高温是制约植物生长发育的主要环境因素之一。近年来,随着全球年平均最低温度和最高温度的持续上升,全球气候变暖和“温室效应”现象日益明显。气候变暖不仅会影响植物的生长发育和植物的分布,还会严重降低很多经济作物的产量和品质,直接威胁种植业的发展[1]。因此,研究高温影响植物生长发育的机制,提高植物抵抗高温的能力对农林业生产具有重要意义。
为了抵抗高温胁迫的危害,植物在长期进化过程中形成了一系列应激反应机制,如热激蛋白等分子伴侣的含量增加,能够帮助维持细胞内蛋白的构象,减少细胞的伤害[2];或产生HSF、DREB等转录因子进行转录调控[3]。此外,也有研究表明,为了适应温度的变化,植物进化出了复杂的表观遗传机制以响应环境温度的胁迫[1]。microRNA(miRNA)调控基因的表达是植物表观遗传调控机制中的一种重要调控方式。microRNA(miRNA)在真核生物基因组中普遍存在,其是一类由20-24个核苷酸组成的小的非编码RNA,通常通过序列互补降解或抑制其靶标基因转录后的翻译过程,从而在转录水平和转录后水平上调控基因的表达[4]。大量研究表明,植物的miRNA在调控植物生长和发育,响应逆境胁迫方面均起重要作用[5-9]。近年来,随着测序技术的快速发展,研究者们研究发现高温胁迫能够影响植物miRNA的形成,大量响应高温胁迫的miRNA在植物中被鉴定出来,部分miRNA在高温胁迫中的调控功能也得到证实[10-13]。
本文综述了近年来在植物研究中发现的与高温胁迫相关的miRNA,总结了miRNA在响应高温胁迫过程中的主要的调控机制,及其与其他调控因子和信号通路之间可能存在内部联系。为深入研究高温胁迫相关miRNA的作用机制和调控通路,利用miRNA提高植物的抗热性提供科学依据。
1 植物响应高温胁迫的分子机制高温是限制植物生长发育的重要胁迫因子。分子生物学和生物技术的飞速发展为我们从分子水平认知植物抵御高温胁迫的机制提供了重要信息和研究手段。研究表明,植物在高温胁迫下发生一系列的应激反应,以减少高温胁迫对于机体的伤害。在分子水平上,植物响应高温胁迫的主要调控途径有活性氧途径(Reactive oxygen species,ROS)和激素调控途径[14]、热激转录因子-热激蛋白(Heat stress transcription factor-heat shock protein、Ca离子-钙调蛋白(Ca2+- CaM)途径。热信号传递在这3个途径中都涵盖3个步骤:先热信号的感知、紧接着信号的传递和放大、再在分子水平上转录调控和功能基因的表达。通过MAPK途径活性氧信号调控下游的转录因子表达,下游的MBF1c、ZAT家族和WRKY转录因子等转录因子表达能被MAPK的磷酸化激活[15]。这些转录因子再调控编码具有抗氧化功能的抗氧化酶类[Ascorbate peroxidase(APX)、Catalase(CAT)等]和非酶类(还原型抗坏血酸、谷胱甘肽、类黄酮等)基因的表达,从而提高植物的抗性[16]。而参与其中的高温胁迫响应基因可大致分为3类:第1类为转录调控基因及参与信号级联导系统,如转录因子(WRKY、HSF、ZAT、DREB等),以及蛋白激酶、泛素化酶和磷酸化酶等;第2类为直接对蛋白及生物膜起作用的功能蛋白,如在植物体内分布最广的是热激蛋白HSPs(Heat shock proteins)和LEA(Late embryo-genesis abundant proteins)等(图 1)。在高温胁迫下,植物体内大部分mRNA的转录及蛋白质的合成会受到抑制,而热激蛋白常常作为“分子伴侣”,参与植物受损蛋白的修复和维持植物细胞的存活[17-18];第3类为水和离子吸收和转运相关的蛋白[14, 19]。
近年来,大量研究表明,高温胁迫除了导致植物编码基因的表达发生改变之外,一些非编码RNA的表达也发生了显著改变,其中,miRNA作为重要的非编码RNA调控手段,参与了编码分子伴侣和转录因子等靶标基因的表达调控,影响了植物的抗热性[20-21]。
2 miRNA的形成过程、作用机制及生物学功能miRNA需要经过转录、加工和复合体装配3个过程才能最终形成。植物中非编码的MIR基因,能够在细胞核中由转录聚合酶Ⅱ转录出初级miRNA转录本(Primary miRNA transcripts),它是一段能形成不完美茎环结构的序列。初级miRNA转录本经过3个步骤加工成为具有转录功能的成熟miRNA:(1)首先在锌指蛋白SE、核糖核酸酶Ⅲ(RNase Ⅲ)DCL1,以及辅酶因子HYL1的共同作用下被加工成miRNA的前体(Precursor of miRNAs);(2)在SE、DCL1及HYL1共同作用下被加工成miRNA和miRNA*的二聚体结构(miRNA/miRNA*duplex)[22];(3)其中被甲基化的一条miRNA*的二聚体会被导出细胞核进入细胞质,然后与Agonaute蛋白(AGO)结合装配成RISC,这时的sRNA具有了转录后沉默功能,是成熟的miRNA。而一般核酸酶会降解另一条被称为miRNA*的二聚体。有研究者发现,其中一条在二聚体的5'端并配对不紧密的miRNA与AGO蛋白结合并行使功能[23]。
miRNA通过碱基互补配对原则识别靶基因[5]。根据其与靶基因的互补程度不同,miRNA通过对靶mRNA切割和翻译抑制2种转录调控机制,指导RISC下调基因表达:在靶基因和miRNA匹配性广泛或者匹配度较高的时候,mRNA会被RISC剪切;而当靶基因和miRNA的匹配度较低且低到不能够让RISC剪切靶基因的时候,靶基因会被RISC通过翻译抑制而沉默[23]。因为植物miRNA和靶基因的匹配属于广泛性匹配,所以miRNA主要是通过RISC选择剪切mRNA来抑制靶基因的表达[24]。不过还是有一小部分植物的靶基因和miRNA的匹配度较低,则miRNA调控RISC通过翻译抑制来沉默靶基因[25-26]。
