2. 中国科学院大学,北京 100049
2. University of Chinese Academy of Sciences, Beijing 100049
土壤盐渍化是荒漠化的形成原因之一[1],也是影响土壤功能的主要威胁[2]。盐渍化导致的土壤盐分积累会降低农作物产量、牧草品质与林木成活率[3],影响农牧民生计选择和土地利用策略[4-5],进而危及农业、牧业和林业的可持续发展。同时,盐分积累会加速植被与土壤退化[6],破坏生态系统的自我调节能力[7],危及地质、资源和国土安全[8-9],进而导致社会经济发展失衡。目前,全球受盐渍化影响的土地面积约1.0×109hm2[2],由此产生的经济损失每年高达2.73×1010美元[10],且目前盐渍化土地面积仍以每年1.5×107-2.0×107hm2的速度增长[3]。
土壤中任何形式盐分的过量积累都会对植物生长及其生理过程产生不利影响[2]。盐胁迫作为自然界中主要的非生物胁迫之一,对植物造成渗透胁迫、离子胁迫和氧化胁迫,阻碍植物对水分和养分的吸收,抑制光合作用的正常进行。这不仅干扰植物种子萌发、幼苗生长和繁殖等各生长阶段的正常生理代谢过程,而且严重影响植物在生态系统中的生产潜力和功能发挥[11]。因此,增强植物耐盐性、促进植物生长和提高盐渍化土地的改良利用,不仅是全球土地退化和干旱区可持续农业研究的重要内容,而且也是保证生态安全、稳定经济增长、实现耕地占补平衡和解决人口—资源—环境问题的必然选择[12]。微生物措施是目前盐渍化土地改良的研究热点[13-14],它利用盐渍生境中植物与有益微生物间的联合作用对土壤进行改良[15],是在尊重植物原有遗传背景的前提下提高植物生长潜力和诱导植物耐盐性[16],具有成本低、效率高、见效快且环保等优势[17-18]。
植物根际促生菌(Plant growth-promoting rhizo-bacteria,PGPR)是指植物根际土壤中具有抑制植物病害和促进植物生长特性的有益细菌[19],也是目前微生物改良措施中应用最为广泛的微生物。已有的大量独立试验研究通过分析不同耐盐指标的变化,证明了PGPR可以改变宿主植物生理代谢途径,以此缓解盐胁迫对宿主植物造成的危害。部分学者也对已有研究中所应用的有效PGPR菌种及其耐盐特性进行了分类与整理[2, 16, 20],为研发和应用微生物菌肥奠定了基础。然而,盐胁迫下PGPR对宿主植物种子萌发、形态结构、生物量和产量等的影响鲜有归纳总结,且PGPR缓解盐胁迫的可能调控路径及作用机制也值得进一步探讨与分析。基于此,本文系统总结了盐胁迫下PGPR对植物生长发育的影响,并探讨了PGPR提高植物耐盐性的相关机制,旨在为进一步筛选高效PGPR菌种及利用PGPR改良盐渍化土地提供理论支撑。
1 盐胁迫下PGPR对植物生长的影响盐分积累影响土壤的理化性质和生物学特性,进而对植物生长、发育及繁殖等过程产生不利影响[21-22]。PGPR接种有利于缓解盐胁迫对植物种子萌发、器官分化、生物量积累与分配以及作物产量与品质等方面造成的不利影响(图 1)。
1.1 种子萌发种子萌发阶段是植物生长周期中最基本和最重要的阶段,影响着植物后续的生长发育[24]。然而,盐分积累使土壤渗透势增加,阻碍种子对水分的吸收[25],干扰种子内部核酸和蛋白质代谢[26-27],影响内源激素合成[28],进而抑制种子萌发[24]。PGPR接种可以促进盐胁迫下种子萌发。例如,在75 mmol/L和100 mmol/L盐胁迫下,含ACC脱氨酶的巨大芽孢杆菌(Bacillus megaterium UPMR2)和肠杆菌(Enterobacter sp. UPMR18)附着于秋葵种子后,能够根据种子产生的氨基酸合成植物激素,进而促进种子萌发[25]。在11 dS/m和14 dS/m盐胁迫下,虽然油菜种子接种含ACC脱氨酶的荧光假单胞菌(Pseudomonas fluorescens)和恶臭假单胞菌(Pseudomonas putida)后发芽率显著升高,但耐盐品种种子的发芽率显著高于不耐盐品种,这可能是由于PGPR菌种与不同耐盐品种种子间的交互作用存在差异所致[29]。在50 mmol/L、100 mmol/L和200 mmol/L盐胁迫下,鹰嘴豆接种可变盐单胞菌(Halomonas variabilis HT1)和产色素海洋细菌(Planococcus rifietoensis RT4)后种子发芽率升高,可能是由于PGPR产生的胞外多糖(Exopolysaccharides,EPS)在种子表面形成了生物被膜,有助于保持种子周围水分充足,为种子萌发创造了有利条件[30]。
1.2 形态结构盐胁迫下,植物细胞伸长率和分裂率降低[24],致使根、茎和叶的生理特征和形态结构发生变化,既不利于根系对水分和养分的吸收,也不利于叶对光能的截获和利用[31-32]。PGPR接种有利于改善根、茎和叶的生理特征和形态结构,提高植物对盐胁迫的适应能力[33](图 1)。例如,在0.2%、0.4%和0.6% NaCl胁迫下,白蜡接种可分泌生长素的阴沟肠杆菌(Enterobacter cloacae TIA062)后,根系活性和根区土壤微生态环境的改善为植物生长提供了良好的环境,根体积、根表面积、植株基茎和株高显著高于对照,且在0.6% NaCl胁迫下的接种效应更为显著[34]。在100 mmol/L盐胁迫下,大豆接种假单胞菌(Pseudomonas sp.)和芽孢杆菌(Bacillus sp.)后,细菌产生的ACC脱氨酶降低了乙烯(Ethylene,ETH)的合成,细菌分泌的生长素(Auxin,IAA)促进了根的发育和茎的生长,使株高、根长、侧根数和叶面积显著增加[35]。可见,盐胁迫下PGPR可以通过调节激素的合成与积累来优化植株形态结构。
1.3 生物量积累与再分配盐胁迫会降低植物的生长速率、抑制生物量的积累并改变生物量的分配模式[23]。盐胁迫条件下,PGPR接种可以提高植物生长速率,并促进不同实验条件中生物量的积累。例如,Meta分析结果表明,在盆栽实验条件中,盐生植物和甜土植物在盐胁迫条件下接种PGPR后根、茎和叶生物量积累均显著增加,且不耐盐植物的促生效应显著高于耐盐植物[18]。在培养液实验条件中,100 mmol/L盐胁迫下拟南芥接种解淀粉芽孢杆菌(Bacillus amyloliquefaciens FZB42)[36]和大豆接种假单胞菌(Pseudomonas sp.)和芽孢杆菌(Bacillus sp.)[35]后生物量积累都显著增加。同时,盐胁迫下PGPR还会诱导根汇效应,影响生物量再分配[14, 23]。例如,在250 mmol/L盐胁迫下,对金合欢接种枯草芽孢杆菌(Bacillus subtilis)后,根干重显著提高了118%,而地上部分干重则提高了40%,根的生物量积累效应显著优于地上部分[37]。
1.4 产量与品质盐胁迫不仅影响植物的生殖生理过程,降低作物产量[2],而且抑制植物对营养物质的吸收,影响作物品质[38]。PGPR接种可以提高盐胁迫下作物的产量与品质,且PGPR引起的增产效应因盐胁迫条件、菌种属性和宿主植物的不同而存在差异。例如,在100 mmol/L和300 mmol/L盐胁迫下,薄荷接种期望盐单胞菌(Halomonas desiderata)和微小杆菌(Exiguobacterium oxidotolerans)后产量和含油量均显著提高[7]。在100 mmol/L、300 mmol/L和500 mmol/L盐胁迫下,山香接种类产碱假单胞菌(Pseudomonas pseudoalcaligenes)和短小芽孢杆菌(Bacillus pumilus)后虽然产量和含油量降低,但由于叶面积和生物量的积累,植株总产油量增加[38]。在4 dS/m、8 dS/m和12 dS/m盐胁迫下,玉米接种含ACC脱氨酶的PGPR菌株后产量显著提高,且在12dS/m盐胁迫下产量提升幅度最大[39]。在盐水灌溉条件下,草莓接种萎缩芽孢杆菌(Bacillus atrophaeus EY6)、克氏葡萄球菌(Staphylococcus kloosii EY37)和考克氏菌(Kocuria erythromyxa EY43)后产量均显著提高,但不同菌株的接种效应存在差异[40]。
