2. 中国农业科学院羊育种工程技术研究中心, 兰州 730050
2. Sheep Breeding Engineering Technology Research Center, Chinese Academy of Agricultural Sciences, Lanzhou 730050
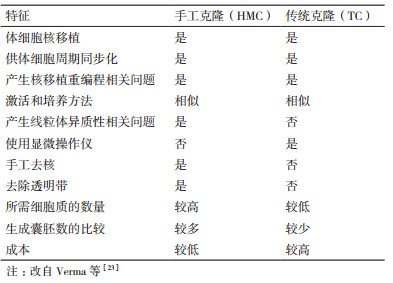
自从美国科学家Briggs和King[1]于1952年第一次通过囊胚细胞核移植成功获得了克隆蝌蚪以来, 科研人员开始了核移植的不断探索。英国科学家Wilmut等[2]在1997年使用绵羊乳腺上皮细胞作为核供体细胞将细胞核移入卵母细胞中, 成功获得世界上第一只克隆绵羊“Dolly”。Dolly的出生证明了高度分化的成年哺乳动物体细胞核在去核卵母细胞胞质中可以发生重新编程恢复其全能性, 并且重组细胞能够发育成与供体细胞遗传物质相同的新个体, 为体细胞核移植的研究奠定了的夯实的理论基础。在此之后科学家利用传统克隆技术先后成功克隆出牛[3]、山羊[4]、猪[5]等多种哺乳动物。然而克隆效率却一直较低, 并且该过程需要使用显微操作仪去除卵母细胞核, 然后通过融合法[6]或注射法[7]使供体细胞或供体细胞核进入去核卵母细胞, 成本较高, 操作复杂, 且囊胚率较低。为此, 科研人员不断对TC进行改进。Vajta等[8]于2001年对Peura的方法进行改进, 首次以体细胞为核供体细胞, 通过手工操作将去透明带核移植技术成功应用于克隆牛。这一改进的核移植技术便是手工克隆(Handm-ade cloning, HMC)技术。该技术不需要显微操作仪、操作较简单、成本较低, 且克隆囊胚率高于基于显微操作的TC[9-10]。表 1总结了HMC和TC之间的异同。从HMC技术的出现直到现在, HMC技术已经用于多种动物的克隆, 但是克隆效率仍较低。许多科研人员不断对体细胞或重构胚进行不同试剂的处理, 尝试通过多种优化方法提高手工克隆效率。本综述介绍了手工克隆技术的发展、操作研究以及影响手工克隆效率的因素。此外, 除了HMC的优势和局限性之外, 本文还讨论了该技术的未来前景, 以期能够提高牛、羊和猪等多种哺乳动物手工克隆的效率。
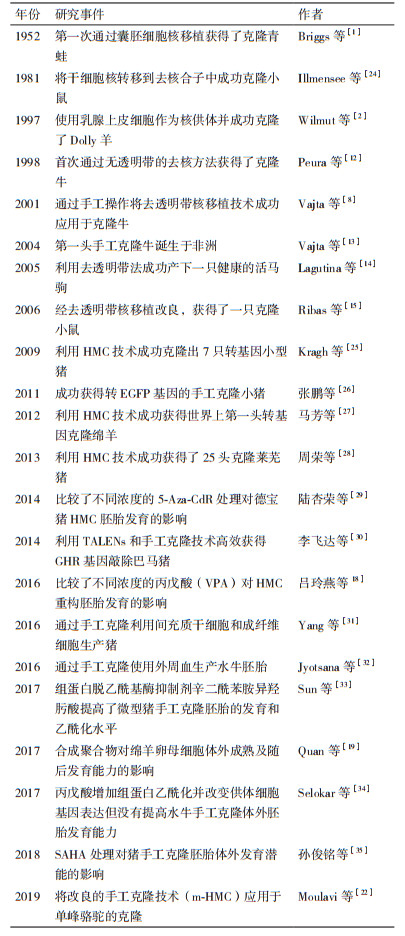
1995年, Tatham等[11]通过用蛋白酶去除透明带改进核移植程序, 但未能成功产下小牛。随后, Peura等[12]于1998年首次通过无透明带的去核方法成功克隆了牛。Vajta等[13]于2004年在没有显微操作仪和CO2培养箱的野外进行实验, 采用HMC技术完成核移植, 成功获得非洲第一头手工克隆牛。Lagutina等[14]于2005年用去透明带法使马卵母细胞获得较高的融合率, 并成功产下一只健康的活马驹。随后克隆出小鼠[15]、猪[16]、绵羊[17]等多种哺乳动物。此后, 科研人员对HMC进行不断研究, 以期进一步优化手工克隆技术。尤其近几年研究较多(表 2), 吕玲燕等[18]于2016年通过实验表明丙戊酸(Valproic acid, VPA)处理可提高猪HMC重构胚胎的囊胚率。Quan等[19]于2017年在绵羊卵母细胞成熟培养基中添加聚乙烯醇(Polyvinyl alcohol, PVA)、聚乙烯吡咯烷(Polyvinylpyrrolidone, PVP)和胎牛血清(Fetal bovine serum, FBS), 并对绵羊卵母细胞的数量及重构胚的卵裂率进行了比较, 实验表明PVA和PVP可以作为血清替代品用于卵母细胞的体外成熟。Zhang等[20]使用HMC生产了肌醇六磷酸酶转基因猪胚泡, 为进一步生产植酸酶转基因猪提供了基础。也有研究表明, 用HMC生产的成纤维细胞在欧洲可以有效地降低脱发性的能力[21]。Moulavi等[22]于2019年将最初用于绵羊的改良的手工克隆(Modified method of handmade cloning, m-HMC)技术应用于单峰骆驼的克隆, 实验结果表明m-HMC技术可能是一种有效生产克隆骆驼和使其商业化的可行的方法。Khan等[10]将通过TC和HMC两种方法获得克隆绵羊囊胚进行比较, 结果HMC相比TC获得的胚泡百分率更高。
核供体细胞的来源和细胞周期的同步化是影响核供体细胞的重要因素。HMC最初利用牛胚胎细胞作为供体核的来源[36]。随后科研人员又研究了许多其他来源, 如囊胚细胞[37]、颗粒细胞[8]、胚胎干细胞[38]、牛[39]、山羊[36]、绵羊[40]成年成纤维细胞、猪[41]、山羊[42]胎儿成纤维细胞。供体细胞的选择对于克服与核重新编程相关的问题非常重要。Saini等[36]研究表明成纤维细胞来源的供体细胞比上皮来源的供体细胞更容易发生重编程。近几年通过对成纤维细胞的研究, Jena等[43]发现胎儿成纤维细胞和成年成纤维细胞对山羊HMC胚胎产生效率无显著差异。Liu等[44]表明成年供体成纤维细胞的HMC胚泡形成率较高, 而胎儿供体成纤维细胞的HMC发育异常率较低, 且仔猪出生效率较高。并且Liu[45]等于2018年通过使用胎儿成纤维细胞进行体细胞核移植成功克隆了猕猴。另外, 受体与供体核细胞周期之间的同步被认为是增加核重编程能力并提高克隆效率所需的关键因素[46]。经处理后处于G0/G1阶段的供体细胞核移植胚胎胚泡发生率更高[47]。
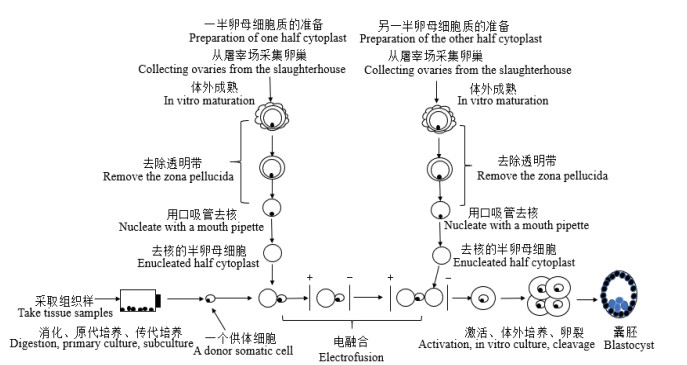
2.2 受体卵母细胞的选择, 体外成熟和去核卵母细胞的质量是在体外条件下胚胎成功发育的主要因素。形态学观察是卵母细胞选择的一种常见的做法, 通过观察卵母细胞周围卵丘细胞层的数目, 紧密度和卵质均一性进行选择[48]。收集的卵母细胞基于观察法可被分为3类: A、B和C级, 研究表明A级卵母细胞成熟率更高并应用于体外胚胎生产[49]。也可以通过亮甲酚蓝染色剂(Brilliant cresyl blue, BCB)染色进行选择。Mohapatra等[50]使用BCB染色卵母细胞用于水牛HMC胚胎生产。根据MⅡ中期阶段是哺乳动物体细胞克隆产生胚胎的最佳时期这一特性[51], 卵母细胞在含有FSH、LH和雌二醇等激素的成熟培养基中体外成熟22 h-24 h[43]。成熟的卵母细胞可以通过链霉蛋白酶处理[13]或透明质酸酶处理[52]来去除透明带。HMC中的去核方法主要包括化学辅助手工去核(Chemically assisted handmade enucleation, CAHE)和极体定向手工去核(Oriented handmade enucleation, OHE)。Li等[53]表明与OHE方法相比, CAHE后的体外发育能力较低。然而, Akshey等[54]研究表明CAHE获得的胞质数明显高于OHE, CAHE是一种用于山羊HMC的高效、可靠的去核方法。