近年来,随着高通量测序技术的发展,越来越多的miRNA从植物中被鉴定。在最新的第22版本miRBase里,一共有326个编码miRNA的位点存在于拟南芥基因组,有588个编码miRNA的位点存在于水稻基因组,有697个编码miRNA的位点存在于杨树基因组(http://www.mirbase.org/)。随着对miRNA研究得不断深入,研究者们发现miRNA在植物的生长和发育中均起着调控作用[5-6],其作为调控因子,广泛地参与到植物生长发育的不同阶段(表 1)。此外,miRNA也参与到了植物响应生物及非生物逆境的调控网络中。近年来,越来越多参与响应各种生物及非生物逆境的miRNA在模式植物被鉴定出来(表 1)。已有大量研究表明,miRNA在植物响应高温胁迫中也充当重要角色[21, 27-44]。
近年来,随着测序技术的快速发展与成熟,通过small RNA深度测序,从不同植物中鉴定出大量响应高温胁迫的保守或者非保守miRNA[12-13, 45]。研究表明,在高温胁迫下,植物miRNA的表达呈现出或上调或下调的表达方式,在部分植物中上调与下调的miRNA的数量差异并不显著:在水稻中鉴定出47个响应高温胁迫的miRNA,其中26个miRNA表达下调,21个miRNA表达上调[11];在番茄中,处于适当升温的环境下,分别有39/38个已知的miRNA出现了显著性的上调/下调,而在剧烈的升温下,则分别有62/57个已知的miRNA出现了显著性的上调/下调[58]。而部分植物中则大部分表现为下调表达:在萝卜中,26个已知的和19个新的miRNA在高温胁迫下出现了显著性差异表达,且大部分表现为下调表达,只有少数上调表达[59]。在中国白杨中,15个高温响应的miRNA家族被鉴定出来,且大部分miRNA的表达量下调[27]。
在不同植物中响应高温胁迫的miRNA不尽相同,目前,已发现在大部分植物中均响应高温胁迫的miRNA包括miR472、miR408、miR398、miR395、miR171、miR169、miR168、miR167、miR166、miR160、miR159和miR156等[28-29]。相同的miRNA在不同植物响应高温胁迫下的的表达模式也不完全相同(表 2)。miR159在木薯和水稻中在受高温胁迫后下调表达[11, 30];而在拟南芥、小麦和大白菜中则表现为上调表达[33, 35, 41]。miR160则在木薯和小麦中下调表达,而在水稻、拟南芥和棉花中上调表达[11, 21, 28, 48, 60]。在高温胁迫下,miR398则在水稻、小麦和拟南芥中上调表达,而在大白菜中下调表达[33, 35, 41]。
还有研究发现,同一家族的miRNA在不同植物中对高温胁迫的响应模式也不同。在小麦、拟南芥和大白菜中,高温胁迫下,miR156家族的miR156a、miR156h和miR156g上调表达;而在桦木、木薯和水稻中,miR156a和miR156d则受高温抑制表达[11, 30, 32-33, 35, 61]。
3.2 miRNA在植物高温胁迫响应中的调控机制miRNA主要是通过抑制靶基因的表达参与到植物的各种代谢和信号途径当中。随着研究得不断深入,明确了部分miRNA的靶向基因,并对其在高温胁迫下的作用机制有了一定的了解。同一miRNA可能存在多个靶向基因,且不同植物受到高温胁迫时,作用的靶向基因不完全一致。转录因子是miRNA作用的重要靶向基因之一。目前发现的植物在高温胁迫下受到miRNA调控较多的有ARF、MYB、WRKY和SPL等家族的转录因子(表 2)。miR160的靶向基因为ARF类转录因子:miR160在拟南芥中抑制其靶基因ARF17,ARF16和ARF10的表达,并通过调控HSP基因的表达从而提高拟南芥的抗热性[21];而在玉米中,miR160参与生长素信号途径,通过抑制其靶基因ARF17和ARF10的表达,从而导致高温胁迫下玉米雄性不育[62]。miR156在拟南芥中作用的靶向转录因子为SPL家族转录因子,miR156通过抑制SPL的表达增强了拟南芥对高温的记忆从而调节抗热性[33]。而在小麦中,高温胁迫下miR156的靶向基因为TaGAMYB1和TaGAMYB2[63]。此外,在耐热性较好的向日葵中还发现miR396通过调控WRKY家族转录因子HaWRKY6的表达,从而帮助向日葵不受到高温胁迫的伤害[60]。除了转录因子以外,在小麦中还发现了miR156、miR159、miR160、miR167、miR319、miR395和miR398等众多miRNA家族参与了HSP17、HSP70和superoxide dismutase等高温胁迫相关基因的表达调控[35]。
大量研究还表明,miRNA通过调控激素响应途径参与到植物响应高温胁迫。miR160、miR167、miR390和miR393在高温胁迫下均参与到了生长素代谢途径中[35-37]。miR159在高温胁迫下能够调控GA3代谢途径[35, 38]。而miR319在高温胁迫下则参与调控茉莉酸代谢途径[35, 39]。
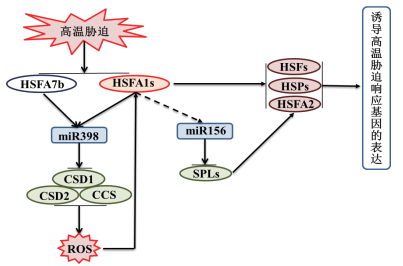
