyles and dietary patterns, which might influence the genetic variant affecting MS. In addition, Yao et al.[56] found that the genetic backgrounds of populations from the south and north of China differed, which might also affect the role of the gene variants in MS.
Our meta-analysis of 2021 cases and 2226 controls showed that rs266729 was not associated with MS in all genetic models in the overall analysis. However, another meta-analysis conducted by Li et al.[57] stated that rs266729 was significantly associated with type 2 diabetes mellitus in the Chinese population. The reason for the inconsistent results of above-mentioned two studies was that MS and type 2 diabetes mellitus are different metabolic status[58] and they have different diagnostic criteria although a highly proportion of type 2 diabetes mellitus patients could have MS[59]. Some studies demonstrated that the genetic variation associated with type 2 diabetes mellitus may not be related to MS[59-60], and it seems that the genetic variation associated with MS might be related to type 2 diabetes mellitus with higher possibility[61]. Subgroup analysis by definition of MS demonstrated that rs266729 could contribute to MS risk using definitions other than the IDF definition, so the definition of MS could affect the results of an analysis of a relationship between the rs266729 polymorphism and MS. MS does not have an optimal definition, and different criteria may lead to discordant results[62-63]. Each set of diagnostic criteria of MS has its own characteristics, and the IDF criteria are more conservative than other criteria for diagnosing MS, which leads to inconsistent results[64]. We could have performed subgroup analysis based on sample size, but the total sample size of the five included studies in our study was < 400[28-29, 36, 38-39], which limited the quality of stratified statistical results.
Our study contains some limitations. First, we did not analyze the effects of gene-gene and gene-environment interactions because of a lack of relevant information, although MS has been reported to result from the interactions of genetic factors and environmental factors such as dietary factors[65-66]. Second, our study was limited to papers published in English and Chinese. Third, our analyses were based primarily on unadjusted effect estimates without controlling for confounding factors because of a lack of original data.
In conclusion, this meta-analysis showed that the ADIPOQ rs2241766 polymorphism was associated with a risk of MS in the Chinese population but failed to reveal any association for the ADIPOQ rs266729 polymorphism in the overall analysis. Further investigations including additional original research with different ethnic populations should be conducted.
| 1. | Kassi E, Pervanidou P, Kaltsas G, et al. Metabolic syndrome:definitions and controversies[J]. BMC Med , 2011, 9 :1–13. doi:10.1186/1741-7015-9-1 |
| 2. | Wang ZW, Wang X, Li X, et al. Prevalence and trend of metabolic syndrome in middle-aged Chinese population[J]. Chin J Epdemiol , 2009, 30 :596–600. |
| 3. | Zheng LP. Progress of research relationship between adiponectin and metabolic syndrome[J]. Mod Diagn Treat , 2013, 24 :321–3. |
| 4. | Henneman P, Aulchenko YS, Frants RR, et al. Prevalence and heritability of the metabolic syndrome and its individual components in a Dutch isolate:the Erasmus Rucphen Family study[J]. J Med Genet , 2008, 45 :572–7. doi:10.1136/jmg.2008.058388 |
