2. 西南大学食品贮藏与物流研究中心,重庆 400715
2. Research Center of Food Storage & Logistics, Southwest University, Chongqing 400715
衰老,是生物随着时间的推移,机体从构成物质、组织结构到生理功能的丧失和退化过程,是复杂的自然现象。在机体正常的衰老过程中会发生许多不良的变化,如皮肤色素沉着、皮肤皱纹等,并会增加对疾病的易感性,与阿尔茨海默氏症、帕金森氏症、糖尿病、动脉粥样硬化、骨质疏松症、骨关节炎和多种癌症等年龄相关疾病的发生有关[1]。
目前,许多天然产物,如多酚类化合物、多糖类化合物的抗衰老功效已经被证实,但其可能存在制备成本高、生物利用效率低等问题。益生菌作为活的微生物,当给予足够量时,会给宿主带来健康益处。益生菌对衰老的作用主要体现在对肠道微生物群的积极调节、免疫调节和寿命延长的作用[2]。因此,益生菌正因其来源广泛、成本低廉、安全性较高的优势,而成为抗衰老研究的热点。论文结合国内外益生菌抗衰老研究现状,从衰老相关机制、抗衰老评价模型以及作用机制等方面综述了益生菌的抗衰老作用。
1 衰老相关机制DNA损伤学说、端粒学说、细胞凋亡学说是阐明衰老机制非常成熟的理论。一般而言,衰老相关机制主要集中在以下几个方面。
1.1 炎症性衰老炎症性衰老是由于氧化应激、炎性因子的过度表达[3]、DNA损伤[4]、自噬[5]和非酶糖化[6]等多种因素的作用,引起一系列由NLRP3和NF-κB激活的炎症反应,使机体处于一种慢性的、逐渐升高的促炎状态。许多研究表明衰老与炎症的普遍增加有关[7-8]。Kuilman等[9]发现用活化癌蛋白BRAFE600诱导人成纤维细胞的过早衰老与炎症细胞因子IL-6的表达水平的上调密切相关,因为IL-6的敲除阻止了其诱导的衰老。
1.2 自由基氧化应激理论活性氧自由基在细胞内的大量积累,会干扰细胞内活性氧物质与抗氧化剂的平衡,对细胞内大分子和细胞本身造成损伤[10],这种由活性氧物质累积的细胞损伤引起的氧化应激在衰老过程中起重要作用,并且是产生慢性炎症状态的主要原因之一[11]。Garrido等[12]通过对早衰(Prematurely aging mice,PAM)小鼠、老年小鼠比较研究发现,PAM小鼠、老年小鼠细胞中过氧化氢酶和谷胱甘肽还原酶活性显著低于NPAM和WT小鼠的相应酶的活性。而黄嘌呤氧化酶的活性和氧化型谷胱甘肽呈相反的趋势。证明衰老细胞中的氧化剂水平明显增多。此外,机体内抗氧化剂水平的提高对衰老具有延缓作用。
1.3 免疫衰老学说随着年龄的增长,适应性免疫系统和先天免疫系统的功能逐渐下降,引起机体慢性炎症,从而加速机体衰老,这种与年龄相关的免疫机能衰退被称为免疫衰老[13-14]。其特征包括:naive CD4+和CD8+T细胞数量减少,T细胞亚群失衡,T细胞受体库和信号减少。相应地,B细胞的淋巴细胞生成减少,抗体生成减少[15]。
1.4 肠道菌群失调人体胃肠道中的微生物数量(约100万亿)约为其体内细胞数量(10万亿)的10倍[16]。随着年龄的增长,在药物、胃肠道感染、饮食等因素的作用下,病理生理功能变化造成肠道微生物群的组成发生改变[15]。肠道通透性的增加可能是这一变化所导致的结果,研究发现,果蝇肠道微生物群的改变发生于肠道屏障功能障碍之前并对其有预示作用[17]。结肠通透性的增加使细菌及其产物逃逸会引起全身性的炎症,增加机体免疫系统的负担,导致机体组织破坏及衰老[18]。Thevaranjan等[19]将来自老年小鼠的微生物群落定殖到无菌幼鼠后,发现无菌幼鼠的肠道细胞通透性增加,明确指出与年龄相关的微生物群落组成的改变可导致肠道通透性增加,同时发现无菌小鼠可免受年龄相关性炎症和巨噬细胞功能失调的影响。肠道微生物群落被认为可能是衰老的决定因素,因此,调节肠道微生物群组成也成为抗衰老研究的有效途径。
2 益生菌抗衰老评价模型益生菌的安全性以及抗衰老能力的评价主要依赖体内和体外两种评价模型。体外评价主要集中在与衰老相关的抗氧化功能研究方面,比如益生菌清除自由基能力、还原力和螯合亚铁离子能力等,以及在细胞水平上益生菌作用于离体细胞的研究[20]。本文主要对建立实验室模型生物的体内模型进行阐述,各模型的优势、缺陷以及评价指标如表 1所示。
借助上述四类标准的实验室模型生物,我们已经对衰老机制的理解方面取得了重大进展,并且这些模式生物将继续发挥重要作用。但是将其结果进行推广时,仍存在寿命短、遗传同质性、实验室存活、缺乏生态环境等多个方面的限制[31],因此在进行相关研究时需要考虑这些因素以适当的补充其他的研究。
3 益生菌抗衰老机制目前,关于益生菌抗衰老作用的研究大多是从其抗氧化、减少炎症、增强免疫力这3个方面进行侧面体现,没有深入探讨其中可能的分子作用途径。本文将从益生菌调节肠道菌群以及抗衰老相关信号通路这两个方面来阐明益生菌的抗衰老作用。
3.1 调节肠道菌群 3.1.1 肠道菌群抗衰老作用简述肠道菌群栖息在人体肠道内,通过对食物中的碳水化合物进行代谢,释放各种代谢产物,对宿主的免疫功能、代谢和稳态维持产生积极作用,进而发挥抗衰老的作用。短链脂肪酸(Short-chain fatty acids,SCFA)是具有抗炎特性的主要代谢产物,在诱导T细胞极化中发挥作用[32-33]。特别是作为上皮细胞的主要能量源的丁酸盐,具有调节基因表达、增殖、分化、凋亡的作用[34]。最新的研究还发现,肠道细菌产生的H2能够抑制羟基自由基诱导的细胞衰老模型中H2O2的增加,从而显示出对细胞衰老的抑制作用[35]。