在这些响应高温胁迫的miRNA中,miR398和miR156在植物响应高温胁迫中的调控途径研究较为深入。在拟南芥中miR398的靶标为控制ROS的产生的COPPER/ZINC SUPEROXIDE DISMUTASE 1(CSD1)、CSD2和COPPER CHAPERONE FOR SOD 1(CCS)基因[40]。miR398超量表达抑制了这些活性氧清除酶的表达,导致ROS的积累,改变细胞内氧化还原状态,从而诱导HSPs及HSFs的表达,最终提高了拟南芥对高温的抗性。还发现HSFA7b和HSFA1b能结合在miR398的启动子区域,从而参与miR398的热诱导过程[40]。因此在拟南芥中,miR398与其靶标CSD1、CCS以及CSD2,HSFs共同组成了一条耐热回路(图 3)。除了拟南芥以外,在大白菜和毛白杨中同样也发现了类似的耐热回路[28, 41]。与miR398不同,在拟南芥中,miR156的靶向基因为SQUAMOSAPROMOTER BINDING-LIKE(SPL)transcription factors。该家族转录因子的下调表达能够促进热击响应基因HsfA2和HSPs的稳定持续表达,从而提高植物的抗热性(图 3)。研究还发现miR156在高温胁迫下特异表达。因此,miR156和SPLs组成的调控模块被认为是植物形成高温胁迫记忆的关键元件,对植物的获得性抗热有促进作用[33]。
|
| 图 3 拟南芥中miR398和miR156与其靶基因参与的耐热性调控途径 |
植物逆境胁迫广泛存在于植物的生长发育过程中,而随着温室效应的愈加严重,高温胁迫对植物的生长和发育影响愈加深远。近年来,对植物中响应高温胁迫的miRNAs研究已经取得一定进展:随着测序技术的不断发展,诸多miRNA及其靶基因被发现和鉴定;已有研究明确了miRNA作为重要的非编码RNA通过转录后调控广泛参与植物高温胁迫响应,其调控的靶基因大多编码转录因子或酶类。然而,目前,miRNA参与植物的高温胁迫的报道主要集中于模式植物中,对在其他观赏植物和经济植物中的研究报道较少。且大多miRNAs及其靶基因的功能仅停留在生物信息学预测与分析上,并没有得到试验证明。miRNA及其靶基因是如何参与到植物响应高温胁迫的分子调控网络途径还有待进一步研究。但不可否认的是,以miRNA差异表达以及对靶基因的调控角度探究植物在高温胁迫下的调控机制为植物抗高温胁迫提供了新的研究思路,对挖掘植物抗高温机制以及培育抗高温新材料具有十分重要的意义。在后续的研究中,miRNA及其靶基因在高温胁迫中的功能验证及其参与植物响应高温胁迫分子调控网络的作用机制将成为植物抗高温育种的研究重点。
| [1] |
刘军钟, 何祖华. 植物响应高温胁迫的表观遗传调控[J]. 科学通报, 2014, 59(8): 631-639. |
| [2] |
裴丽丽, 徐兆师, 尹丽娟, 等. 植物热激蛋白90的分子作用机理及其利用研究进展[J]. 植物遗传资源学报, 2013, 14(1): 109-114. DOI:10.3969/j.issn.1672-1810.2013.01.017 |
| [3] |
Ohama N, Sato H, Shinozaki K, et al. Transcriptional regulatory network of plant heat stress response[J]. Trends in Plant Science, 2016, 22(1): 53-65. |
| [4] |
罗小宁, 翟立娟, 李想, 等. 园林植物microRNA研究进展[J]. 生物技术通报, 2018, 34(8): 23-32. |
| [5] |
Bartel D. MicroRNAs :Target recognition and regulatory functions[J]. Cell, 2009, 136(2): 215-233. DOI:10.1016/j.cell.2009.01.002 |
| [6] |
Chuck G, O'Connor D. Small RNAs going the distance during plant development[J]. Current Opinion in Plant Biology, 2010, 13(1): 40-45. DOI:10.1016/j.pbi.2009.08.006 |
| [7] |
Yue W, Ying Y, Wang C, et al. OsNLA1, a RING-type ubiquitin ligase, maintains phosphate homeostasis in Oryza sativa via degradation of phosphate transporters[J]. The Plant Journal, 2017, 90(6): 1040-2051. DOI:10.1111/tpj.13516 |
| [8] |
Aravind J, Rinku S, Pooja B, et al. Identification, characterization, and functional validation of drought-responsive microRNAs in subtropical maize inbreds[J]. Frontiers in Plant Science, 2017, 8: 941. DOI:10.3389/fpls.2017.00941 |
| [9] |
Zeng X, Xu Y, Jiang J, et al. Identification of cold stress responsive microRNAs in two winter turnip rape(Brassica rapa L.)by high throughput sequencing[J]. BMC Plant Biology, 2018, 18(1): 52. DOI:10.1186/s12870-018-1242-4 |