2.3 融合和胚胎培养去核的卵母细胞与供体细胞的融合激活主要通过电激活和化学激活。Vajta等[8]通过电脉冲进行HMC胚胎的激活, Borah等[49]用钙离子载体和6-(二氨基)嘌呤(6-dimethylamino purine, 6-DMAP)处理进行化学激活。Akshey等[55]研究表明, 电脉冲激活对山羊HMC胚胎卵裂率和囊胚率优于钙离子载体激活。科研人员对融合参数进行了优化, 如对于水牛HMC, Selokra等[56]已经优化了电融合条件, 使融合率超过95%, 胚泡率达到52.0%。融合后, 胚胎培养体系和培养基的选择对胚胎发育至关重要。融合后的胚胎培养体系包括微穴法(Well of the well, WOW)[57], 微滴法(Microdrop, MD)[58]和平面培养法(the flat surface culture system, FS)[59], 目前WOW和FS使用较多。培养基不仅为胚胎的体外发育提供营养, 而且还会影响胚胎体内的发育。培养基主要包括(Modified charles rosenkrans 2, mCR2), 改良的合成输卵管液(Modified synthetic oviductal fluid, mSOF)和(Research vitro cleave, RVCL)。Shah[59]等比较了mCR2, mSOF和RVCL三种培养基, 发现在RVCL培养基中观察到的水牛HMC卵裂率和囊胚率高于其他两种培养基。另外培养基的选择也取决于胚胎的发育阶段, 培养体系, 胚胎类型和物种。mSOF培养基已经被广泛用于MD的水牛HMC中[60-61], 而RVCL培养基最适合用于使用FS的手工克隆中[62-63]。
3 限制手工克隆效率的主要因素影响HMC效率的因素众多, 包括体细胞的选择, 卵母细胞的选择, 卵母细胞的去核程序, 重构胚的培养条件, 重构胚的融合-激活方案和表观遗传重编程等。影响HMC效率的主要因素为异常的表观遗传重编程。Kang等[64]表明造成体细胞克隆效率低以及克隆动物各类异常表型的主要原因之一是表观遗传修饰在克隆胚胎早期发育阶段的异常重编程。基因组的重新编程是高度复杂的过程, 需要及时激活和关闭决定蛋白质正确表达的基因。异常的表观遗传重编程包括DNA甲基化、组蛋白修饰、X染色体失活、基因组印记[65]等。Jyotsana等[32]表明水牛克隆胚胎中存在高度的DNA甲基化和组蛋白乙酰化, 其基因表达模式与体外受精胚胎中的表达模式不同。Suteevun等[66]表明DNA的甲基化水平在2细胞期到8细胞期不断降低, 到桑椹胚开始不断增加, 在囊胚期达到最高。同时, 桑椹胚期组蛋白乙酰化水平最低。另外, 近期研究表明手工克隆囊胚与体外受精相比, 表观遗传修饰不同[32, 63]。此外经研究发现组蛋白修饰中组蛋白3赖氨酸9(Histone 3 Lysine 9, H3K9)甲基化是细胞重编程的主要障碍之一[67]。同样, 组蛋白3赖氨酸27(Histone 3 Lysine 27, H3K27)的甲基化水平高被认为是细胞重编程的重要障碍[68]。同时, 克隆胚胎也会发生异常的组蛋白乙酰化修饰。也有科研人员确定组蛋白3赖氨酸4(Histone 3 Lysine 4, H3K4)甲基化为主要的表观遗传学障碍[69]。众多科研人员的研究结果为提高HMC克隆效率奠定了理论基础。
4 手工克隆效率的提高对于体细胞的选择, 卵母细胞的选择, 卵母细胞的去核程序, 重构胚的培养条件, 重构胚的融合-激活, 科研人员已经将其优化, 文中已详细阐述。对于异常的表观遗传修饰, 科研人员已通过不同方法来修复异常重编程。这些方法主要通过表观遗传修饰剂或者表观遗传抑制调控因子来修复供体细胞或胚胎的表观遗传编程状态, 对囊胚率和胚胎质量有明显改善[70]。研究证明, S-腺苷高半胱氨酸[71]、5-氮杂-2'-脱氧胞苷[72]和RG108[73]能够降低DNA甲基化水平, 并且提高囊胚率。对于提高组蛋白乙酰化水平方面, 科研人员直到目前已经进行了大量研究, 如通过提高组蛋白赖氨酸各位点乙酰化水平来提高HMC囊胚率。吴霄等[74]通过体外转录获得赖氨酸特异性脱甲基酶4B(Lysine-specific demethylase 4B, KDM4B)和赖氨酸特异性脱甲基酶4D(Lysine-specific demethylase 4D, KDM4D)mRNA并将其分别显微注射至猪克隆胚胎, 结果表明过表达KDM4B可以显著提高猪克隆胚胎的囊胚率。Zhang等[75]于2018年用重组人KDM4D蛋白对供体绵羊胎儿成纤维细胞进行处理, 结果显示H3K9三甲基化和H3K9二甲基化水平均降低。Liu等[45]于2018年用TC在猴供体细胞中注射KDM4D mRNA并用组蛋白去乙酰基酶抑制剂曲古抑菌素A(Trichostatin A, TSA)处理, 大大提高了代孕猴的胚泡发育和核移植胚胎的妊娠率。目前用HMC技术对供体细胞或胚胎进行特异性去甲基化酶处理的研究甚少。众多科研人员的研究为HMC克隆效率的提高提供了新思路。但目前没有确切的数据能够表明特异性去甲基化酶可以提高HMC胎儿出生率。表观遗传因子对HMC胚胎中的重编程的作用仍需研究。