2 PGPR提高植物耐盐性相关机制盐胁迫对植物造成渗透胁迫、离子胁迫和氧化胁迫,阻碍植物对水分和养分的吸收,抑制光合作用[17]。PGPR可以诱导宿主植物系统耐性,通过增强渗透调节能力、提高水分和养分吸收水平,保持离子和活性氧平衡,促进光合作用与利用微生物间的对抗和协同关系等途径,提高植物对盐胁迫环境的适应能力(图 1)。
2.1 增强渗透调节,促进水分吸收盐分积累降低了土壤水的能量状态,影响根对土壤水分的吸收,对植物造成渗透胁迫[17]。盐胁迫下,PGPR通过调节宿主植物有机渗透溶质的合成、增强根系的吸水能力与改善根区水分状况等方式来缓解渗透胁迫对植物的伤害。
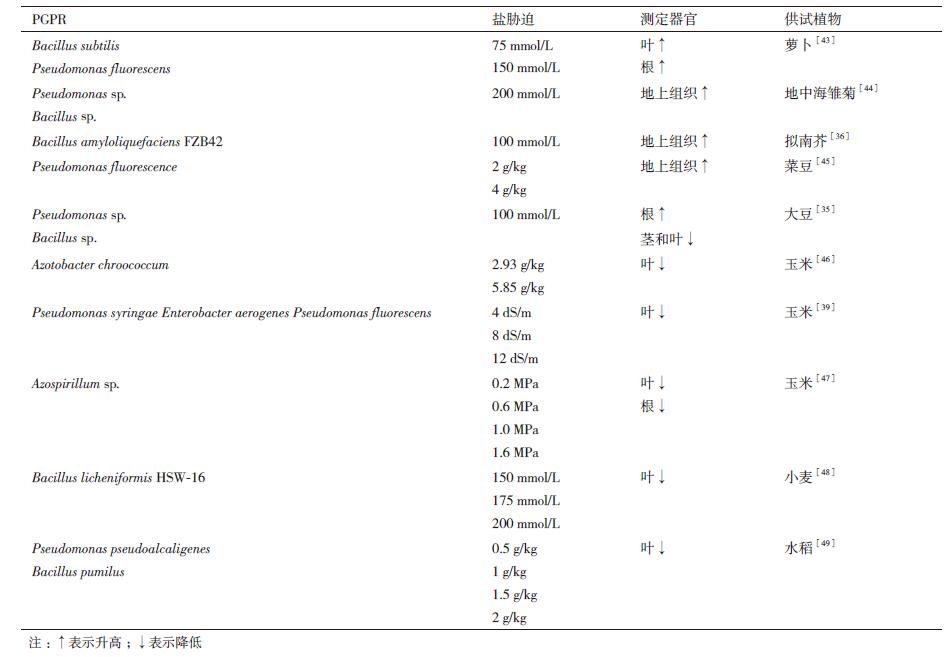
2.1.1 合成有机渗透溶质,提高渗透调节能力渗透胁迫下,植物体内有机渗透溶质(如脯氨酸、可溶性糖、可溶性蛋白和甜菜碱等)的合成有助于降低细胞渗透势,改善植株内生理干旱状况[17]。其中,脯氨酸是目前研究最为广泛的渗透调节物质[41-42]。一些研究者认为,脯氨酸的积累是植物在盐胁迫下进行渗透调节的重要指标。盐胁迫下PGPR接种显著促进了脯氨酸的合成与积累,有助于维持细胞水势[7],缓解渗透胁迫对植物造成的伤害[16]。植物接种PGPR后脯氨酸含量的增加可能是由于:(1)PGPR接种增强了宿主植物营养吸收水平,从而加快了脯氨酸的合成速率;(2)PGPR引起脯氨酸合成相关基因表达上调[35-36]。然而,另一些研究者则认为,脯氨酸的积累是盐胁迫对植物造成伤害的标志,脯氨酸含量的显著降低意味着PGPR接种缓解了渗透胁迫对植株造成的伤害[39]。可见,盐胁迫下不同植物接种PGPR后各器官中脯氨酸含量的变化各异(表 1)。关于PGPR对盐胁迫下植物脯氨酸含量的影响及脯氨酸对植物耐盐性的作用都是有待进一步研究的关键问题。
可溶性糖、可溶性蛋白和甜菜碱也是植物体内重要的有机渗透溶质和酶保护剂,其含量的积累有利于保持宿主植物体内的渗透平衡,缓解植物生理干旱[37, 42, 50]。盐胁迫下,PGPR合成渗透调节物质的速度高于其宿主植物,可以有效地促进宿主植物对渗透调节物质的吸收[23]。Meta分析结果表明,盐胁迫下,PGPR接种可促进耐盐植物和盐敏感植物叶中可溶性糖和可溶性蛋白的积累,且耐盐植物中可溶性糖的接种效应显著高于盐敏感植物[18]。在250 mmol/L盐胁迫下,金合欢接种枯草芽孢杆菌(Bacillus subtilis)后甜菜碱含量显著高于对照38.4%,有利于植物保持渗透平衡[37]。在0.5 g/kg、1 g/kg、1.5 g/kg、2 g/kg和2.5 g/kg盐胁迫下,水稻接种类产碱假单胞菌(Pseudomonas pseudoalcaligenes)和短小芽孢杆菌(Bacillus pumilus)后叶中类甜菜碱季铵化合物含量
均显著升高,且混合接种效应显著高于单独接种;类甜菜碱季铵化合物的积累可以保护植株亚细胞结构,提高渗透调节水平,改善叶中水分状况[49]。
2.1.2 调节根系生理与结构,增强水分吸收与运输能力根系形态结构和生理特征影响着植物对水分的吸收与运输[24]。盐胁迫下,PGPR接种能够改变根系结构和生理状态,提高根对水分的吸收和运输速率,进而缓解渗透胁迫[14]。PGPR在土壤中可以产生挥发性物质(Volatile Organic Compounds,VOCs)。当PGPR与根无物理接触时,通过释放VOCs促进根系生长和扩大根系吸收面积[51],以此缓解由盐分积累导致的渗透胁迫对植物造成的伤害。当PGPR与根有物理接触时,通过释放植物激素、诱导宿主植物体内激素合成的基因表达与加强植物激素间的协同作用来促进根系生长和改善根系形态,促进盐胁迫下植物对水分和养分的吸收利用,缓解植物生理干旱[52-54]。PGPR接种还可以通过改善根系生理状态来促进水分运输。例如,在2.56 dS/m盐胁迫下,玉米接种巨大芽孢杆菌(Bacillus megaterium)后根中水孔蛋白PIP水平和蛋白丰度的增加提高了根系水力导度[55],有利于根系对水分的运输和对细胞内渗透压的调节[56]。
2.1.3 改善根区土壤环境,调节根区水分状况盐分在土壤中的积累会破坏土壤结构,影响根际土壤水分微环境[57-58]。部分PGPR在维持其自身生长发育的过程中能够产生EPS[59]。EPS的吸湿性和胶体稳定性有利于生物被膜的形成和PGPR的定殖,进而增加土壤保水性能和团粒结构,提高植物根系周围水势,缓解由于土壤盐分积累对植株根系造成的渗透胁迫,为植物生长提供良好的水分微环境和抵御渗透胁迫的能力[30, 60-61]。因此,盐胁迫下,PGPR接种不仅有利于促进植物根系生长和提高根际微生物数量,而且可以改善盐渍土壤结构和性质,调节根区水分状况,促进根系对水分的吸收,间接缓解渗透胁迫对植物造成的伤害。
2.2 促进营养吸收,降低盐离子毒害盐分伴随着根系对水分的吸收进入植物体内,影响钠离子(Na+)与其它营养离子的代谢,降低植物对营养离子的吸收和运移,从而对植物造成离子毒害[17]。PGPR通过提高土壤养分可得性、促进根系营养吸收和调控Na+的吸收与运移等途径,重建宿主植物离子平衡状态[18]。
2.2.1 提高土壤供肥能力,增强土壤营养可得性盐胁迫下,部分PGPR可以分解土壤中难溶性矿物质,并将其转化为可被植物吸收利用的矿质元素形态,增加土壤中营养元素的可得性[41, 54, 62]。具有固氮作用的PGPR可以增加土壤中氮(N)含量并促进植物对N的吸收。例如,在-0.3 MPa盐胁迫下,玉米接种具有固氮能力的产脂固氮螺菌(Azospirillum lipoferum)后地上部分N产量显著提高[63]。具有溶磷作用的PGPR通过分泌有机酸来降低土壤pH值,将土壤中难溶性磷酸盐转变为可被植物吸收利用的形态。在9 dS/m盐胁迫下,绿豆接种具有溶磷作用的蜡状芽孢杆菌(Bacillus cereus Pb25)后,根际土壤中的微生物碳、脱氢酶、磷酸酶和有机磷的含量都显著增加,叶中磷(P)含量显著高于对照48%[54]。解钾细菌既可以溶出矿物中的元素用于自身生长,又可以释放出钾(K)元素以增加土壤中K的可利用性和促进植物对K的吸收[64]。已有结果表明,植物接种解钾细菌后叶中K浓度在低盐胁迫下提高了6%-14%,在高盐胁迫下提高了4%-8%[65]可见,部分PGPR可以发挥生物肥料的作用,提高土壤供肥能力,改善根区土壤养分状况。
2.2.2 调节根系生理和形态,促进营养吸收增强植物的营养吸收能力是提高植物耐盐性的关键[17]。根系生长状况和形态结构直接影响植物对营养物质的吸收、转化和合成[66]。PGPR可以通过改变根系生理状况、刺激根系发育和改善根系形态结构等途径,增强根对土壤营养元素的利用,缓解盐胁迫下植物体内营养匮缺[67-69]。例如,在250 mmol/L盐胁迫下,金合欢接种枯草芽孢杆菌(Bacillus subtilis)后根瘤数量、重量和豆血红蛋白含量的显著增加有助于增强根对N的固定,根中硝酸还原酶、亚硝酸还原酶和固氮酶活性的增强有利于增强根中N代谢能力[37],根中酸性和碱性磷酸酶含量的显著升高有助于有机磷化合物的水解,增强植物对P的吸收和转运[58]。在50 mmol/L和70 mmol/L NaCl胁迫下,大豆接种芽孢杆菌(Bacillus japonicum USDA110)和恶臭假单胞菌(Pseudomonas putida TSAU1)后,根长、根表面积、根直径和根体积等均显著增加,有利于促进大豆对N和P的吸收[67]。Meta分析结果表明,盐胁迫下植物接种PGPR后N、P、K、钙(Ca)和镁(Mg)等营养元素的显著增加主要得益于:(1)根系分泌物加速了根区养分循环;(2)根系结构的改善扩大了根系吸收范围[18]。养分吸收能力的增强既可以通过促进生长来稀释植株内盐离子浓度,又可通过提高K+/Na+比、Ca2+/Na+比和Mg2+/Na+比来重建植株离子平衡状态[24, 54]。此外,植物接种PGPR后根生物量、分泌物及根际微生物数量的增加还有助于促进好氧微生物的生长繁殖,增强根及根际微生物的呼吸作用,改善根区土壤通气状况[60, 70],加快有机质分解和土壤矿化,为根系吸收养分创造良好条件[71]。