3.1.2 益生菌对肠道菌群的调节作用益生菌通过在肠道中的定植而向机体引入具有健康作用的菌株,并抑制病原菌的入侵,同时调节衍生代谢物而实现其延缓衰老的作用[16, 36]。用清酒乳杆菌处理可降低小鼠肠道中变形杆菌门和厚壁菌门的数量,以及变形杆菌门与拟杆菌门和厚壁菌门与拟杆菌门的比率[37]。用植物乳杆菌LC27、长双歧杆菌LC67治疗可显著减少乙醇诱导小鼠肠道中变形杆菌门数量,增加乙醇抑制的拟杆菌门和放线菌门数量[38]。此类结果同时表明,这些改变都能有效地减少代谢产物LPS的产生。另外,发现长期摄入含德氏乳杆菌的发酵酸奶使小鼠肠道内拟杆菌门与厚壁菌门的比例增加的同时,丁酸盐和丙酸盐的产生也增加[28]。
益生菌可以调节紧密连接蛋白的表达,对保护肠道屏障完整性和功能发挥有益作用。植物乳杆菌LC27和长双歧杆菌LC67处理乙醇诱导的小鼠,可以增加被抑制的紧密连接蛋白occludin和claudin-1表达[38]。同样地,在芽孢杆菌处理的小鼠中,紧密连接蛋白ZO-1和occludin的表达显著增加[39]。该作用有利于预防微生物及其代谢产物进入血液循环而引起的全身性炎症。另外,有研究表明,益生菌混合物IRT5可以通过抑制肠道微生物群LPS的产生来抑制炎症疾病的发生[40]。SCFA可通过G蛋白偶联受体或是抑制组蛋白脱乙酰基酶而发挥其强抗炎作用[41]。
3.2 益生菌抗衰老相关信号通路 3.2.1 腺苷一磷酸激活蛋白激酶(Adenosine 5’-mo-nophosphate-activated protein kinase,AMPK)途径AMPK是一种介导寿命延长的细胞内能量传感器[42],其作为细胞能量稳态的主开关,通过抑制内质网应激和炎症性疾病,以及在衰老过程中诱导自噬来减少氧化应激而发挥作用[43-44]。最近的一项研究发现,发酵乳杆菌DR9、副干酪乳杆菌OFS 0291和瑞士乳杆菌OFS 1515的无细胞上清液显示出很强的促进AMPK的磷酸化活性[45]。在体外,利用清酒乳杆菌对caco-2细胞的处理也发现了AMPK活性增强的现象[37]。因此,许多益生菌已经证明具有提高AMPK活性的作用。
据报道,AMPK的激活能够调节肌肉中的线粒体相关基因,与老年小鼠运动功能的改善相关[46]。Hor等[45]研究发现乳酸杆菌除了在上坡运动试验期间提高D-半乳糖大鼠模型的运动性能外,还降低了骨骼和肌肉中的衰老标记物p53基因的表达。表明乳酸杆菌能以AMPK依赖性方式保护骨骼和肌肉免受衰老。此外,已有研究证实肝细胞、胆管细胞的衰老是引发非酒精性肝病(NAFLD)肝纤维化的进展因素[47],而Hitoshi等[48]的研究显示,对于采用胆碱缺乏、L-氨基酸补充饮食方式诱导的NAFLD大鼠,通过喂养MIYAIRI 588丁酸梭菌菌株,表现出AMPK的激活,从而通过调节脂质和能量代谢、胰岛素敏感性以及氧化应激反应来显著抑制NAFLD进展。因此,表明乳酸杆菌和丁酸梭菌能以AMPK依赖性方式分别延缓骨骼细胞、肌肉细胞以及肝细胞、胆管细胞的衰老。益生菌通过AMPK通路的调节途径如图 1所示。

|
| 图 1 益生菌对AMPK信号通路的影响 |
NF-κB途径是衰老的关键媒介之一,该通路的激活与引起衰老的相关因素密切相关。氧化应激过程活性氧水平的升高,使抑制蛋白Iκb被I kappa b激酶磷酸化,再经过泛素连接酶的泛素化而降解[49],NF-κB发生核转位而增强其信号传导[50]。炎症也可以调节NF-κB家族成员的激活,从而触发组织特异性衰老相关的退行性过程[51]。并且NF-κB信号的激活也被证实是细胞DNA损伤的标志之一[52]。
通过老化组织的生物信息学分析发现,NF-κB被认为是老化过程中基因表达变化最相关的转录因子[53],控制着细胞中一些细胞因子、生长因子、趋化因子和细胞黏附分子的基因表达[54]。此外,Warnier等[55]新发现的一种对衰老有调节作用的SCN9A钠通道也被证实其由NF-κB诱导表达,引起质膜去极化,从而表现出促衰老特性。
随着年龄的增长,各种组织中的NF-κB都会增加,通过抑制NF-κB通路的活性对衰老的干预成为可能,益生菌就是NF-κB的有效抑制剂之一。研究发现,植物乳杆菌C29通过抑制NF-κB信号传导途径改善老年小鼠的年龄依赖性结肠炎[56]。与Jang等[37]在清酒乳杆菌对长期高脂饮食诱导肥胖小鼠作用的研究结果相一致。其可能是通过作用于NF-κB的上游信号物质而抑制NF-κB的激活。已知Toll样受体(Toll-like receptors,TLRs)可以介导NF-κB通路信号传导的激活[57],其中,TLR4在脂多糖(Lipopolysaccharide,LPS)诱导下可通过激活髓系分化因子88(MyD88)依赖性和myd88独立途径,导致信号传导产生各种炎症性细胞因子[58]。有研究表明,用乳酸菌处理后的caco-2细胞显示出TLR信号负性调节因子Tollip、SIGIRR和IRAKM-1的表达增加[57]。在TLR-NF-κB信号通路中,也有研究发现鼠李糖乳杆菌LGG可以通过降低IκB的降解速率来抑制NF-κB的核转位,从而导致IL-8表达水平下降[59]。植物乳杆菌HY115和短乳杆菌HY7401可以通过抑制IκB的磷酸化来降低NF-κB的激活,进而抑制IL-1β、TNF-α等mRNA的表达,降低炎症反应[60]。
另外,在外源应激下,由巨噬细胞产生的TNF-α、IL-6和IL-1β可额外激活NF-κB通路[61],而Wu等[62]发现含有植物乳杆菌的发酵乳能够抑制LPS诱导这些因子的产生,通过荧光素酶法发现,含有植物乳杆菌的发酵乳可显著抑制NF-κB的转录活性。因此,益生菌也可以通过抑制NF-κB的转录活性而减少促炎症基因的表达。益生菌对NF-κB信号通路的影响如图 2所示。
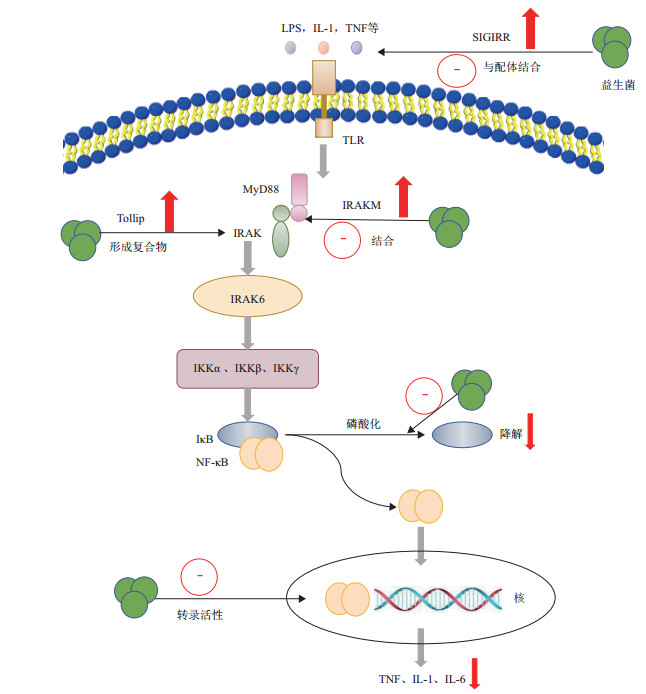