| 5. | Bellia A, Giardina E, Lauro D, et al. "The Linosa Study":epidemiological and heritability data of the metabolic syndrome in a Caucasian genetic isolate[J]. Nutr Metab Cardiovasc Dis , 2009, 19 :455–61. doi:10.1016/j.numecd.2008.11.002 |
| 6. | Povel CM, Boer JMA, Reiling E, et al. Genetic variants and the metabolic syndrome:a systematic review[J]. Obes Rev , 2011, 12 :952–67. doi:10.1111/obr.2011.12.issue-11 |
| 7. | Kissebah AH, Sonnenberg GE, Myklebust J, et al. Quantitative trait loci on chromosomes 3 and 17 influence phenotypes of the metabolic syndrome[J]. Pros Natl Acad Sci USA , 2000, 97 :14478–83. doi:10.1073/pnas.97.26.14478 |
| 8. | Ling H, Waterworth DM, Stirnadel HA, et al. Genome-wide linkage and association analyses to identify genes influencing adiponectin levels:the GEMS Study[J]. Obesity (Silver Spring) , 2009, 17 :737–44. doi:10.1038/oby.2008.625 |
| 9. | Wu JJ, Liu Z, Meng K, et al. Association of Adiponectin Gene (ADIPOQ) rs2241766 Polymorphism with Obesity in Adults:A Meta-Analysis[J]. PLoS One , 2014, 9 :e95270. doi:10.1371/journal.pone.0095270 |
| 10. | Motawi T, Salman T, Shaker O, et al. Association of polymorphism in adiponectin (+45 T/G) and leptin (-2548 G/A) genes with type 2 diabetes mellitus in male Egyptians[J]. Arch Med Sci , 2015, 11 :937–44. |
| 11. | Li P, Jiang R, Li L, et al. Correlation of serum adiponectin and adiponectin gene polymorphism with metabolic syndrome in Chinese adolescents[J]. Eur J Clin Nutr , 2015, 69 :62–7. doi:10.1038/ejcn.2014.152 |
| 12. | Lu JF, Zhou Y, Huang GH, et al. Association of ADIPOQ polymorphisms with obesity risk:a meta-analysis[J]. Hum Immunol , 2014, 75 :1062–8. doi:10.1016/j.humimm.2014.09.004 |
| 13. | Wang SJ, Jia WP, Bao YQ, et al. Association of adiponectin gene polymorphism with metabolic syndrome[J]. Joural of Chinese Practical Diagnosis and Therapy , 2012, 26 :659–61. |
| 14. | Zhang H, Mo XB, Hao YC, et al. Association of polymorphisms in the adiponectin gene with stroke and hypertension:a meta-analysis[J]. Molecular Cardiology of China , 2012, 12 :136–9. |
| 15. | Zadjali F, Al-Yahyaee S, Hassan MO, et al. Association of adiponectin promoter variants with traits and clusters of metabolic syndrome in Arabs:family-based study[J]. Gene , 2013, 527 :663–9. doi:10.1016/j.gene.2013.06.057 |
| 16. | Gu FH, Abulaiti A, Ostenson CG, et al. Single Nucleotide Polymorphisms in the Proximal Promoter Region of the Adiponectin (APM1) Gene Are Associated With Type 2 Diabetes in Swedish Caucasians[J]. Diabetes , 2003, 53 :S31–S5. |
| 17. | Kaftan AN, Hussain MK. Association of adiponectin gene polymorphism rs266729 with type two diabetes mellitus in Iraqi population.A pilot study[J]. Gene , 2015, 570 :95–9. doi:10.1016/j.gene.2015.06.004 |
| 18. | de Ferranti SD, Gauvreau K, Ludwig DS, et al. Prevalence of the metabolic syndrome in American adolescents:findings from the Third National Health and Nutrition Examination Survey[J]. Circulation , 2004, 110 :2494–7. doi:10.1161/01.CIR.0000145117.40114.C7 |
| 19. | Wells G, Shea B, O'Connell D, et al.The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyse.http://wwwohrica/programs/clinical_epide-miology/oxfordasp |
| 20. | Bu RF, Wu WJ, Hua JL, et al. The association of adiponectin gene polymorphism and serum adiponectin level with metabolic syndrome in older[J]. Chin J Gerontology , 2011, 31 :4112–4. |