2.2.3 产生EPS和VOCs,调控Na+的吸收与运移部分PGPR产生EPS和释放VOCs。EPS可以提高土壤阳离子交换量、土壤孔隙度、聚合度和持水力,增强根际土壤养分的可得性和可利用性,降低Na+对植物的伤害[16, 23]。例如,在100 mmol/L盐胁迫下,拟南芥接种产EPS的解淀粉芽孢杆菌(Bacillus amyloliquefaciens FZB42)后,EPS在根部形成的生物被膜结合土壤中的Na+,限制了根对Na+的吸收,从而降低了体内Na+含量[36]。VOCs作为一种信号传递的信号分子,参与并调节植物与微生物互作[72]。盐胁迫下,PGPR释放的VOCs可以通过调节高亲和性K+转运蛋白(High Affinity K Transport 1,HK1)来保持植株内Na+稳态。例如,在100 mmol/L盐胁迫下,拟南芥接种枯草芽孢杆菌(Bacillus subtilis GB03)后,VOCs通过下调根部HK1的表达限制Na+进入根系,通过上调地上部分基因表达降低植株中Na+含量和Na+循环,从而降低Na+对植物的伤害[16, 73]。
2.3 减少活性氧伤害,提高抗氧化能力渗透胁迫和离子胁迫相互作用会破坏植物体内活性氧(Reactive oxygen species,ROS)产生与清除间的动态平衡,引起膜脂过氧化,进而对植物造成氧化胁迫[17]。PGPR可通过减少盐胁迫初期ROS的产生与积累、提高抗氧化酶活性和增加非酶类抗氧化物等途径,提高植物抗氧化能力。
2.3.1 减少ROS的产生与积累减轻盐胁迫初期渗透胁迫和离子胁迫对植物的伤害,可相应地降低氧化胁迫对植物造成的伤害[74]。丙二醛(Malondialdehyde,MDA)是膜脂过氧化的产物,也是植物遭受氧化胁迫的标志[7],其含量越低表明植物的耐盐性越高[75]。盐胁迫下,PGPR能有效抑制植株内ROS的产生,降低MDA含量,提高植物耐盐性。例如,在5 dS/m和10 dS/m盐胁迫下,绿豆接种分散泛菌(Pantoea dispersa)和屎肠球菌(Enterococcus faecium)后Na+吸收受到抑制,这有利于减少叶中过氧化氢(Hydrogen peroxide,H2O2)的产生,从而降低叶中MDA的积累[76]。Meta分析结果显示,盐胁迫下耐盐植物和盐敏感植物接种PGPR后MDA含量分别显著降低了23.01%和23.96%,且MDA的减少与Na+吸收的减少呈正相关,表明植物接种PGPR后盐离子胁迫的缓解可以相应地减少盐胁迫初期ROS的产生与积累,降低由ROS导致的氧化胁迫带来的不利影响[18]。
2.3.2 提高抗氧化酶活性植物通过酶类和非酶类抗氧化系统对ROS进行调节,增强植物抗氧化能力[74, 77]。在酶类抗氧化系统中,超氧化物歧化酶(Superoxide dismutase,SOD)是植物应对氧化胁迫的“第一道防线”,它将超氧阴离子(Superoxide anion,O2-)转化为水和H2O2;过氧化物酶(Peroxidase,POD)、过氧化氢酶(Catalase,CAT)和抗坏血酸过氧化物酶(Ascorbate peroxidase,APX)都是H2O2清除酶;谷胱甘肽过氧化物酶(Glutathione peroxidase,GPX)和谷胱甘肽还原酶(Gutathione reductase,GR)等是重要的氧化还原调节酶[24, 78]。盐胁迫下,部分植物接种PGPR后抗氧化酶活性显著升高[77]。例如,在150 mmol/L盐胁迫下,小麦接种碱湖迪茨氏菌(Dietzia natronolimnaea STR1)后叶和根中MnSOD、POD、CAT、APX、GPX和GR均显著增加,叶中抗氧化酶基因表达显著高于对照3倍,而根中抗氧化酶基因表达显著高于对照2-3倍[79]。在150mmol/L、175 mmol/L和200 mmol/L盐胁迫下,小麦接种克雷伯氏菌(Klebsiella sp. SBP-8)后叶中SOD、POD和CAT活性显著提高,而MDA、H2O2和O2-均显著降低[80]。可见,PGPR可通过诱导抗氧化酶基因上调来提高抗氧化酶活性,增强宿主植物抗氧化能力。
2.3.3 增加非酶类抗氧化物非酶类抗氧化物质(如脯氨酸、甜菜碱、多酚、抗坏血酸和谷胱甘肽等)的合成,可以保护植物体内不稳定大分子免受或少受各种代谢反应所形成的ROS攻击[66, 78, 81]。PGPR可以显著提高植物体内非酶类抗氧化物含量,缓解ROS对植物造成的氧化损伤。例如,在9 dS/m盐胁迫下,绿豆接种蜡样芽孢杆菌(Bacillus cereus Pb25)后叶中脯氨酸含量的显著增加有利于维持亚细胞结构(如膜和蛋白质)的稳定,清除多余ROS[54]。在0.5%-2.5% NaCl下,水稻接种假单胞菌(Pseudomonas sp.)和芽孢杆菌(Bacillus sp.)后甘氨酸-甜菜碱类季铵化合物显著增加,可以防止调节性外源蛋白解离,稳定光系统Ⅱ蛋白复合物的析氧活性[49]。在2.93 g/kg和5.85 g/kg盐胁迫下,玉米接种固氮菌(Azotobacter sp.)后叶中多酚含量的升高可以有效降低ROS对叶的伤害[46]。在5 dS/m和10 dS/m盐胁迫下,绿豆接种分散泛菌(Pantoea dispersa)和屎肠球菌(Enterococcus faecium)后叶中抗坏血酸和谷胱甘肽的显著提高有助于维持细胞内ROS的动态平衡,减少ROS造成的膜损伤[76]。
2.4 促进光合色素合成,改善光合性能光合作用是植物生长的基础,盐胁迫下影响植物生长的因素都会影响光合作用[82-83]。PGPR接种可以促进光合色素合成,提高光合速率,进而改善植物光合性能。
2.4.1 促进光合色素合成渗透胁迫诱导植物内源激素水平发生变化,抑制根对营养物质的吸收和叶对光能的截取,不利于光合色素的合成[33, 62, 84]。离子胁迫降低光合色素合成的特异性酶活性,造成光合色素降解增多[85]。氧化胁迫下ROS增多会破坏叶绿体超微结构和叶绿素相关蛋白的合成,造成色素光氧化[58, 86]。可见,由盐胁迫导致的次级胁迫不利于光合色素的合成。Meta分析结果表明,盆栽实验中耐盐植物和盐敏感植物在盐胁迫下接种PGPR后,类胡萝卜素含量分别显著增加了27.77%和55.02%,叶绿素含量分别显著增加了34.89%和34.99%[18]。盐胁迫下植物接种PGPR后叶绿素含量增加的原因可能是:(1)叶面积的增加促进了植物对光能的截取和利用[54];(2)根对Na+的吸收减少和对N、P和Mg2+等营养元素的吸收增加,有利于促进光合色素的合成[18, 58];(3)PGPR的ACC脱氨酶抑制了植物体内ETH合成,减缓了叶绿素的降解[54]。