|
| 图 2 益生菌对NF-κB信号通路的影响 |
但是,并不是所有的益生菌都能抑制NF-κB通路,如Jensen等[63]研究发现罗伊氏乳杆菌能显著引起NF-κB活化,这可能与不同菌株的细胞壁组分肽聚糖和脂磷壁酸、不同环境下菌株菌毛的表达[64]、表面层SLPA蛋白[18]存在着差异性有关。这也暗示益生菌抗衰老的研究应该在株水平上进行。
4 展望目前,益生菌抗衰老以及预防年龄相关的退行性疾病的作用已经成为研究热点,但是大多数研究都是通过益生菌的抗氧化能力、提高免疫力、减少炎症这3方面来侧面表征抗衰老作用,因此需要进一步明确益生菌体内抗衰老作用以及相关的分子机制,深入研究益生菌对其作用的信号通路,为益生菌在缓解衰老方面的应用提供理论基础。此外,有必要明确益生菌中发挥缓解衰老的功效成分,针对不同益生菌菌株所具备的不同作用效应,开发复合益生菌产品以增强抗衰老作用将成为未来研究的趋势。
| [1] |
Georges J, Liesbeth V. Evidence for the hallmarks of human aging in replicatively aging yeast[J]. Microbial Cell, 2016, 3(7): 263-274. DOI:10.15698/mic2016.07.510 |
| [2] |
Park MR, Yun HS, Son SJ, et al. Short communication: Development of a direct in vivo screening model to identify potential probiotic bacteria using Caenorhabditis elegans[J]. Journal of Dairy Science, 2014, 97(11): 6828-6834. DOI:10.3168/jds.2014-8561 |
| [3] |
Pangrazzi L, Meryk A, Naismith E, et al. "Inflamm-aging" influences immune cell survival factors in human bone marrow[J]. European Journal of Immunology, 2017, 47(3): 481-492. DOI:10.1002/eji.201646570 |
| [4] |
Shao P, Guo NF, Wang C, et al. Aflatoxin G(1)induced TNF-alpha-dependent lung inflammation to enhance DNA damage in alveolar epithelial cells[J]. Journal of Cellular Physiology, 2019, 234(6): 9194-9206. DOI:10.1002/jcp.27596 |
| [5] |
Zhao J, Zheng HY, Zhong G, et al. Ursolic acid exhibits anti-inflammatory effects through blocking TLR4-MyD88 pathway mediated by autophagy[J]. Cytokine, 2019, 123: 154726. DOI:10.1016/j.cyto.2019.05.013 |
| [6] |
Watanabe M, Toyomura T, Wake H, et al. Advanced glycation end products attenuate the function of tumor necrosis factor-like weak inducer of apoptosis to regulate the inflammatory response[J]. Molecular and Cellular Biochemistry, 2017, 434(1-2): 153-162. DOI:10.1007/s11010-017-3045-6 |
| [7] |
Bui TT, Rodriguez BE, Talero E, et al. Anti-inflammatory effect of resveratrol in old mice liver[J]. Experimental Gerontology, 2015, 64: 1-7. DOI:10.1016/j.exger.2015.02.004 |
| [8] |
Kasuya A, Tokura Y. Attempts to accelerate wound healing[J]. Journal of Dermatological Science, 2014, 76(3): 169-172. DOI:10.1016/j.jdermsci.2014.11.001 |
| [9] |
Kuilman T, Michaloglou C, Vredeveld LC, et al. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network[J]. Cell, 2008, 133(6): 1019-1031. DOI:10.1016/j.cell.2008.03.039 |
| [10] |