5 HMC的展望相比TC, HMC在克隆过程中所需设备成本较低, 操作较简单, 需要掌握的专业知识较少, 并且囊胚率和活产率较高。因此HMC得到不断发展并克隆出多种动物。目前HMC的操作技术方法已经得到完善, 但是克隆效率仍较低, 另外使用异质性细胞质及透明带的缺失可能会影响HMC胚胎生产效率, 同时由于异常表观遗传重编程的存在, HMC的克隆效率相比体内受精和体外受精较低。研究已发现H3K9甲基化和H3K27甲基化水平高是细胞重编程的主要障碍, 并且可以通过添加H3K9去甲基化酶和H3K27去甲基化酶降低甲基化水平从而提高克隆效率。但是异常的表观遗传机制仍不明确, 是目前研究HMC的存在问题, 今后需要科研人员对表观遗传的机制进行不断研究。另外, 对于去甲基化酶的研究仍不够深入, 特异性去甲基化酶对供体细胞或胚胎的不同处理方式对HMC克隆效率的影响仍需今后对其进行研究。通过克服HMC中存在的问题和难题来更好地优化HMC体系, 提高HMC的克隆效率, 更加广泛地应用到畜牧生产实践中去, 为家畜的品种改良及优良品种的扩繁提供技术支撑, 为拯救濒危物种铺平了道路, 还可以利用HMC结合基因编辑技术为生产转基因动物用于疾病模型和生物制药、用于生产所需患者特异性干细胞再生疗法中。
| [1] |
Briggs R, King TJ. Transplantation of living nuclei from blastula cells into enucleated frogs' eggs[J]. Proc Natl Acad Sci USA, 1952, 38(5): 455-463. DOI:10.1073/pnas.38.5.455 |
| [2] |
Wilmut I, Schnieke AE, McWhir J, et al. Viable offspring derived from fetal and adult mammalian cells[J]. Nature(London), 1997, 385(6619): 810-813. DOI:10.1038/385810a0 |
| [3] |
Robl JM, Prather R, Barnes F, et al. Nuclear transplantation in bovine embryos[J]. J Anim Sci, 1987, 64(2): 642-647. DOI:10.2527/jas1987.642642x |
| [4] |
Baguisi A, Behboodi E, Melican DT, et al. Production of goats by somatic cell nuclear transfer[J]. Nat Biotechnol, 1999, 17(5): 456-461. DOI:10.1038/8632 |
| [5] |
Onishi A, Iwamoto M, Akita T, et al. Pig cloning by microinjection of fetal fibroblast nuclei[J]. Science, 2000, 289(5482): 1188-1190. DOI:10.1126/science.289.5482.1188 |
| [6] |
Willadsen SM. Nuclear transplantation in sheep embryos[J]. Nature, 1986, 320(6057): 63-65. DOI:10.1038/320063a0 |
| [7] |
Wakayama T, Perry AC, Zuccotti M, et al. Full-term development of mice from enucleated oocytes injected with cumulus cell nuclei[J]. Nature, 1998, 394(6691): 369-374. DOI:10.1038/28615 |
| [8] |
Vajta G, Lewis IM, Hyttel P, et al. Somatic cell cloning without micromanipulators[J]. Cloning, 2001, 3(2): 89-95. DOI:10.1089/15204550152475590 |
| [9] |
Vajta G. Handmade cloning: the future way of nuclear transfer?[J]. Trends in Biotechnology, 2007, 25(6): 250-253. DOI:10.1016/j.tibtech.2007.04.004 |
| [10] |
Khan S, Tali M, Khan A, et al. Comparison of efficiency of in vitro cloned sheep embryo production by conventional somatic cell nuclear transfer and handmade cloning technique[J]. Reproduction in Domestic Animals, 2018, 53(2): 512-518. DOI:10.1111/rda.13138 |
| [11] |
Tatham BG, Dowsing AT, Trounson AO. Enucleation by centrifugation of in vitro-matured bovine oocytes for use in nuclear transfer[J]. Biology of reproduction, 1995, 53(5): 1088-1094. DOI:10.1095/biolreprod53.5.1088 |
| [12] |
Peura TT, Lewis IM, Trounson AO. The effect of recipient oocyte volume on nuclear transfer in cattle[J]. Molecular reproduction and development, 1998, 50(2): 185-191. DOI:10.1002/(SICI)1098-2795(199806)50:2<185::AID-MRD9>3.0.CO;2-G |
| [13] |