2.4.2 提高光合速率盐胁迫下,受缺水和盐害的双重影响,根中合成的脱落酸通过木质部液流在叶中积累,导致气孔导度(Stomatal conductance,Gs)、蒸腾速率(Transpiration rate,Tr)和光合速率(Photosynthetic rate,Pn)降低,光合作用受到抑制[24, 87-88]。盐胁迫下,植物接种PGPR后Pn提高[33]。例如,在250 mmol/L和350 mmol/L NaCl胁迫下,大麦接种巴西固氮螺菌(Azospirillum brasilense NO40)后叶绿素含量、Pn、Gs和Tr都显著增加,且不耐盐品种的接种效应优于耐盐品种,叶绿素含量增加有利于提高Pn[89]。在40 mmol/L、80 mmol/L和120 mmol/L盐胁迫下,甜椒接种巴西固氮螺菌(Azospirillum brasilense)和分散泛菌(Pantoea dispersa)后Pn、Gs和CO2同化速率均显著增加,而PSII的光化学效率和叶绿素含量无显著变化,Pn的增加主要受Gs影响而非受PSII效率或叶绿素的影响[90]。可见,不同植物接种PGPR后叶绿素含量的变化对光合速率的影响不同。此外,盐胁迫下植物接种PGPR后光合作用相关的基因表达上调也会增强光合作用[36]。
2.5 抑制有害微生物,发挥有益微生物间的协同作用盐胁迫下,盐分积累影响土壤微生物群落结构的演替和功能多样性的稳定。PGPR可抑制根际土壤中病原微生物的繁殖,增强有益微生物的定殖,利用微生物间的对抗和协同关系为植物生长创造良好的土壤条件。
2.5.1 降低病原微生物对植物的胁迫盐分积累使土壤有益微生物群落逐渐转变为微生物活性低、竞争性弱和多样性差的原核微生物群落[91],致使土壤潜在病原微生物在根际存活且富集[52]。部分PGPR具有较高耐盐性,当其到达根表面后,呈线状成对地包裹于整个根被皮,后密集覆盖于整个根系,可在高度竞争的根际土壤微环境中迅速占据生态位[52, 92],保证自身在植物根际的成功定殖。随后,PGPR通过消耗营养物质或产生抗生素等途径,减少根际病原微生物的生存和繁衍,提高有益微生物的数量及整体活性,使宿主植物免受或少受病原微生物的侵害[52, 62]。例如,盐胁迫下,PGPR不仅可以通过诱导和调节植物内源激素促进小麦生长,而且可以通过分泌细胞壁降解酶或裂解酶来抑制植物病原微生物活性和降低病原微生物对植物的侵害,为植物的生长创造有力环境[52]。同时,细菌的群体效应现象和群体效应猝灭也是PGPR抑制病原菌生长繁殖和促进宿主植物生长的策略[93],但相关机制有待今后进一步研究。
2.5.2 利用微生物间的协同作用促进植物生长根际有益微生物的协同作用可以提高植物耐盐性并改善其生长性能。PGPR和丛枝菌根真菌(Arbuscular mycorrhizal fungi,AMF)是根际土壤微生物的重要组成者,具有促进生长与防治病害的双重潜力,二者也可相互促进对方在根际的定殖和发展[94-95]。PGPR有利于AMF孢子萌发和菌丝生长,增强根的感受性及其与AMF间的相互识别,有利于菌根效应的发挥[96-97]。反之,菌根分泌物也可加强PGPR与AMF的联系[96]。例如,菌丝延伸可以带动菌根周围PGPR的扩散,促进PGPR效应的发挥[94]。已有研究结果表明,在盐渍土壤中,燕麦同时接种AMF和PGPR后生物量积累、抗氧化能力和土壤酶活性均显著提高,且AMF和PGPR混合接种效应显著优于PGPR单一接种[98],表明微生物间的协同作用可以促进植物生长并提高植物对盐渍生境的修复潜力。
3 总结及展望PGPR既可提高植物的耐盐性使其适应盐渍生境,又可利用其与宿主植物间的相互作用来改良盐渍化土地,使其适应更多植物生长。然而,任何PGPR都不可能适应所有环境并拥有对所有植物有效的促生机制,而同种植物对不同PGPR的响应也受生长环境影响而存在差异。目前,已有试验多集中对植物接种微生物后形态特征和生理指标的响应进行研究,而对植物接种PGPR后分子水平的相关机理探索较少。若加强分子水平的相关基因表达方面的研究,有利于进一步揭示盐胁迫下PGPR接种对植物耐盐性的影响及二者的互作机制。同时,目前关于PGPR提高植物耐盐性的研究多局限于温室环境和控制条件,而对天然盐渍生境中PGPR对植物的促生潜力评估较少,今后应加强不同盐胁迫条件下不同PGPR菌种对不同植物种属的影响研究,这有助于不同作物专用PGPR菌肥的研发和PGPR菌肥在盐渍化地区农业发展和生态建设中的应用。此外,目前研究中选用的植物多为耐盐性较低的农作物,而对盐渍荒漠地区中维持生态系统稳定、促进能量循环与物质转化且具有农业生产潜力的盐生植物研究较少。今后可加强盐胁迫下PGPR对盐生植物的影响研究,这对改善和修复干旱半干旱地区盐渍化土地具有重要的生态学意义和应用价值。
| [1] |
Zhang H, Xiang Y, Irving LJ, et al. Nitrogen addition can improve seedling establishment of N-sensitive species in degraded saline soils[J]. Land Degradation and Development, 2019, 30: 119-127. |
| [2] |
Egamberdieva D, Wirth S, Bellingrath-Kimura SD, et al. Salt-Tolerant Plant growth promoting rhizobacteria for enhancing crop productivity of saline soils[J]. Front Microbiol, 2019, 10: 2791. |
| [3] |
Sardo V, Hamdy A. Halophytes-A previous resource[M]. Hamdy A.Non-conventional water use : WASAMED project. Bari : CIHEAM / EU DG Research, 2005.
|
| [4] |
Kosová K, Vítámvás PA, Urban AMO, et al. Plant proteome responses to salinity stress - comparison of glycophytes and halophytes[J]. Functional Plant Biology, 2013, 40: 775-786. |
| [5] |
Anik AR, Ranjan R, Ranganathan T. Estimating the impact of salinity stress on livelihood choices and incomes in Rural Bangladesh[J]. Journal of International Development, 2018, 30(8): 1414-1438. |
| [6] |
Estrada B, Aroca R, Azcón-Aguilar C, et al. Importance of native arbuscular mycorrhizal inoculation in the halophyte Asteriscus maritimus for successful establishment and growth under saline conditions[J]. Plant Soil, 2013, 370(1-2): 175-185. |
| [7] |
Bharti N, Barnawal D, Awasthi A, et al. Plant growth promoting rhizobacteria alleviate salinity induced negative effects on growth, oil content and physiological status in Mentha arvensis[J]. Acta Physiologiae Plantarum, 2014, 36: 45-60. |