Lee JY, Paik IY, Kim JY. Voluntary exercise reverses immune aging induced by oxidative stress in aging mice[J]. Experimental Gerontology, 2019, 115: 148-154. DOI:10.1016/j.exger.2018.08.009 |
| [11] |
Baeeri M, Bahadar H, Rahimifard M, et al. α-Lipoic acid prevents senescence, cell cycle arrest, and inflammatory cues in fibroblasts by inhibiting oxidative stress[J]. Pharmacological Research, 2019, 141: 214-223. DOI:10.1016/j.phrs.2019.01.003 |
| [12] |
Garrido A, Cruces J, Ceprian N, et al. Oxidative-inflammatory stress in immune cells from adult mice with premature aging[J]. Int J Mol Sci, 2019, 20(3): 769-792. DOI:10.3390/ijms20030769 |
| [13] |
Salminen A, Kaarniranta K, Kauppinen A. Immunosenescence: the potential role of myeloid-derived suppressor cells(MDSC)in age-related immune deficiency[J]. Cellular and Molecular Life Sciences, 2019, 76(10): 1901-1918. DOI:10.1007/s00018-019-03048-x |
| [14] |
Vida C, Martinez DT, Cruces J, et al. Role of macrophages in age-related oxidative stress and lipofuscin accumulation in mice[J]. Redox Biology, 2017, 12: 423-437. DOI:10.1016/j.redox.2017.03.005 |
| [15] |
Landete José María, Pilar G, Rodríguez Eva, et al. Probiotic bacteria for healthier aging: immunomodulation and metabolism of phytoestrogens[J]. BioMed Research International, 2017, 2017. DOI:10.1155/2017/5939818 |
| [16] |
Michael C, Anthony B. The impact of diet and lifestyle on gut microbiota and, human health[J]. Nutrients, 2014, 7(1): 17-44. DOI:10.3390/nu7010017 |
| [17] |
Clark R, Salazar A, Yamada R, et al. Distinct shifts in microbiota composition during drosophila aging impair intestinal function and drive mortality[J]. Cell Reports, 2015, 12(10): 1656-1667. DOI:10.1016/j.celrep.2015.08.004 |
| [18] |
Wasilewska E, Zlotkowska D, Wroblewska B. Yogurt starter cultures of Streptococcus thermophilus and Lactobacillus bulgaricus ameliorate symptoms and modulate the immune response in mouse model of dextran sulfate sodium-induced colitis[J]. Journal of Dairy Science, 2019, 102(1): 37-53. DOI:10.3168/jds.2018-14520 |
| [19] |
Thevaranjan N, Puchta A, Schulz C, et al. Age-associated microbial dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction[J]. Cell Host & Microbe, 2017, 21(4): 455-466. |
| [20] |
郑晓楠, 张昊, 郭慧媛, 等. 益生菌抗衰老功能的研究进展[J]. 中国乳业, 2012(2): 50-53. DOI:10.3969/j.issn.1671-4393.2012.02.012 |
| [21] |
Bilinski T, Bylak A, Zadragtecza R. The budding yeast Saccharomyces cerevisiae as a model organism: possible implications for gerontological studies[J]. Biogerontology, 2017, 18(4): 631-640. DOI:10.1007/s10522-017-9712-x |