| 21. | Cai Q, Liu HJ, Chen T, et al. Mononucleotide polymorphism at+45 and+276 site of adiponectin gene in aircrew with metabolic syndrome[J]. Clin J Med Officers , 2010, 38 :813–5. |
| 22. | Chen F, Su Y, Li XX, et al. Relationship of the adiponectin level and gene polymorphism with the metabolic syndrome[J]. J Clin Cardiol (China) , 2011, 27 :845–8. |
| 23. | Chen F, Wu HL, Yang QL, et al. Study on the correlation of adiponectin gene polymorphism and coronary heart disease in the patients with metabolic syndrome in Han nationlity population in Ningxia[J]. Clin J Crit Care Med , 2012, 32 :24–9. |
| 24. | Gao M, Ding DX, Huang JH, et al. Association of Genetic Variants in the Adiponectin Gene with Metabolic Syndrome:A Case-Control Study and a Systematic Meta-Analysis in the Chinese Population[J]. PLoS One , 2013, 8 :e58412. doi:10.1371/journal.pone.0058412 |
| 25. | Leu HB, Chung CM, Lin SJ, et al. Adiponectin Gene Polymorphism Is Selectively Associated with the Concomitant Presence of Metabolic Syndrome and Essential Hypertension[J]. PLoS One , 2011, 6 :e19999. doi:10.1371/journal.pone.0019999 |
| 26. | Huang FZ, Liu J, Pang D, et al.The association of adiponectin gene polymorphism with metabolic syndrome and plasma adiponectin concentration.Chinese Journal of Gerontology, 2014;34.(In Chinese) |
| 27. | Li XT, Wei DY, He HJ, et al. Association of the adiponectin gene (ADIPOQ)+45T>G polymorphism with the metabolic syndrome among Han Chinese in Sichuan province of China[J]. Asia Pac J Clin Nutr , 2012, 21 :296–301. |
| 28. | Li YP, Yang M, Xiong YX, et al. Adiponectin gene SNP-11377 and SNP-4522 haplotypes are related to metabolic syndrome[J]. Basic Clin Med , 2010, 30 :1288–92. |
| 29. | Li YP, Zhang Y, Li XL, et al. The association of adiponectin gene SNP+45, SNP+276 and SNP-11377 with metabolic syndrome[J]. Clin Focus , 2009, 24 :786–8. |
| 30. | Li YP, Zhang Y, Li XL, et al. Assocoation study of adiponectin gene polymorphisms with metabolic syndrome[J]. Journal Kunming Medical University , 2008, 29 :127–30. |
| 31. | Liu DX, Hua Q, Guo JC, et al. Difference of adiponectin gene exon 2 Glyl5Gly genotype and allele frequency between patients with metabolic syndrome and normal persons[J]. Chin J Clin Rehabilitation , 2006, 10 :4–6. |
| 32. | Xu J, Zhuo Q, Tian Y, et al. Association of adiponectin gene polymorphism with metabolic syndrome in older Han adults from major cities in China[J]. Journal of Hygiene Research , 2013, 42 :353–9. |
| 33. | Yao M.The relationship between adiponectin gene and metabolic syndrome.Chinese Doctor's Dissertations Full-Text Database, 2004.(In Chinese) |
| 34. | Zhu XW, Wu WJ, Bu RF, et al. Studies on adiponectin gene polymorphisms in patients with metabolic syndrome[J]. Shandong Med J , 2010, 50 :10–2. |
| 35. | Li P.Metabolic Syndrome And CardioVascular Disease Related Risk Factors in Urban Adolescents of Northeast China.Chinese Doctor's Dissertations Full-Text Database, 2012.(In Chinese) |
| 36. | Lin CH, Ho CY, Liu CS, et al. Influence of Adiponectin Gene Polymorphisms on Adiponectin Serum Level and Insulin Resistance Index in Taiwanese Metabolic Syndrome Patients[J]. Chin J Physiol , 2012, 55 :405–11. doi:10.4077/CJP.2012.BAA081 |
| 37. | Du J, Ye XH, Li Q, et al. Genetic Variants in the ADIPOQ Gene and the Risk of Metabolic Syndrome:A Case-Control Study of a Chinese Han population[J]. Ann Hum Genet , 2011, 76 :101–9. |