| [8] |
李建国, 濮励杰, 朱明, 等. 土壤盐渍化研究现状及未来研究热点[J]. 地理学报, 2012, 67(9): 1233-1245. Li JG, Pu LJ, Zhu M, et al. The present situation and hot issues in the salt-affected soil research[J]. Acta Geographica Sinica, 2012, 67(9): 1233-1245. |
| [9] |
胡涛, 张鸽香, 郑福超, 等.植物盐胁迫响应的研究进展[J].分子植物育种, 2018, 16(9):264-273. Hu T, Zhang GX, Zheng FC, Cao Y. Research progress in plant salt stress response [J]. Molecular Plant Breeding, 2018, 16(9): 264-273. |
| [10] |
Qadir M, Quillérou E, Nangia V, et al. Economics of salt-induced land degradation and restoration[J]. Natural Resources Forum, 2014, 38(4): 282-295. |
| [11] |
Hasanuzzaman M, Nahar K, Alam MM, et al. Potential use of halophytes to remediate saline soils[J]. Journal of Biomedicine and Biotechnology, 2014, 2014(8): 589341. |
| [12] |
郧文聚, 杨劲松, 鞠正山. 以科技创新对接国家战略——聚焦"一带一路"沿线盐碱地治理[J]. 国土资源, 2015, 9: 44-46. Yun WJ, Yang JS, Ju ZS. Docking national strategy with scientific and technological innovation-Focusing on the treatment of saline and alkaline land along the"Belt and Road"[J]. Land and Resources, 2015, 9: 44-46. |
| [13] |
De-Bashan LE, Hernandez JP, Bashan Y. The potential contribution of plant growth-promoting bacteria to reduce environmental degradation-a comprehensive evaluation[J]. Applied Soil Ecology, 2012, 61: 171-189. |
| [14] |
Dodd IC, Perez-Alfocea F. Microbial amelioration of crop salinity stress[J]. J Exp Bot, 2012, 63: 3415-3428. |
| [15] |
Lau JA, Lennon JT. Evolutionary ecology of plant-microbe interactions :soil microbial structure alters selection on plant traits[J]. New Phytol, 2011, 192(1): 215-224. |
| [16] |
刘少芳, 王若愚. 植物根际促生细菌提高植物耐盐性研究进展[J]. 中国沙漠, 2019, 39(2): 1-12. Liu SF, Wang RY. Advance in research on plant salt tolerance improved by plant-growth-promoting rhizobacteria[J]. Journal of desert research, 2019, 39(2): 1-12. |
| [17] |
潘晶, 黄翠华, 罗君, 等. 盐胁迫对植物的影响及AMF提高植物耐盐性的机制[J]. 地球科学进展, 2018, 33(4): 361-372. Pan J, Huang CH, Luo J, et al. Effects of salt stress on plant and the mechanism of Arbuscular Mycorrhizal Fungi enhancing salt tolerance of plants[J]. Advances in earth science, 2018, 33(4): 361-372. |
| [18] |
Pan J, Peng F, Xue X, et al. The growth promotion of two salt-tolerant plant groups with PGPR inoculation :a meta-analysis[J]. Sustainability, 2019, 11(2): 378. |
| [19] |
康贻军, 程洁, 梅丽娟, 等. 植物根际促生菌作用机制研究进展[J]. 应用生态学报, 2010, 21(1): 232-238. Kang YJ, Cheng J, Mei LJ, et al. Action mechanisms of plant growth-promoting rhizobacteria(PGPR):A review[J]. Chinese Journal of Applied Ecology, 2010, 21(1): 232-238. |
| [20] |
Wansik S, Siddikee MA, Joe MM, et al. Halotolerant plant growth promoting bacteria mediated salinity stress amelioration in plants[J]. Korean Journal of Soil Science and Fertilizer, 2016, 49(4): 355-367. |
| [21] |
Munns R, Gilliham M. Salinity tolerance of crops - what is the cost?[J]. New Phytologist, 2015, 208(3): 668-673. |
| [22] |
Munns R, Tester M. Mechanisms of salinity tolerance[J]. Annual Review of Plant Biology, 2008, 59: 651-681. |
| [23] |
Ilangumaran G, Smith DL. Plant growth promoting rhizobacteria in amelioration of salinity stress :a systems biology perspective[J]. Frontiers in Plant Science, 2017, 8: 1768. |
| [24] |
Ahmad P, Azooz MM, Prasad MNV. Ecophysiology and responses of plants under salt stress[M]. Springer New York Heidelberg Dordrecht London, 2013.