| [22] |
Imai S. The NAD World 2. 0: the importance of the inter-tissue communication mediated by NAMPT/NAD+/SIRT1 in mammalian aging and longevity control[J]. NPJ Systems Biology and Applications, 2016, 2: UNSP 16018. DOI:10.1038/npjsba.2016.18 |
| [23] |
Renata ZT, Magdalena KM, Malgorzata A, et al. Cell size influences the reproductive potential and total lifespan of the & IT Saccharomyces cerevisiae & iT yeast as revealed by the analysis of polyploid strains[J]. Oxidative Medicine and Cellular Longevity, 2018. DOI:10.1155/2018/1898421 |
| [24] |
Leung MCK, Williams PL, Benedetto A, et al. Caenorhabditis elegans: An emerging model in biomedical and environmental toxicology[J]. Toxicological Sciences, 2008, 106(1): 5-28. DOI:10.1093/toxsci/kfn121 |
| [25] |
Chalfie M, Tu Y, Euskirchen G, et al. Green fluorescent protein as a marker for gene expression[J]. Science, 1994, 263(5148): 802-805. DOI:10.1126/science.8303295 |
| [26] |
翟畅, 叶波平. 秀丽隐杆线虫与药物筛选[J]. 药物生物技术, 2017(5): 91-94. |
| [27] |
Lee HY, Lee SH, Min KJ. Insects as a model system for aging studies[J]. Entomological Research, 2015, 45(1). DOI:10.1111/1748-5967.12088 |
| [28] |
Cheng X, Zhou W, Zhang Y. The behavioral, pathological and therapeutic features of the senescence-accelerated mouse prone 8 strain as an Alzheimer's disease animal model[J]. Ageing Research Reviews, 2014, 13: 13-37. DOI:10.1016/j.arr.2013.10.002 |
| [29] |
Usui Y, Kimura Y, Satoh T, et al. Effects of long-term intake of a yogurt fermented with Lactobacillus delbrueckii subsp bulgaricus 2038 and Streptococcus thermophilus 1131 on mice[J]. International Immunology, 2018, 30(7): 319-331. DOI:10.1093/intimm/dxy035 |
| [30] |
Kolosova NG, Vitovtov AO, Muraleva NA, et al. Rapamycin suppresses brain aging in senescence-accelerated OXYS rats[J]. Aging, 2013, 5(6): 474-484. DOI:10.18632/aging.100573 |
| [31] |
Cohen AA. Aging across the tree of life: the importance of a comparative perspective for the use of animal models in aging[J]. Biochimica et Biophysica Acta(BBA)-Molecular Basis of Disease, 2017, 1864(9): 2680-2689. |
| [32] |
Levy M, Thaiss CA, Elinav E. Metabolites: messengers between the microbiota and the immune system[J]. Genes & Development, 2016, 30(14): 1589-1597. |
| [33] |