| 38. | Wang LJ, Yu J, Ye Y, et al. Metabolic syndrome and adiponectin gene polymorphism in Han population of northern Guangxi Zhuang Autonomous Region[J]. Chin J Geriatr Heart Brain Vessel Dis , 2013, 15 :1133–8. |
| 39. | Ye P.Association between Polymorphisms of PPARγProl2Ala, C1431T, ADIPOQ-11377C>G, Physical Activity, and Metabolic Syndrome in Children.Chinese Master's Dissertations Full-Text Database, 2013.(In Chinese) |
| 40. | Shen J, Wu HL, Liu YJ, et al. Relation of metabolic syndrome with plasma adiponectin level and gene polymorphism in Ningxia Hui subjects[J]. Chin J Geriatr Heart Brain Vseeel Dis , 2013, 15 :7–10. |
| 41. | Kadowaki T, Yamauchi T, Kubota N, et al. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome[J]. J of Clin Invest , 2006, 116 :1784–92. doi:10.1172/JCI29126 |
| 42. | Heid IM, Henneman P, Hicks A, et al. Clear detection of ADIPOQ locus as the major gene for plasma adiponectin:results of genome-wide association analyses including 4659 European individuals[J]. Atherosclerosis , 2010, 208 :412–20. doi:10.1016/j.atherosclerosis.2009.11.035 |
| 43. | Yang WS, Tsou PL, Lee WJ, et al. Allele-specific differential expression of a common adiponectin gene polymorphism related to obesity[J]. J Mol Med (Berl) , 2003, 81 :428–34. doi:10.1007/s00109-002-0409-4 |
| 44. | Pollin TI, Tanner K, Shuldiner AR, et al. Linkage of Plasma Adiponectin Levels to 3q27 Explained by Association With Variation in the APM1 Gene[J]. Diabetes , 2005, 54 :268–74. doi:10.2337/diabetes.54.1.268 |
| 45. | Vasseur F, Helbecque N, Dina C, et al. Single-nucleotide polymorphism haplotypes in the both proximal promoter and exon 3 of the APM1 gene modulate adipocyte-secreted adiponectin hormone levels and contribute to the genetic risk for type 2 diabetes in French Caucasians[J]. Hum Mol Genet , 2002, 11 :2607–14. doi:10.1093/hmg/11.21.2607 |
| 46. | Berthier MT, Houde A, Cote M, et al. Impact of adiponectin gene polymorphisms on plasma lipoprotein and adiponectin concentrations of viscerally obese men[J]. J Lipid Res , 2005, 46 :237–44. |
| 47. | Guzman-Ornelas MO, Chavarria-Avila E, Munoz-Valle JF, et al. Association of ADIPOQ+45T>G polymorphism with body fat mass and blood levels of soluble adiponectin and inflammation markers in a Mexican-Mestizo population[J]. Diabetes Metab Syndr Obes , 2012 :369–78. doi:10.2147/DMSO |
| 48. | Li L, Liu ZH, Yu FX, et al.Correlation of the Adiponectin Gene+45 in Exon 2 Polymorphisms and Type 2 Diabetes Mellitus in Chinese Population:A Meta-Analysis.Chin J Evid-based Med 2012;12, 1235-40.(In Chinese) |
| 49. | Hara K, Boutin P, Mori Y, et al. Genetic Variation in the Gene Encoding Adiponectin Is Associated With an Increased Risk of Type 2 Diabetes in the Japanese Population[J]. Diabetes , 2002, 51 :536–40. doi:10.2337/diabetes.51.2.536 |
| 50. | Suriyaprom K, Phonrat B, Tungtrongchitr R. Association of adiponectin gene-11377C>G polymorphism with adiponectin levels and the metabolic syndrome in Thais[J]. Asia Pac J Clin Nutr , 2014, 23 :167–73. |
| 51. | Karmelic I, Lovric J, Bozina T, et al. Adiponectin level and gene variability are obesity and metabolic syndrome markers in a young population[J]. Arch Med Res , 2012, 43 :145–53. doi:10.1016/j.arcmed.2012.02.004 |
| 52. | Ranjith N, Pegoraro RJ, Shanmugam R. Obesity-associated genetic variants in young Asian Indians with the metabolic syndrome and myocardial infarction[J]. Cardiovasc J Afr , 2011, 22 :25–30. |