|
| [25] |
Habib SH, Kausar H, Saud H. Plant growth-promoting rhizobacteria enhance salinity stress tolerance in Okra through ROS-scavenging enzymes[J]. Biomed Research International, 2016(1-4): 1-10. |
| [26] |
Gomes-Filho E, Lima CR, Costa JH, et al. Cowpea ribonuclease : properties and effect of NaCl-salinity on its activation during seed germination and seedling establishment[J]. Plant Cell Reports, 2008, 27(1): 147-157. |
| [27] |
Dantas BF, Ribeiro LdS, Aragão CA. Germination, initial growth and cotyledon protein content of bean cultivars under salinity stress[J]. Revista Brasileira de Sementes, 2007, 29(2): 106-110. |
| [28] |
严青青, 张巨松, 李星星, 等. 盐碱胁迫对海岛棉种子萌发及幼苗根系生长的影响[J]. 作物学报, 2019, 45(1): 104-114. Yan QQ, Zhang JS, Li XX, et al. Effects of salinity stress on seed germination and root growth of seedlings in island cotton[J]. Acta Agronomica Sinica, 2019, 45(1): 104-114. |
| [29] |
Jalili F, Khavazi K, Pazira E, et al. Isolation and characterization of acc deaminase-producing fluorescent pseudomonads, to alleviate salinity stress on canola(Brassica napus L.)growth[J]. Journal of Plant Physiology, 2009, 166(6): 667-674. |
| [30] |
Waheed QA, Nasim SA. Bacterial exopolysaccharide and biofilm formation stimulate chickpea growth and soil aggregation under salt stress[J]. Brazilian Journal of Microbiology, 2012, 43(3): 1183-1191. |
| [31] |
Storey R, Walker RR. Citrus and salinity :a review[J]. ScientiaHorticulturae, 1999, 78(1): 39-81. |
| [32] |
Osman KT. Saline and sodic soils[M]. Switzeland: Springer International Publishing AG, 2018.
|
| [33] |
Pan J, Huang C. Peng F, et al. Effect of arbuscular mycorrhizal fungi(AMF)and plant growth-promoting bacteria(PGPR) inoculations on Elaeagnus Angustifolia L. in saline soil[J]. Applied Sciences, 2020, 10: 945. |
| [34] |
井大炜, 马海林, 刘方春, 等. 盐胁迫环境下接种根际促生细菌对白蜡树根际生物学特征及其生长的影响[J]. 水土保持通报, 2018, 38(1): 76-81. Jing DW, Ma HL, Liu FC, et al. Effects of inoculating plant growth-promoting rhizobacteria on biological characteristics of rhizosphere and growth of Fraxinus chinensis under salt stress[J]. Bulletin of Soil and Water Conservation, 2018, 38(1): 76-81. |
| [35] |
Kumari S, Vaishnav A, Jain S, et al. Bacterial-mediated induction of systemic tolerance to salinity with expression of stress alleviating enzymes in Soybean(Glycine max L. Merrill)[J]. Journal of Plant Growth Regulation, 2015, 34(3): 558-573. |
| [36] |
Liu S, Hao H, Lu X, et al. Transcriptome profiling of genes involved in induced systemic salt tolerance conferred by Bacillus amyloliquefaciens FZB42 in Arabidopsis thaliana[J]. Scientific Reports, 2017, 7(1): 10795. |
| [37] |
Abeer H, Abd_Allah EF, Alqarawi AA, et al. Induction of osmoregulation and modulation of salt stress in Acacia gerrardii Benth by arbuscular mycorrhizal fungi and Bacillus subtilis(BERA 71)[J]. Biomed Research International, 2016, 1: 1-11. |
| [38] |
Jha Y, Subramanian RB. Rhizobacteria enhance oil content and physiological status of Hyptis suaveolens under salinity stress[J]. Rhizosphere, 2016(1): 33-35. |
| [39] |
Nadeem SM, Zahir ZA, Naveed M, et al. Preliminary investigations on inducing salt tolerance in maize through inoculation with rhizobacteria containing ACC deaminase activity[J]. Canadian Journal of Microbiology, 2007, 53: 1141-1149. |
| [40] |
Karlidag H, Yildirim E, Turan M, et al. Plant growth-promoting rhizobacteria mitigate deleterious effects of salt stress on strawberry plants(Fragaria ananassa)[J]. Hortscience A Publication of the American Society for Horticultural Science, 2013, 5(48): 563-567. |
| [41] |
Arora NK, Tewari S, Singh S, et al. PGPR for protection of plant health under saline conditions[M]. Maheshwari DK, Bacteria in Agrobiology: Stress Management. Springer Berlin Heidelberg, 2012.