Vajta G, Bartels P, Joubert J, et al. Production of a healthy calf by somatic cell nuclear transfer without micromanipulators and carbon dioxide incubators using the Handmade Cloning(HMC)and the Submarine Incubation System(SIS)[J]. Theriogenology, 2004, 62(8): 1465-1472. DOI:10.1016/j.theriogenology.2004.02.010 |
| [14] |
Lagutina I, Lazzari G, Duchi R, et al. Somatic cell nuclear transfer in horses: Effect of oocyte morphology, embryo reconstruction method and donor cell type[J]. Reproduction, 2005, 130(4): 559-567. DOI:10.1530/rep.1.00772 |
| [15] |
Ribas R, Oback B, Ritchie W, et al. Modifications to Improve the Efficiency of Zona-Free Mouse Nuclear Transfer[J]. Cloning and Stem Cells, 2006, 8(1): 10-15. |
| [16] |
Du Y, Kragh PM, Zhang Y, et al. Piglets born from handmade clon-ing, an innovative cloning method without micromanipulation[J]. Theriogenology, 2007, 68(8): 1104-1110. DOI:10.1016/j.theriogenology.2007.07.021 |
| [17] |
Lagutina I, Lazzari G, Duchi R, et al. Comparative aspects of somatic cell nuclear transfer with conventional and zona-free method in cattle, horse, pig and sheep[J]. Theriogenology, 2006, 67(1): 90-98. |
| [18] |
吕玲燕, 吴永绍, 陈宝剑, 等. 猪手工克隆重构胚胎发育效果影响的最佳VPA浓度初探[J]. 基因组学与应用生物学, 2016, 35(9): 2336-2341. |
| [19] |
Quan GB, Ma Y, Ni YN, et al. Effects of synthetic polymers on in vitro maturation of sheep oocytes and subsequent developmental capacity after parthenogenetic activation or fertilization[J]. Small Ruminant Research, 2017, 153: 153-157. DOI:10.1016/j.smallrumres.2017.06.008 |
| [20] |
Zhang MY, Yang HW, et al. Production of Phytase Transgenic Porcine Blastocysts by Handmade Cloning[J]. Journal of Biobased Materials and Bioenergy, 2018, 12(7): 168-174. |
| [21] |
Gómez N, Ramirez M, Ruiz Cortés Z. Primary fibroblast cell cycle synchronization and effects on handmade cloned(HMC)bovine embryos[J]. Ciência Animal Brasileira, 2018, 19(19): 1-17. |
| [22] |
Moulavi F, Hosseini SM. Development of a modified method of handmade cloning in dromedary camel[J]. PLoS One, 2019, 14(4): e0213737. DOI:10.1371/journal.pone.0213737 |
| [23] |
Verma G, Arora JS, Sethi RS, et al. Handmade cloning: Recent advances, potential and pitfalls[J]. Journal of animal science and biotechnology, 2015, 6(1): 43-43. |
| [24] |
Illmensee K, Hoppe PC. Nuclear transplantation in mus musculus: Developmental potential of nuclei from preimplantation embryos[J]. Cell, 1981, 23(1): 9-18. |
| [25] |
Kragh PM, Nielsen AL, Li J, et al. Hemizygous minipigs produced by random gene insertion and handmade cloning express the Alzheimer's disease-causing dominant APPsw[J]. Transgenic Research, 2009, 18(4): 545-558. |
| [26] |
张鹏, 杨珍珍, 窦红伟, 等. 利用改进的手工克隆技术生产转GFP基因猪克隆胚胎[J]. 遗传, 2011, 33(5): 117-122. |
| [27] |
马芳, 刘旭林. 世界首例转基因手工克隆绵羊成功诞生[J]. 北京农业, 2012, 39(14): 67-67. |
| [28] |