Smith PM, Howitt MR, Panikov N, et al. The microbial metabolites, short-chain fatty acids, regulate colonic T-reg cell homeostasis[J]. Science, 2013, 341(6145): 569-573. DOI:10.1126/science.1241165 |
| [34] |
Kim CH, Jeongho P, Myunghoo K. Gut microbiota-derived short-chain fatty acids, T Cells, and inflammation[J]. Immune Network, 2014, 14(6): 277-288. DOI:10.4110/in.2014.14.6.277 |
| [35] |
Sakai T, Kurokawa R, Hirano S, et al. Hydrogen indirectly suppresses increases in hydrogen peroxide in cytoplasmic hydroxyl radical-induced cells and suppresses cellular senescence[J]. Int J Mol Sci, 2019, 20(2): 456. DOI:10.3390/ijms20020456 |
| [36] |
Hor YY, Lew LC, Jaafar MH, et al. Lactobacillus sp. improved microbiota and metabolite profiles of aging rats[J]. Pharmacological Research, 2019, 146: 104312. DOI:10.1016/j.phrs.2019.104312 |
| [37] |
Jang HM, Han SK, Kim JK, et al. Lactobacillus sakei alleviates high-fat-diet-induced obesity and anxiety in mice by inducing AMPK activation and SIRT1 expression and inhibiting gut microbiota-mediated NF-kappa B activation[J]. Molecular Nutrition & Food Research, 2019, 63(6): 1800978. |
| [38] |
Kim WG, Kim HI, Kwon EK, et al. Lactobacillus plantarum LC27 and Bifidobacterium longum LC67 mitigate alcoholic steatosis in mice by inhibiting LPS-mediated NF-κB activation through restoration of the disturbed gut microbiota[J]. Food & Function, 2018, 9(8): 4255-4265. |
| [39] |
Kim B, Kwon J, Kim MS, et al. Protective effects of Bacillus probiotics against high-fat diet-induced metabolic disorders in mice[J]. PLoS One, 2018, 13(12): e0210120. DOI:10.1371/journal.pone.0210120 |
| [40] |
Jeong JJ, Woo JY, Ahn YT, et al. The probiotic mixture IRT5 ameliorates age-dependent colitis in rats[J]. International Immunopharmacology, 2015, 26(2): 416-422. DOI:10.1016/j.intimp.2015.04.021 |
| [41] |
Gyu JS, Hisako K, Yoshiyasu U, et al. Probiotic bifidobacterium breve induces IL-10-producing Tr1 cells in the colon[J]. PLoS Pathogens, 2012, 8(5): e1002714. DOI:10.1371/journal.ppat.1002714 |
| [42] |
Ulgherait M, Rana A, Rera M, et al. AMPK modulates tissue and organismal aging in a non-cell-autonomous manner[J]. Cell Reports, 2014, 8(6): 1767-1780. DOI:10.1016/j.celrep.2014.08.006 |
| [43] |
Jang HJ, Yang KE, Oh WK, et al. Nectandrin B-mediated activation of the AMPK pathway prevents cellular senescence in human diploid fibroblasts by reducing intracellular ROS levels[J]. Aging-US, 2019, 11(11): 3731-3749. DOI:10.18632/aging.102013 |