| [10] |
Pan Y, Niu M, Liang J, et al. Identification of heat-responsive miRNAs to reveal the miRNA-mediated regulatory network of heat stress response in Betula luminifera[J]. Trees, 2017, 31(5): 1635-1652. DOI:10.1007/s00468-017-1575-x |
| [11] |
Li J, Wu LQ, Zheng WY, et al. Genome-wide identification of microRNAs responsive to high temperature in rice(Oryza sativa) by high-throughput deep sequencing[J]. Journal of Agronomy and Crop Science, 2014, 201(2015): 379-388. |
| [12] |
Liang C, Zhang X, Lei S, et al. Conserved and novel heat stressresponsive microRNAs identified by deep sequencing in Pyropia yezoensis[J]. Journal of Applied Phycology, 2018, 30(1): 685-696. DOI:10.1007/s10811-017-1260-x |
| [13] |
Liu Q, Yang TF, Yu T, et al. Integrating small RNA sequencing with QTL mapping for identification of miRNAs and their target genes associated with heat tolerance at the flowering stage in rice[J]. Frontiers in Plant Science, 2017, 8: 43. |
| [14] |
Mittler R, Finka A, Goloubinoff P. How do plants feel the heat?[J]. Trends in Biochemical Sciences, 2012, 37(3): 118-125. DOI:10.1016/j.tibs.2011.11.007 |
| [15] |
Fiil BK, Petersen K, Petersen M, et al. Gene regulation by MAP kinase cascades[J]. Current Opinion in Plant Biology, 2009, 12(5): 615-621. DOI:10.1016/j.pbi.2009.07.017 |
| [16] |
Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants[J]. Plant Physiology & Biochemistry, 2010, 48(12): 909-930. |
| [17] |
Liu J, Qin Q, Zhang Z, et al. OsHSF7 gene in rice, Oryza sativa L. encodes a transcription factor that functions as a high temperature receptive and responsive factor[J]. BMB Reports, 2009, 42(1): 16-21. DOI:10.5483/BMBRep.2009.42.1.016 |
| [18] |
Gong XQ, Hu JB, Liu JH. Cloning and characterization of FcWRKY40, A WRKY transcription factor from Fortunella crassifolia, linked to oxidative stress tolerance[J]. Plant Cell Tissue & Organ Culture, 2014, 119(1): 1-14. |
| [19] |
Qu AL, Ding YF, Jiang Q, et al. Molecular mechanisms of the plant heat stress response[J]. Biochemical & Biophysical Research Communications, 2013, 432(2): 203-207. |
| [20] |
Debernardi JM, Lin H, Chuck G, et al. microRNA172 plays a crucial role in wheat spike morphogenesis and grain threshability[J]. Development, 2017, 144(11): 1966-1975. DOI:10.1242/dev.146399 |
| [21] |