周荣, 周秀, 罗绿花, 等. 利用手工克隆技术培育体细胞克隆莱芜猪[J]. 广东畜牧兽医科技, 2013, 38(2): 26-29. |
| [29] |
陆杏蓉, 孙俊铭, 李志鹏, 等. 甲基化酶抑制剂5-Aza-CdR处理对德保猪手工克隆胚胎体外发育潜能的影响[J]. 畜牧兽医学报, 2014, 45(08): 1274-1281. |
| [30] |
李飞达, 李勇, 等. 利用TALENs和手工克隆技术高效获得GHR基因敲除巴马猪[J]. 遗传, 2014, 36(9): 903-911. |
| [31] |
Yang ZZ, Vajta G, Xu Y, et al. Production of pigs by Hand-made cloning using mesenchymal stem cells and fibroblasts[J]. Cellular Reprogramming, 2016, 18(4): 256-263. DOI:10.1089/cell.2015.0072 |
| [32] |
Jyotsana B, Sahare AA, Raja AK, et al. Use of peripheral blood for production of buffalo(Bubalus bubalis)embryos by Hand-made cloning[J]. Theriogenology, 2016, 86(5): 1318-1324. DOI:10.1016/j.theriogenology.2016.04.073 |
| [33] |
Sun JM, Cui QK, Li ZP, et al. Suberoylanilide hydroxamic acid, a novel histone deacetylase inhibitor, improves the development and acetylation level of miniature porcine Handmade cloning embryos[J]. Reproduction in Domestic Animals, 2017, 52(5): 763-774. DOI:10.1111/rda.12977 |
| [34] |
Selokar NL, Saini M, Agrawal H, et al. Valproic acid increases histone acetylation and alters gene expression in the donor cells but does not improve the in vitro developmental competence of Buffalo(Bubalus bubalis)embryos produced by hand-made cloning[J]. Cellular Reprogramming, 2017, 19(1): 10-18. DOI:10.1089/cell.2016.0029 |
| [35] |
孙俊铭, 崔奎青, 李志鹏, 等. SAHA处理对猪手工克隆胚胎体外发育潜能的影响[J]. 黑龙江畜牧兽医, 2018(5): 104-108. |
| [36] |
Saini M, Selokar NL, Raja AK, et al. Effect of donor cell type on developmental competence, quality, gene expression, and epigenetic status of interspecies cloned embryos produced using cells from wild buffalo and oocytes from domestic buffalo[J]. Theriogenology, 2015, 84(1): 101-108. DOI:10.1016/j.theriogenology.2015.02.018 |
| [37] |
Elsheikh AS, Takahashi Y, Hishinuma M, et al. Developmental ability of mouse late 2-cell stage blastomeres fused to chemically enucleated oocytes in vitro[J]. J Vet Med Sci, 1997, 59(2): 107-113. DOI:10.1292/jvms.59.107 |
| [38] |
Mohapatra SK, Sandhu A, Neerukattu VS, et al. Buffalo embryos produced by handmade cloning from oocytes selected using brilliant cresyl blue staining have better developmental competence and quality and are closer to embryos produced by in vitro fertilization in terms of their epigenetic status and gene expression pattern[J]. Cellular Reprogramming, 2015, 17(2): 141-150. DOI:10.1089/cell.2014.0077 |
| [39] |
Ribeiro ES, Gerger RP, Ohlweiler LU, et al. Developmental potential of bovine hand-made clone embryos reconstructed by aggregation or fusion with distinct cytoplasmic volumes[J]. Cloning and Stem Cells, 2009, 11(3): 377-386. DOI:10.1089/clo.2009.0022 |
| [40] |