| [44] |
Wang ZW, Chen ZL, Jiang ZY, et al. Cordycepin prevents radiation ulcer by inhibiting cell senescence via NRF2 and AMPK in rodents[J]. Nature Communications, 2019, 10: UNSP 2538. DOI:10.1038/s41467-019-10386-8 |
| [45] |
Hor YY, Ooi CH, Khoo BY, et al. Lactobacillus strains alleviated aging symptoms and aging-induced metabolic disorders in aged rats[J]. Journal of Medicinal Food, 2019, 22(1): 1-13. DOI:10.1089/jmf.2018.4229 |
| [46] |
Kobilo T, Guerrieri D, Zhang Y, et al. AMPK agonist AICAR improves cognition and motor coordination in young and aged mice[J]. Learning & Memory, 2014, 21(2): 119-126. |
| [47] |
张晶, 王炳元, 施军平. 细胞衰老在非酒精性脂肪性肝病发生发展中的作用[J]. 临床肝胆病杂志, 2016, 32(3): 442-445. DOI:10.3969/j.issn.1001-5256.2016.03.008 |
| [48] |
Hitoshi E, Maki N, Noriko K, et al. Butyrate-producing probiotics reduce nonalcoholic fatty liver disease progression in rats: New insight into the probiotics for the gut-liver axis[J]. PLoS One, 2013, 8(5): e63388. DOI:10.1371/journal.pone.0063388 |
| [49] |
聂炼, 张爱忠, 姜宁, 等. 益生菌对Toll样受体-核转录因子-κB信号通路调控作用的研究进展[J]. 动物营养学报, 2018, 30(11): 4342-4348. DOI:10.3969/j.issn.1006-267x.2018.11.009 |
| [50] |
Sultuybek GK, Soydas T, Yenmis G. NF-kappa B as the mediator of metformin's effect on ageing and ageing-related diseases[J]. Clinical and Experimental Pharmacology and Physiology, 2019, 46(5): 413-422. DOI:10.1111/1440-1681.13073 |
| [51] |
Rendra E, Riabov V, Mossel DM, et al. Reactive oxygen species(ROS)in macrophage activation and function in diabetes[J]. Immunobiology, 2019, 224(2): 242-253. DOI:10.1016/j.imbio.2018.11.010 |
| [52] |
Wu Z, Miyamoto S. Many faces of NF-κB signaling induced by genotoxic stress[J]. Journal of Molecular Medicine, 2007, 85(11): 1187-1202. DOI:10.1007/s00109-007-0227-9 |
| [53] |
Adler AS, Sinha S, Kawahara TLA, et al. Motif module map reveals enforcement of aging by continual NF-kB activity[J]. Genes & Development, 2007, 21(24): 3244-3257. |
| [54] |
Dong QG, Sclabas GM, Fujioka S, et al. The function of multiple IkappaB: NF-kappaB complexes in the resistance of cancer cells to Taxol-induced apoptosis[J]. Oncogene, 2002, 21(42): 6510-6519. DOI:10.1038/sj.onc.1205848 |
| [55] |
Warnier M, Flaman JM, Chouabe C, et al. The SCN9A channel and plasma membrane depolarization promote cellular senescence through Rb pathway[J]. Aging Cell, 2018, 17(3): e12736. DOI:10.1111/acel.12736 |
| [56] |