Lin JS, Kuo CC, Yang IC, et al. MicroRNA160 modulates plant development and heat shock protein gene expression to mediate heat tolerance in Arabidopsis[J]. Front Plant Sci, 2018, 9: 68. DOI:10.3389/fpls.2018.00068 |
| [22] |
Dong Z, Han MH, Fedoroff N. The RNA-binding proteins HYL1 and SE promote accurate in vitro processing of pri-miRNA by DCL1[J]. Proceedings of the National Academy of Sciences of the United States of America, 2008, 105(29): 9970-9975. DOI:10.1073/pnas.0803356105 |
| [23] |
Bartel DP. MicroRNAs :genomics, biogenesis, mechanism, and function[J]. Cell, 2004, 116(2): 281-297. DOI:10.1016/S0092-8674(04)00045-5 |
| [24] |
Voinnet O. Origin, biogenesis, and activity of plant microRNAs[J]. Cell, 2009, 136(4): 669-687. DOI:10.1016/j.cell.2009.01.046 |
| [25] |
Dugas DV, Bartel B. Sucrose induction of Arabidopsis miR398 represses two Cu/Zn superoxide dismutases[J]. Plant Mol Biol, 2008, 67(4): 403-417. DOI:10.1007/s11103-008-9329-1 |
| [26] |
Lanet E, Delannoy E, Sormani R, et al. Biochemical evidence for translational repression by Arabidopsis microRNAs[J]. The Plant Cell, 2009, 21(6): 1762-1768. DOI:10.1105/tpc.108.063412 |
| [27] |
Chen L, Ren Y, Zhang Y, et al. Genome-wide identification and expression analysis of heat-responsive and novel microRNAs in Populus tomentosa[J]. Gene, 2012, 504(2): 160-165. DOI:10.1016/j.gene.2012.05.034 |
| [28] |
Zhao JG, He QS, Chen G, et al. Regulation of non-coding RNAs in heat stress responses of plants[J]. Frontiers in Plant Science, 2016, 7: 18. |
| [29] |
Sunkar R, Li YF, Jagadeeswaran G. Functions of microRNAs in plant stress responses[J]. Trends in Plant Science, 2012, 17(4): 196-203. DOI:10.1016/j.tplants.2012.01.010 |
| [30] |
Ball-Taborda C, Plata G, Ayling S, et al. Identification of cassava microRNAs under abiotic stress[J]. Int J Genomics, 2013, 2013: 857-986. |
| [31] |
Kumar R. Role of microRNAs in biotic and abiotic stress responses in crop plants[J]. Applied Biochemistry & Biotechnology, 2014, 174(1): 93-115. |
| [32] |
Sailaja B, Voleti SR, Subrahmanyam D, et al. Prediction and expression analysis of mirnas associated with heat stress in Oryza sativa[J]. Rice Science, 2014, 21(1): 3-12. |
| [33] |
Stief A, Altmann S, Hoffmann K, et al. Arabidopsis miR156 regulates tolerance to recurring environmental stress through SPL transcription factors[J]. The Plant Cell, 2014, 26(4): 1792. DOI:10.1105/tpc.114.123851 |
| [34] |