Zhang P, Liu P, et al. Handmade cloned transgenic sheep rich in omega-3 fatty acids[J]. PLoS One, 2013, 8(2): e55941. DOI:10.1371/journal.pone.0055941 |
| [41] |
Zhang P, Zhang Y, Dou H, et al. Handmade cloned transgenic piglets expressing the nematode fat-1 gene[J]. Cellular Reprogramming, 2012, 14(3): 258-266. DOI:10.1089/cell.2011.0073 |
| [42] |
Akshey YS, Malakar D, De AK, et al. Effect of roscovitine treated donor cells and different activation methods on development of handmade cloned goat(Capra hircus)embryos[J]. Theriogenology, 2011, 75(8): 1516-1524. DOI:10.1016/j.theriogenology.2010.12.015 |
| [43] |
Jena MK, Malakae D, De AK, et al. Handmade cloned and parthenogenetic goat embryos - A comparison of different culture media and donor cells[J]. Small Ruminant Research, 2012, 105(1-3): 255-262. DOI:10.1016/j.smallrumres.2012.03.001 |
| [44] |
Liu T, Dou HW, Xiang X, et al. Factors determining the efficiency of porcine somatic cell nuclear transfer: Data analysis with over 200, 000 reconstructed embryos[J]. Cellular reprogramming, 2015, 17(6): 463-471. DOI:10.1089/cell.2015.0037 |
| [45] |
Liu Z, Cai Z, Wang YJ, et al. Cloning of macaque monkeys by somatic cell nuclear transfer[J]. Cell, 2018, 172(4): 881-887. DOI:10.1016/j.cell.2018.01.020 |
| [46] |
Campbell KH, Loi P, Otaegui PJ, et al. Cell cycle co-ordination in embryo cloning by nuclear transfer[J]. Reviews of reproduction, 1996, 1(1): 40-46. DOI:10.1530/ror.0.0010040 |
| [47] |
Selokar NL, Saini M, Muzaffer M, et al. Roscovitine treatment improves synchronization of donor cell cycle in G0/G1 stage and in vitro development of handmade cloned buffalo(Bubalus bubalis)embryos[J]. Cellular Reprogramming, 2012, 14(2): 146-154. DOI:10.1089/cell.2011.0076 |
| [48] |
Loos FD, Vliet CV, Maurik PV, et al. Morphology of immature bovine oocytes[J]. Molecular Reproduction & Development, 1989, 24(2): 197-204. |
| [49] |
Borah M, Nath NC, et al. Handmade cloning for embryo production in goat, Assam, India[J]. International Journal of Current Microbiology and Applied Sciences, 2018, 7(5): 2518-2529. DOI:10.20546/ijcmas.2018.705.290 |
| [50] |
Mohapatra SK, Sandhu A, Neerukattu VS, et al. Buffalo embryos produced by handmade cloning from oocytes selected using brilliant cresyl blue staining have better developmental competence and quality and are closer to embryos produced by in vitro fertilization in terms of their epigenetic status and gene expression pattern[J]. Cellular Reprogramming, 2015, 17(2): 141-150. DOI:10.1089/cell.2014.0077 |
| [51] |
Campbell KH, RitchieI WA, Wilmut I. Nuclear-cytoplasmic interactions during the first cell cycle of nuclear transfer reconstructed bovine embryos: implications for deoxyribonucleic acid replication and development[J]. Biol Reprod, 1993, 49(5): 933-942. DOI:10.1095/biolreprod49.5.933 |