Jeong JJ, Kim KA, Jang SE, et al. Orally administrated Lactobacillus pentosus var. plantarum C29 ameliorates age-dependent colitis by inhibiting the uuclear factor-kappa B signaling pathway via the regulation of lipopolysaccharide production by gut microbiota[J]. PLoS One, 2015, 10(11): e0142521. DOI:10.1371/journal.pone.0142521 |
| [57] |
Kanmani P, Kim H. Functional capabilities of probiotic strains on attenuation of intestinal epithelial cell inflammatory response induced by TLR4 stimuli[J]. BioFactors, 2019, 45(2): 223-235. DOI:10.1002/biof.1475 |
| [58] |
Jin X, Zhang M, Yang YF, et al. Saccharomyces cerevisiae β-glucan-induced SBD-1 expression in ovine ruminal epithelial cells is mediated through the TLR-2-MyD88-NF-κB/MAPK pathway[J]. Veterinary Research Communications, 2019, 43(2): 77-89. DOI:10.1007/s11259-019-09747-x |
| [59] |
Zhang LY, Li N, Caicedo R, et al. Alive and dead Lactobacillus rhamnosus GG decrease tumor necrosis factor-alpha-induced interleukin-8 production in Caco-2 cells[J]. Journal of Nutrition, 2005, 135(7): 1752-1756. DOI:10.1093/jn/135.7.1752 |
| [60] |
Lee HS, Han SY, Bae EA, et al. Lactic acid bacteria inhibit proinflammatory cytokine expression and bacterial glycosaminoglycan degradation activity in dextran sulfate sodium-induced colitic mice[J]. International Immunopharmacology, 2008, 8(4): 574-580. DOI:10.1016/j.intimp.2008.01.009 |
| [61] |
Jang SE, Hyam SR, Han MJ, et al. Lactobacillus brevis G-101 ameliorates colitis in mice by inhibiting NF-κB, MAPK and AKT pathways and by polarizing M1 macrophages to M2-like macrophages[J]. Journal of Applied Microbiology, 2013, 115(3): 888-896. DOI:10.1111/jam.12273 |
| [62] |
Wu SJ, Fang JY, Ng CC, et al. Anti-inflammatory activity of Lactobacillus-fermented adlay-soymilk in LPS-induced macrophages through suppression of NF-kappa B pathways[J]. Food Research International, 2013, 52(1): 262-268. DOI:10.1016/j.foodres.2013.02.053 |
| [63] |
Jensen H, Dromtorp SM, Axelsson L, et al. Immunomodulation of monocytes by probiotic and selected lactic acid bacteria[J]. Probiotics and Antimicrobial Proteins, 2015, 7(1): 14-23. DOI:10.1007/s12602-014-9174-2 |
| [64] |
Douillard FP, Ribbera A, Jarvinen HM, et al. Comparative genomic and functional analysis of Lactobacillus casei and Lactobacillus rhamnosus strains marketed as probiotics[J]. Applied and Environmental Microbiology, 2013, 79(6): 1923-1933. DOI:10.1128/AEM.03467-12 |