| 53. | Lau CH, Muniandy S. Adiponectin and Resistin Gene Polymorphisms in Association with Their Respective Adipokine Levels[J]. Ann of Hum Genet , 2011, 75 :370–82. doi:10.1111/ahg.2011.75.issue-3 |
| 54. | Goni L, Cuervo M, Milagro FI, et al. Gene-Gene Interplay and Gene-Diet Interactions Involving the MTNR1B rs10830963 Variant with Body Weight Loss[J]. J Nutrigenet Nutrigenomics , 2014, 7 :232–42. |
| 55. | Luis DAd, Aller R, Izaola O, et al. Effects of a High-Protein/Low-Carbohydrate Diet versus a Standard Hypocaloric Diet on Weight and Cardiovascular Risk Factors:Role of a Genetic Variation in the rs9939609 FTO Gene Variant[J]. J Nutrigenet Nutrigenomics , 2015, 8 :128–36. doi:10.1159/000441142 |
| 56. | Yao YG, Kong QP, Bandelt HJ, et al. Phylogeographic differentiation of mitochondrial DNA in Han Chinese[J]. Am J Hum Genet , 2002, 70 :635–51. doi:10.1086/338999 |
| 57. | Li YY, Yang ZJ, Zhou CW, et al. Adiponectin-11377CG gene polymorphism and type 2 diabetes mellitus in the Chinese population:a meta-analysis of 6425 subjects[J]. PLoS One , 2013, 8 :e61153. doi:10.1371/journal.pone.0061153 |
| 58. | Pei F, Sun J. New insight into improvement of cardiovascular outcomes with intensive glycemic control in patients with metabolic syndrome and type 2 diabetes mellitus included[J]. Cell Biochem Biophys , 2015, 71 :9–15. doi:10.1007/s12013-014-0175-2 |
| 59. | Nikzamir A, Nakhjavani M, Golmohamadi T, et al. Association of Angiotensin-Converting Enzyme Gene Insertion/Deletion Polymorphism with Metabolic Syndrome in Iranians with Type 2 Diabetes Mellitus[J]. Arch Iranian Med , 2008, 11 :3–9. |
| 60. | Peters KE, Beilby J, Cadby G, et al. A comprehensive investigation of variants in genes encoding adiponectin (ADIPOQ) and its receptors (ADIPOR1/R2), and their association with serum adiponectin, type 2 diabetes, insulin resistance and the metabolic syndrome[J]. BMC Med Genet , 2013, 14 :1–12. |
| 61. | Chen YL, Pei D, Hung YJ, et al. Associations between genetic variants and the severity of metabolic syndrome in subjects with type 2 diabetes[J]. Genet and Mol Res , 2015, 14 :2518–26. doi:10.4238/2015.March.30.10 |
| 62. | Pokharel DR, Khadka D, Sigdel M, et al. Prevalence of metabolic syndrome in Nepalese type 2 diabetic patients according to WHO, NCEP ATPⅢ, IDF and Harmonized criteria[J]. J of Diabetes Metab Disord , 2014, 13 :1–13. doi:10.1186/2251-6581-13-1 |
| 63. | Wang R, Peng NC, Shi LX, et al. Comparison of four different diagnostic criteria on metabolic syndrome in all epidemiological study in Guiyang city[J]. Chin J Diabetes Mellitus , 2014, 6 :606–10. |
| 64. | Ye PY, Yan YK, Ding WQ, et al. Prevalence of metabolic syndrome in Chinese children and adolescents:a Meta-analysis[J]. Chin J Epidemiol , 2015, 36 :884–8. |
| 65. | Edelenyi FSd, Goumidi L, Bertrais S, et al. Prediction of the metabolic syndrome status based on dietary and genetic parameters, using Random Forest[J]. Genes Nutr , 2008, 3 :173–6. doi:10.1007/s12263-008-0097-y |
| 66. | Ferguson JF, Phillips CM, Tierney AC, et al. Gene-nutrient interactions in the metabolic syndrome:single nucleotide polymorphisms in ADIPOQ and ADIPOR1 interact with plasma saturated fatty acids to modulate insulin resistance[J]. Am J Clin Nutr , 2010, 91 :794–801. doi:10.3945/ajcn.2009.28255 |
 2016, Vol. 29
2016, Vol. 29