| [52] |
Vajta G, Lewis IM, Trounson AO, et al. Handmade somatic cell cloning in cattle: Analysis of factors contributing to high efficiency in vitro[J]. Biology of reproduction, 2003, 68(2): 571-578. |
| [53] |
Li J, Villemoes K, Zhang Y, et al. Efficiency of two enucleation methods connected to handmade cloning to produce transgenic porcine embryos[J]. Reproduction in Domestic Animals, 2008, 44(1): 122-127. |
| [54] |
Akshey YS, Malakar D, De A, et al. Study of the efficiency of chemically assisted enucleation method for handmade cloning in goat(Capra hircus)[J]. Reproduction in Domestic Animals, 2010, 46(4): 699-704. |
| [55] |
Akshey YS, Malakar D, De AK, et al. Effect of roscovitine treated donor cells and different activation methods on development of handmade cloned goat(Capra hircus)embryos[J]. Theriogenology, 2011, 75(8): 1516-1524. DOI:10.1016/j.theriogenology.2010.12.015 |
| [56] |
Selokra N, Shah R, Saha A, et al. Effect of post-fusion holding time, orientation and position of somatic cell-cytoplasts during electrofusion on the development of handmade cloned embryos in buffalo(Bubalus bubalis)[J]. Theriogenology, 2012, 78(4): 930-936. DOI:10.1016/j.theriogenology.2012.03.018 |
| [57] |
Vajta G, Peura TT, Holm P, et al. New method for culture of zona-included or zona-free embryos: The Well of the Well(WOW)system[J]. Molecular Reproduction and Development, 2000, 55(3): 256-264. DOI:10.1002/(SICI)1098-2795(200003)55:3<256::AID-MRD3>3.0.CO;2-7 |
| [58] |
Kaith S, Saini M, Raja AK, et al. Early cleavage of handmade cloned Buffalo(Bubalus bubalis)embryos is an indicator of their developmental competence and quality[J]. Reproduction in Domestic Animals, 2015, 50(2): 214-220. DOI:10.1111/rda.12472 |
| [59] |
Shah R, George A, Singh MK, et al. Hand-made cloned buffalo(Bubalus bubalis)embryos: Comparison of different media and culture systems[J]. Cloning and Stem Cells, 2008, 10(4): 435-442. |
| [60] |
Kitiyanant Y, Saikhun J, Chaisalee B, et al. Somatic cell cloning in buffalo(Bubalus bubalis): Effects of interspecies cytoplasmic recipients and activation procedures[J]. Cloning and Stem Cells, 2001, 3(3): 97-104. |
| [61] |
Shi D, Lu F, Wei Y, et al. Buffalos(Bubalus bubalis)cloned by nuclear transfer of somatic cells[J]. Biology of Reproduction, 2007, 77(2): 285-291. DOI:10.1095/biolreprod.107.060210 |
| [62] |
George A, Sharma R, Singh KP, et al. Production of cloned and transgenic embryos using buffalo(Bubalus bubalis)embryonic stem cell-like cells isolated from in vitro fertilized and cloned blastocysts[J]. Cell Reprogram, 2011, 13(3): 263-272. |
| [63] |