|
| [42] |
Auge RM, Toler HD, Saxton AM. Arbuscular mycorrhizal symbiosis and osmotic adjustment in response to NaCl stress :a meta-analysis[J]. Frontiers in Plant Science, 2014, 5: 562. |
| [43] |
Mohamed HI, Gomaa EZ. Effect of plant growth promoting Bacillus subtilis and Pseudomonas fluorescens on growth and pigment composition of radish plants(Raphanus sativus)under NaCl stress[J]. Photosynthesis, 2012, 50(2): 263-272. |
| [44] |
Hidri R, Barea JM, Mahmoud MB, et al. Impact of microbial inoculation on biomass accumulation by Sulla carnosa provenances, and in regulating nutrition, physiological and antioxidant activities of this species under non-saline and saline conditions[J]. Journal of Plant Physiology, 2016, 201: 28-41. |
| [45] |
Younesi O, Moradi A. Effects of plant growth-promoting rhizobacterium(PGPR)and arbuscular mycorrhizal fungus(AMF) on antioxidant enzyme activities in salt-stressed bean(Phaseolus vulgaris L.)[J]. Agriculture, 2014, 60(1): 10-21. |
| [46] |
Rojas-Tapias D, Moreno-Galván A, Pardo-Díaz S, et al. Effect of inoculation with plant growth-promoting bacteria(PGPB)on amelioration of saline stress in maize(Zea mays)[J]. Applied Soil Ecology, 2012, 61: 264-272. |
| [47] |
Hamdia MAES, Shaddad MAK, Doaa MM. Mechanisms of salt tolerance and interactive effects of Azospirillum brasilense inoculation on maize cultivars grown under salt stress conditions[J]. Plant Growth Regulation, 2004, 44: 165-174. |
| [48] |
Singh RP, Jha PN. A halotolerant bacterium Bacillus licheniformis HSW-16 augments induced systemic tolerance to salt stress in wheat plant(Triticum aestivum)[J]. Frontiers in Plant Science, 2016(7): 1890. |
| [49] |
Jha Y, Subramanian RB, Patel S. Combination of endophytic and rhizospheric plant growth promoting rhizobacteria in Oryza sativa shows higher accumulation of osmoprotectant against saline stress[J]. Acta Physiologiae Plantarum, 2010, 33(3): 797-802. |
| [50] |
Ashraf M, Harris PJC. Potential biochemical indicators of salinity tolerance in plants[J]. Plant Science, 2004, 166: 3-16. |
| [51] |
Zhang H, Kim MS, Sun Y, et al. Soil bacteria confer plant salt tolerance by tissue-specific regulation of the sodium transporter HKT1[J]. Molecular Plant-Microbe Interactions, 2008, 21: 737-744. |
| [52] |
Egamberdieva D, Kucharova Z. Selection for root colonising bacteria stimulating wheat growth in saline soils[J]. Biology and Fertility of Soils, 2009, 45: 563-571. |
| [53] |
Raheem A, Ali B. Halotolerant rhizobacteria :beneficial plant metabolites and growth enhancement of Triticum aestivum L. in salt-amended soils[J]. Archives of Agronomy and Soil Science, 2015, 61: 1691-1705. |
| [54] |
Islam F, Yasmeen T, Arif MS, et al. Plant growth promoting bacteria confer salt tolerance in Vigna radiata by up-regulating antioxidantdefense and biological soil fertility[J]. Plant Growth Regulation, 2016, 80: 23-36. |
| [55] |
Marulanda A, Azcón R, Chaumont F, et al. Regulation of plasma membrane aquaporins by inoculation with a Bacillus megateriumstrain in maize(Zea mays L.)plants under unstressedand salt-stressed conditions[J]. Planta, 2010, 232(2): 533-543. |
| [56] |
王文铖, 崔克辉. 非生物逆境对植物水孔蛋白表达调控的研究进展[J]. 植物生理学报, 2016, 52(4): 423-430. Wang WC, Cui KH. Research progress in the expression and regulation of aquaporins under abiotic stresses[J]. Plant Physiology Journal, 2016, 52(4): 423-430. |
| [57] |
Eida AA, Hirt H, Saad MM. Challenges faced in field application of phosphate-solubilizing bacteria[M]. Mehnaz S. Rhizotrophs: Plant growth promotion to bioremediation. Springer Nature Singapore Pte Ltd, 2017: 125-143.
|
| [58] |
Hashem A, Abd Allah EF, Alqarawi AA, et al. The interaction between arbuscular mycorrhizal fungi and endophytic bacteria enhances plant growth of Acacia gerrardii under salt stress[J]. Front Microbiol, 2016, 7: 1089. |
| [59] |
卢翔, 王若愚. 干旱对解淀粉芽孢杆菌(Bacillus amylolique-faciens)FZB42生物被膜的形成及根际定殖能力的影响[J]. 中国沙漠, 2019, 39(3): 199-205. Lu X, Wang RY. Effects of drought stress on the biofilm formation and root colonization ability of Bacillus amyloliquefaciens FZB42[J]. Journal of Desert Research, 2019, 39(3): 199-205. |
| [60] |
Sandhya V, Ali SZ, Grover M, et al. Alleviation of drought stress effects in sunflower seedlings by the exopolysaccharides producing Pseudomonas putida strain GAP-P45[J]. Biology and Fertility of Soils, 2009, 46(1): 17-26. |
| [61] |
Kohler J, Caravaca F, Carrasco L, et al. Contribution of Pseudomonas mendocina and Glomus intraradices to aggregate stabilization and promotion of biological fertility in rhizosphere soil of lettuce plants under field conditions[J]. Soil Use and Management, 2006, 22(3): 298-304. |
| [62] |
Dodd IC, Zinovkina NY, Safronova VI, et al. Rhizobacterial mediation of plant hormone status[J]. Annals of Applied Biology, 2010, 157: 361-379. |
| [63] |
El-Samad HA, El-Komy HM. Effect of salinity, gibberellic acid and Azospirillum inoculation on growth and nitrogen uptake of Zea mays[J]. Biologia Plantarum, 1997, 41(1): 109-120. |
| [64] |
连宾, 傅平秋, 莫德明, 等. 硅酸盐细菌解钾作用机理的综合效应[J]. 矿物学报, 2002, 22(2): 179-183. Lian B, Fu PQ, Mo DM, et al. A Comprehensive review of the mechanism of potassium releasing by silicate bacteria[J]. Acta Mineralogica Sinica, 2002, 22(2): 179-183. |
| [65] |
Jha Y, Subramanian RB. Regulation of plant physiology and antioxidant enzymes for alleviating salinity stress by potassium-mobilizing bacteria[M]. Meena VS. Potassium solubilizing microorganisms for sustainable agriculture.Springer India, 2016: 149-162.
|
| [66] |
Bai L, Wang P, Song CP. Reactive oxygen species(ROS)and ABA signaling[M]. Zhang DP. Abscisic acid: metabolism, transport and signaling. Springer, 2014: 191-223.
|
| [67] |
Egamberdieva D, Wirth S, Jabborova D, et al. Coordination between Bradyrhizobium and Pseudomonas alleviates salt stress in soybean through altering root system architecture[J]. Journal of Plant Interaction, 2017, 12: 100-107. |
| [68] |
Irizarry I, White JF. Application of bacteria from non-cultivated plants to promote growth, alter root architecture and alleviate salt stress of cotton[J]. Journal of Applied Microbiology, 2017, 122(4): 1110-1120. |
| [69] |
Berg G, Alavi M, Schmidt CS, et al. Biocontrol and osmoprotection for plants under saline conditions[M]. de Bruijn FJ. Molecular Microbial Ecology of the Rhizosphere. Wiley -Blackwell, 2013: 587-592.