Wang Y, Sun F, Cao H, et al. TamiR159, directed wheat, TaGAMYB, cleavage and its involvement in anther development and heat response[J]. PLoS One, 2012, 7(11): e48445. DOI:10.1371/journal.pone.0048445 |
| [35] |
Goswami S, Kumar RR, Rai RD. Heat-responsive microRNAs regulate the transcription factors and heat shock proteins in modulating thermo stability of starch biosynthesis enzymes in wheat (Triticum aestivum L.)under the heat stress[J]. Australian Journal of Crop Science, 2014, 8(5): 697-705. |
| [36] |
Kruszka K, Pacak A, Swidabarteczka A, et al. Transcriptionally and post-transcriptionally regulated microRNAs in heat stress response in barley[J]. Journal of Experimental Botany, 2014, 65(20): 6123-6135. DOI:10.1093/jxb/eru353 |
| [37] |
Lin JS, Kuo CC, Yang IC, et al. MicroRNA160 modulates plant development and heat shock protein gene expression to mediate heat tolerance in Arabidopsis[J]. Front Plant Sci, 2018, 9: 68. DOI:10.3389/fpls.2018.00068 |
| [38] |
Li H, Wang Y, Wang Z, et al. Microarray and genetic analysis reveals that csa-miR159b plays a critical role in abscisic acidmediated heat tolerance in grafted cucumber plants[J]. Plant, Cell & Environment, 2016, 39(8): 1790-1804. |
| [39] |
Hivrale V, Yun Z, Puli COR, et al. Characterization of drought- and heat-responsive microRNAs in switchgrass[J]. Plant Sci, 2016, 242: 214-223. DOI:10.1016/j.plantsci.2015.07.018 |
| [40] |
Guan Q, Lu X, Zeng H, et al. Heat stress induction of miR398 triggers a regulatory loop that is critical for thermotolerance in Arabidopsis[J]. Plant Journal, 2013, 74(5): 840-851. DOI:10.1111/tpj.12169 |
| [41] |
Yu X, Wang H, Lu Y, et al. Identification of conserved and novel microRNAs that are responsive to heat stress in Brassica rapa[J]. Journal of Experimental Botany, 2012, 63(2): 1025-1038. DOI:10.1093/jxb/err337 |
| [42] |
Liu F, Wang W, Sun X, et al. Conserved and novel heat stressresponsive microRNAs were identified by deep sequencing in Saccharina japonica(Laminariales, Phaeophyta)[J]. Plant Cell & Environment, 2015, 38(7): 1357-1367. |
| [43] |
Gupta OP, Meena NL, Sharma I, et al. Differential regulation of microRNAs in response to osmotic, salt and cold stresses in wheat[J]. Mol Biol Rep, 2014, 41(7): 4623-4629. DOI:10.1007/s11033-014-3333-0 |
| [44] |
Yamasaki H, Hayashi M, Fukazawa M, et al. SQUAMOSA promoter binding protein-like7 is a central regulator for copper homeostasis in Arabidopsis[J]. Plant Cell, 2009, 21(1): 347-361. DOI:10.1105/tpc.108.060137 |