Saini M, Selokar NL, Agrawal H, et al. Treatment of donor cells and reconstructed embryos with a combination of trichostatin-A and 5-aza-2'-Deoxycytidine Improves the Developmental competence and quality of buffalo embryos produced by handmade cloning and alters their epigenetic status and gene expression[J]. Cell Reprogram, 2017, 19(3): 208-215. |
| [64] |
Kang YK, Koo DB, Park JS, et al. Aberrant methylation of donor genome in cloned bovine embryos[J]. Nature Genetics, 2001, 28(2): 173-177. DOI:10.1038/88903 |
| [65] |
杨正田, 沈伟, 邓继先. 体细胞核移植胚胎核重编程的研究进展[J]. 遗传学报, 2004, 31(6): 641-646. |
| [66] |
Suteevun T, Parnpai R, Marjani SL, et al. Epigenetic characteristics of cloned and in vitro-fertilized swamp buffalo(Bubalus bubalis)embryos[J]. Journal of Animal Science, 2006, 84(8): 2065-2071. DOI:10.2527/jas.2005-695 |
| [67] |
Matoba S, Liu Y, Lu F, et al. Embryonic development following somatic cell nuclear transfer impeded by persisting histone methylation[J]. Cell, 2014, 159(4): 884-895. DOI:10.1016/j.cell.2014.09.055 |
| [68] |
Xie B, Zhang H, Wei R, et al. Histone H3 lysine 27 trimethylation acts as an epigenetic barrier in porcine nuclear reprogramming[J]. Reproduction, 2015, 151(1): 9-16. |
| [69] |
Matoba S, Zhang Y. Somatic cell nuclear transfer reprogramming: Mechanisms and applications[J]. Cell Stem Cell, 2018, 23(4): 471-485. DOI:10.1016/j.stem.2018.06.018 |
| [70] |
Sadeesh EM, Fozia S, Meena K. Combined positive effect of oocyte extracts and brilliant cresyl blue stained recipient cytoplasts on epigenetic reprogramming and gene expression in buffalo nuclear transfer embryos[J]. Cytotechnology, 2017, 69(2): 289-305. DOI:10.1007/s10616-016-0057-0 |
| [71] |
Jafari S, Hosseini MS, Hajian M, et al. Improved in vitro develop-ment of cloned bovine embryos using S-adenosylhomocysteine, a non-toxic epigenetic modifying reagent[J]. Molecular Reproduction and Development, 2011, 78(8): 576-584. DOI:10.1002/mrd.21344 |
| [72] |
Wang YS, Xiong XR, An ZX, et al. Production of cloned calves by combination treatment of both donor cells and early cloned embryos with 5-aza-2/-deoxycytidine and trichostatin A[J]. Theriogenology, 2010, 75(5): 819-825. |
| [73] |
Xu W, Li Z, Yu B, et al. Effects of DNMT1 and HDAC inhibitors on gene-specific methylation reprogramming during porcine somatic cell nuclear transfer[J]. PloS one, 2013, 8(5): e64705. DOI:10.1371/journal.pone.0064705 |
| [74] |
吴霄, 李果, 敖政, 等. 过表达H3K9me3去甲基化酶对猪克隆胚胎体外发育效率的影响[J]. 广东农业科学, 2017, 44(10): 96-101. |
| [75] |
Zhang Y, Wang Q, Liu K, et al. Treatment of donor cells with recombinant KDM4D protein improves preimplantation development of cloned ovine embryos[J]. Cytotechnology, 2018, 70(5): 1-9. |