|
| [70] |
Mirza BS, Mirza MS, Bano A, et al. Coinoculation of chickpea with rhizobium isolates from roots and nodules and phytohormone-producing enterobacter strains[J]. Australian Journal of Experimental Agriculture, 2007, 47(8): 1008-1015. |
| [71] |
郑小兰, 王瑞娇, 赵群法, 等. 根际氧含量影响植物生长的生理生态机制研究进展[J]. 植物生态学报, 2017, 41(7): 805-814. Zheng XL, Wang RJ, Zhao QF, et al. Ecophysiological mechanisms of plant growth under the influence of rhizosphere oxygen concentration :A review[J]. Chinese Journal of Plant Ecology, 2017, 41(7): 805-814. |
| [72] |
李敏, 张鹏鹏, 刘凯, 等. 几株烟草根际促生细菌的挥发性物质对拟南芥根构型的影响[J]. 山东农业大学学报, 2015, 46(3): 347-352. Li M, Zhang PP, Liu K, et al. Effects of volatiles produced by some plant growth-promoting rhizobacteria of tobacco on root architecture of Arabidopsis thaliana[J]. Journal of Shandong Agricultural University, 2015, 46(3): 347-352. |
| [73] |
Yang J, Kloepper JW, Ryu CM. Rhizosphere bacteria help plants tolerate abiotic stress[J]. Trends Plant Sci, 2009, 14(1): 1-4. |
| [74] |
Mittler R. Oxidative stress, antioxidants and stress tolerance[J]. Trends Plant Sci, 2002, 7(9): 405-410. |
| [75] |
Kaur N, Dhawan M, Sharma I, et al. Interdependency of reactive oxygen species generating and scavenging system in salt sensitive and salt tolerant cultivars of rice[J]. BMC Plant Biology, 2016, 16: 131. |
| [76] |
Panwar M, Tewari R, Nayyar H. Native halo-tolerant plant growth promoting rhizobacteria Enterococcus and Pantoea sp. improve seed yield of Mungbean(Vigna radiata L.)under soil salinity by reducing sodium uptake and stress injury[J]. Physiology and Molecular Biology of Plants, 2016, 22(4): 445-459. |
| [77] |
Naz R, Bano A. Influence of Exogenously applied salicylic acid and plant growth promotion rhizobacteria inoculation on the growth and physiology of sunflower(Helianthus annuus L.)under salt stress[J]. Pakistan Journal of Botany, 2013, 45(2): 367-373. |
| [78] |
Bose J, Rodrigo-Moreno A, Shabala S. ROS homeostasis in halophytes in the context of salinity stress tolerance[J]. J Exp Bot, 2014, 65(5): 1241-1257. |
| [79] |
Bharti N, Pandey SS, Barnawal D, et al. Plant growth promoting rhizobacteria Dietzia natronolimnaea modulates the expression of stress responsive genes providing protection of wheat from salinity stress[J]. Scientific Reports, 2016, 6: 34768. |
| [80] |
Singh RP, Jha PN. Analysis of fatty acid composition of PGPR Klebsiella sp. SBP-8 and its role in ameliorating salt stress in wheat[J]. Symbiosis, 2017, 73: 213-222. |
| [81] |
Bharti N, Yadav D, Barnawal D, et al. Exiguobacterium oxidotolerans, a halotolerant plant growth promoting rhizobacteria, improves yield and content of secondary metabolites in Bacopa monnieri(L.)Pennell under primary and secondary salt stress[J]. World Journal of Microbiology and Biotechnology, 2013, 29: 379-387. |
| [82] |
Ashraf M, Harris PJC. Photosynthesis under stressfulenvironments:an overview[J]. Photosynthetica, 2013, 51(2): 163-190. |
| [83] |
Parida AK, Das AB. Salt tolerance and salinity effects on plants :areview[J]. Ecotoxi Environ Safety, 2005, 60(3): 324-349. |
| [84] |
Bano A, Fatima M. Salt tolerance in Zea mays L. following inoculation with Rhizobium and Pseudomonas[J]. Biology and Fertility of Soils, 2009, 45: 405-413. |
| [85] |
Murkute AA, Sharma S, Singh SK. Studies on salt stress tolerance of citrus rootstock genotypes with arbuscular mycorrhizal fungi[J]. Horticultural Science, 2006, 33(2): 70-76. |
| [86] |
Jha Y, Subramanian RB. Paddy physiology and enzymes level is regulated by rhizobacteria under saline stress[J]. Journal of Applied Botany and Food Quality, 2012, 85: 168-173. |
| [87] |
Barnawal D, Bharti N, Pandey SS, et al. Plant growth-promoting rhizobacteria enhance wheat salt and drought stress tolerance by altering endogenous phytohormone levels and TaCTR1 / TaDREB2 expression[J]. Physiologia Plantarum, 2017, 161: 502-514. |
| [88] |
Deinlein U, Stephan AB, Horie T, et al. Plant salt-tolerance mechanisms[J]. Trends Plant Sci, 2014, 19: 371-379. |
| [89] |
Omar MNA, Osman MEH, Kasim WA, et al. Improvement of salt tolerance mechanisms of barley cultivated under salt stress using Azospirillum brasilense[M]. Ashraf M. Salinity and Water Stress. Springer Netherlands, 2009: 133-147.
|
| [90] |
Delel Amor FM, Cuadra-Crespo P. Plant growth-promoting bacteria as a tool to improve salinity tolerance in sweet pepper[J]. Functional Plant Biology, 2012, 39: 82-90. |
| [91] |
Wong VNL, Greene RSB, Dalal RC, et al. Soil carbon dynamics in saline and sodic soils :a review[J]. Soil Use and Management, 2009, 26(1): 2-11. |
| [92] |
孙真, 郑亮, 邱浩斌. 植物根际促生细菌定殖研究进展[J]. 生物技术通报, 2017, 33(2): 8-15. Sun Z, Zheng L, Qiu HB. Research advances on colonization of plant growth-promoting rhizobacteria[J]. Biotechnology Bulletin, 2017, 33(2): 8-15. |
| [93] |
吴林坤, 林向民, 林文雄. 根系分泌物介导下植物-土壤-微生物互作关系研究进展与展望[J]. 植物生态学报, 2014, 38(3): 298-310. Wu LK, Lin XM, Lin WX. Advances and perspective in research on plant-soil-microbe interactions mediated by root exudates[J]. Chinese Journal of Plant Ecology, 2014, 38(3): 298-310. |
| [94] |
刘润进, 陈应龙. 菌根学[M]. 北京: 科学出版社, 2007. Liu RJ, Chen YL. Mycorrhizology[M]. Beijing: Science Press, 2007. |
| [95] |
龙伟文, 王平, 冯新梅, 等. PGPR与AMF相互关系的研究进展[J]. 应用生态学报, 2000, 11(2): 311-314. Long WW, Wang P, Feng XM, et al. Research progress on PGPR/ AMF interactions[J]. Chinese Journal of Applied Ecology, 2000, 11(2): 311-314. |
| [96] |
Artursson V, Finlay RD, Jansson JK. Interactions between arbuscular mycorrhizal fungi and bacteria and their potential for stimulating plant growth[J]. Environmental Microbiology, 2006, 8(1): 1-10. |
| [97] |
Richardson AE, Barea JM, McNeill AM, et al. Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms[J]. Plant Soil, 2009, 321(1): 305-339. |
| [98] |
Xun F, Xie B, Liu S, et al. Effect of plant growth-promoting bacteria (PGPR)and arbuscular mycorrhizal fungi(AMF)inoculation on oats in saline-alkali soil contaminated by petroleum to enhance phytoremediation[J]. Environmental Science and Pollution Research, 2015, 22: 598-608. |