| [45] |
潘樱, 张仪平, 朱敏慧, 等. 光皮桦miR393及其靶基因在低氮胁迫中的表达分析[J]. 核农学报, 2017, 31(10): 1921-1930. DOI:10.11869/j.issn.100-8551.2017.10.1921 |
| [46] |
Wu G, Poethig RS. Temporal regulation of shoot development in Arabidopsis thaliana by miR156 and its target SPL3[J]. Development, 2006, 133(18): 3539-3547. DOI:10.1242/dev.02521 |
| [47] |
Dotto M, Mez MS, Soto MS, et al. UV-B radiation delays flowering time through changes in the PRC2 complex activity and miR156 levels in Arabidopsis thaliana[J]. Plant Cell & Environment, 2018, 41(6): 1394-1406. |
| [48] |
Ren W, Wang H, Bai J, et al. Association of microRNAs with types of leaf curvature in Brassica rapa[J]. Frontiers in Plant Science, 2018, 9: 73. DOI:10.3389/fpls.2018.00073 |
| [49] |
Yang CH, Li DY, Ma DH, et al. Overexpression of microRNA319 impacts leaf morphogenesis and leads to enhanced cold tolerance in rice(Oryza sativa L.)[J]. Plant Cell & Environment, 2013, 36(12): 2207-2218. |
| [50] |
Anwar N, Ohta M, Yazawa T, et al. miR172 downregulates the translation of cleistogamy 1 in barley[J]. Annals of Botany, 2020, 36(2): 251-265. |
| [51] |
Zhang YC, Yu Y, Wang CY, et al. Overexpression of microRNA OsmiR397 improves rice yield by increasing grain size and promoting panicle branching[J]. Nature Biotechnology, 2013, 31(9): 848-852. DOI:10.1038/nbt.2646 |
| [52] |
Xu J, Li J, Cui L, et al. New insights into the roles of cucumber TIR1 homologs and miR393 in regulating fruit/seed set development and leaf morphogenesis[J]. BMC Plant Biology, 2017, 17(1): 130. |
| [53] |
Kim JY, Kwak KJ, Jung HJ, et al. MicroRNA402 affects seed germination of Arabidopsis thaliana under stress conditions via targeting DEMETER-LIKE protein3 mRNA[J]. Plant and Cell Physiology, 2010, 51(6): 1079-1083. DOI:10.1093/pcp/pcq072 |
| [54] |
Gao P, Bai X, Yang L, et al. Over-expression of osa-MIR396c decreases salt and alkali stress tolerance[J]. Planta, 2010, 231(5): 991-1001. DOI:10.1007/s00425-010-1104-2 |
| [55] |
Zhou M, Li D, Li Z, et al. Constitutive expression of a miR319 gene alters plant development and enhances salt and drought tolerance in transgenic creeping bentgrass[J]. Plant Physiology, 2014, 161(4): 1375-1391. |
| [56] |
Zhang W, Gao S, Zhou X, et al. Bacteria-responsive microRNAs regulate plant innate immunity by modulating plant hormone networks[J]. Plant Mol Biol, 2011, 75(1/2): 93-105. |
| [57] |
Zhao J, Yuan S, Zhou M, et al. Transgenic creeping bentgrass overexpressing Osa-miR393a exhibits altered plant development and improved multiple stress tolerance[J]. Plant Biotechnology Journal, 2019, 17(1): 233-251. DOI:10.1111/pbi.12960 |
| [58] |
Zhou R, Wang Q, Jiang F, et al. Identification of miRNAs and their targets in wild tomato at moderately and acutely elevated temperatures by high-throughput sequencing and degradome analysis[J]. Scientific Reports, 2016, 6: 33777. DOI:10.1038/srep33777 |
| [59] |
Wang R, Xu L, Zhu X, et al. Transcriptome-wide characterization of novel and heat-stress-responsive microRNAs in radish(Raphanus sativus L.)using next-generation sequencing[J]. Plant Molecular Biology Reporter, 2014, 33(4): 867-880. |
| [60] |
Giacomelli JI, Weigel D, Chan RL, et al. Role of recently evolved miRNA regulation of sunflower HaWRKY6 in response to temperature damage[J]. New Phytol, 2012, 195(4): 766-773. DOI:10.1111/j.1469-8137.2012.04259.x |
| [61] |
Pan Y, Niu M, Liang J, et al. Identification of heat-responsive miRNAs to reveal the miRNA-mediated regulatory network of heat stress response in Betula luminifera[J]. Trees, 2017, 31(5): 1635-1652. DOI:10.1007/s00468-017-1575-x |
| [62] |
Xin M, Wang Y, Yao Y, et al. Diverse set of microRNAs are responsive to powdery mildew infection and heat stress in wheat (Triticum aestivum L.)[J]. BMC Plant Biology, 2010, 10: 123. DOI:10.1186/1471-2229-10-123 |
| [63] |
Ding Y, Ma Y, Liu N, et al. microRNAs involved in auxin signalling modulate male sterility under high-temperature stress in cotton (Gossypium hirsutum)[J]. Plant J, 2017, 91: 977-994. DOI:10.1111/tpj.13620 |
| [64] |
May P, Liao W, Wu Y, et al. The effects of carbon dioxide and temperature on microRNA expression in Arabidopsis development[J]. Nature Communications, 2013, 4: 2145. DOI:10.1038/ncomms3145 |
| [65] |
Mahale BM, Fakrudin B. LNA mediated in situ hybridization of miR171 and miR397a in leaf and ambient root tissues revealed expressional homogeneity in response to shoot heat shock in Arabidopsis thaliana[J]. Journal of Plant Biochemistry and Biotechnology, 2014, 23(1): 93-103. DOI:10.1007/s13562-013-0191-0 |
| [66] |
Li S, Liu J, Liu Z, et al. HEAT-INDUCED TAS1 TARGET1 mediates thermotolerance via HEAT STRESS TRANSCRIPTION FACTOR A1a-directed pathways in Arabidopsis[J]. The Plant Cell, 2014, 26(4): 1764-1780. DOI:10.1105/tpc.114.124883 |